Abstract
Scutellarin (SCU), a flavonoid glucuronide derived from Scutellaria barbata and Erigeron breviscapus, exhibits broad pharmacological effects with promising therapeutic potential in treating various chronic diseases. It has demonstrated efficacy in modulating multiple biological pathways, including antioxidant, anti-inflammatory, anti-apoptotic, and vasodilatory mechanisms. These protective roles make SCU a valuable compound in treating chronic diseases such as cerebrovascular diseases, cardiovascular diseases, neurodegenerative disorders, and metabolic diseases. Despite its multi-targeted effects, SCU faces challenges such as low bioavailability and limited clinical data, which hinder its widespread therapeutic application. Current research supports its potential to prevent oxidative stress, reduce inflammatory responses, and enhance cell survival in cells and rats. However, more comprehensive studies are required to clarify its molecular mechanisms and to develop strategies that enhance its bioavailability for clinical use. SCU could emerge as a potent therapeutic agent for the treatment of chronic diseases with complex pathophysiological mechanisms. This review examines the current literature on Scutellarin to provide a comprehensive understanding of its pharmacological activity, mechanisms of action, and therapeutic potential in treating chronic diseases.
Keywords: Scutellarin, pharmacological effects, therapeutic potential, chronic diseases, mechanisms
1 Introduction
Chronic diseases are the major cause of premature adult deaths globally, and older adults are more susceptible to most chronic diseases than younger adults (Su et al., 2023). According to the World Health Organization’s global report, 80% of chronic disease deaths occur in low- and middle-income countries. Approximately one in five people in China was older than 60 in 2020. Currently, Long-term pharmacotherapy is used increasingly to control symptoms and slow disease progression. However, the drugs used in the prevention and treatment have clear targets and certain efficacy, Long-term pharmacotherapy carries the risk of adverse drug reactions that account for more than 5% of acute admissions (Huang and Grady, 2022). Therefore, it is crucial to investigate more potent and safer pharmaceuticals for managing chronic diseases.
Erigeron breviscapus (Vant.) Hand.-Mazz. has been used by the Yi minority in Southwest China for treating stroke-induced paralysis and rheumatic joint pain. More recently, modern extraction techniques, such as gas chromatography (GC) and high-performance liquid chromatography (HPLC), have been employed to isolate active components like Scutellarin (SCU) from E. breviscapus (Fan et al., 2021). Moreover, it is a flavonoid glycoside compound, named by IUPAC as (2S,3S,4S,5R, 6S)-6-[5,6-dihydroxy-2-(4-hydroxyphenyl)-4-oxochromen-7-yl]oxy-3,4,5- trihy droxyoxane-2-carboxylic acid, with molecular formula C21H18O12 and the molecular weight 462.4 g/mol (Figure 1). Due to its low toxicity and wide availability, SCU exerts a range of diverse pharmacological activities in previous studies, including anti-inflammatory, anti-tumor, anti-apoptotic, anti-oxidation stress, and vasodilatory activities (Ma et al., 2024; Xie et al., 2023; Yuan et al., 2024), making it a promising compound in treating chronic diseases such as cerebrovascular diseases, cardiovascular diseases, neurodegenerative disorders, and metabolic diseases by regulating multiple biological pathways.
FIGURE 1.
SCU in the dried whole plant of Erigeron breviscapus.
However, while the therapeutic potential of SCU is evident, there are still significant barriers to its widespread clinical use. Low bioavailability limits its efficacy when administered orally. Additionally, the current research of SCU remains limited in terms of clinical trials, making it difficult to fully understand its therapeutic mechanisms and long-term effects in humans. More comprehensive studies are required to enhance its bioavailability and clarify its molecular mechanisms in various chronic diseases.
The research on SCU in the past 3 decades has led to the accumulated evidence that establishes its effectiveness in treating chronic diseases. This review aims to provide a comprehensive overview of the pharmacological effects, mechanisms of action, and therapeutic potential of SCU, with a focus on its role in treating chronic diseases. By examining the current literature, we highlight the promise of SCU as a multi-target therapeutic agent and identify the challenges that must be addressed to facilitate its clinical application. This review will serve as a resource for future investigations and facilitate the development of SCU as a therapeutic agent for chronic disease.
2 Pharmacological effect and potential mechanism of SCU
Over the past 3 decades, a large-scale effort was made to investigate the pharmacological effects of SCU. A comprehensive literature search was conducted using several databases, including Google Scholar (https://scholar.google.com), Web of Knowledge (https://www.webofknowledge.com), NCBI (https://www.ncbi.nlm.nih.gov), Springer Online Journals (https://link.springer.com), Elsevier Science Direct, and CNKI (https://www.cnki.net), covering publications up to 30 June 2024. Titles, abstracts, and full-text articles were screened to determine their relevance. After removing duplicates and excluding non-relevant articles, the remaining studies were synthesized to form the basis of this review.
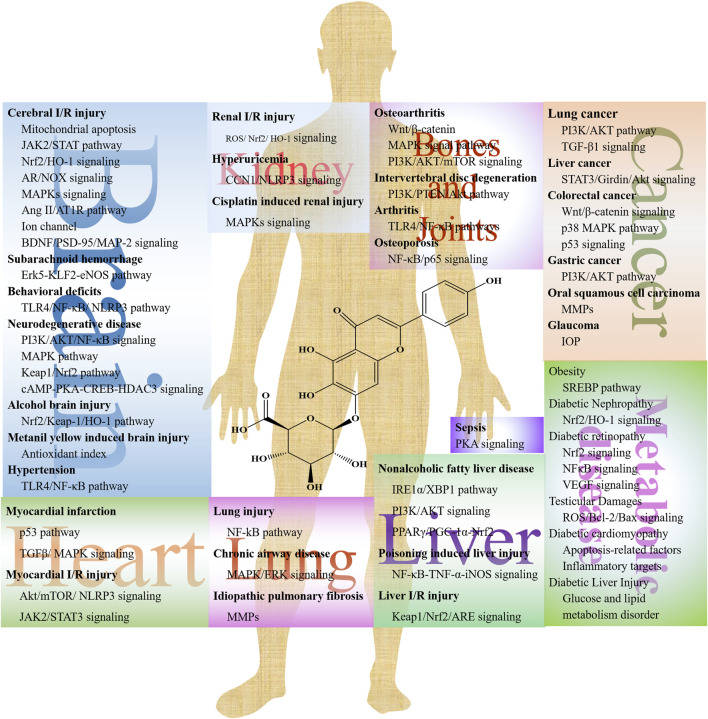
Numerous studies present the efficacy of SCU on cerebrovascular disease, cardiovascular disease, lung injury, and kidney injury (Figure 2). SCU has been reported to have a broad range of pharmacological effects, including vasodilation, anti-thrombotic action, anti-inflammatory, scavenging of free radicals, and improvement in microcirculation through in vivo and in vitro experiments. However, its underlying mechanism is still unclear. Table 1 shows the efficacy of SCU on different models, main targets, and diseases.
FIGURE 2.
The potential mechanisms of SCU in different diseases.
TABLE 1.
Summary of preclinical studies evaluating the effects of SCU in different diseases.
| Model | Main Target | Disease | Tissue | Reference | ||
|---|---|---|---|---|---|---|
| Male SD rats | MCAO-induced brain damage | In vivo | PARP↓, NAD↓ | I/R injury | Brain | Zhang et al. (2009) |
| Female SD rats | — | In vivo | GAP43↑, PTN↓, JAK2↓, STAT↓ | I/R injury | Brain | Niu et al. (2021) |
| Primary cortical neurons | In vitro | |||||
| SD rats | MCAO-induced brain damage | In vivo | p65↓, p38↓, ROS↓, MDA↓, SOD↑, CAT↑ | I/R injury | Brain | Zhang et al. (2022f) |
| GSH-Px↑, GSH↑, IL-1↓, IL-6↓, TNF-α↓ | ||||||
| Rats | MCAO-induced brain damage | In vivo | SOD↑, CAT↑, GSH↑, ROS↓ | I/R injury | Brain | Guo et al. (2011a) |
| Rat cortical neurons | In vitro | |||||
| C57BL/6N mice | MCAO-induced brain damage | In vivo | NOX1↓, NOX2↓, NOX4↓ | I/R injury | Brain | Deng et al. (2022) |
| AR−/− mice | ||||||
| Male SD rats | MCAO-induced brain damage | In vivo | eNOS↑, VEGF↓, bFGF↓, iNOS↓ | I/R injury | Brain | Hu et al. (2005) |
| Male SD rats | MCAO-induced brain damage | In vivo and in vitro | NOX2↓, 8-OHdG↓, 4-HNE↓, 3-NT↓ | Sun et al. (2018c) | ||
| Primary astrocytes | caspase-3↓, NeuN↓, connexin 43↑ | |||||
| Wistar rat, Neuronal cells | Neuron damage induced by hydrogen peroxide | In vitro | cNOS↓, NO↑ | Neuron damage | Brain | Liu et al. (2005) |
| SD rats | MCAO-induced brain damage | In vivo | PKG↑ | Cerebral ischemia | Brain | Chen et al. (2021b) |
| Rat brain microvascular | — | In vitro | NO↑, CD63↑, claudin 5↑, occludin↑, ZO1↑, LDH↓, ROS↓ | Cerebral ischemia | Brain | Zhong et al. (2019) |
| Endothelial cells | ||||||
| Rats | MCAO-induced brain damage | In vivo | XOD↓, ALT↓, AST↓, MDA↓ | I/R injury | Brain/Liver | Yang et al. (2003) |
| BV-2 cells, TNC1 astrocytes | MCAO-induced brain damage | In vitro | GFAP↑, Notch-1↑, NICD↑, HES-1↑, TNF-α↑, IL-1β↑, iNOS↑ | Cerebral ischemia | Brain | Fang et al. (2015) |
| Male SD rats, BV-2 cells | MCAO-induced brain damage | In vivo and in vitro | NF-κB↓, Notch-1↓, NICD↓, RBP-JK↓, Hes-1↓ MCP-1↓ | Cerebral ischemia | Brain | Yuan et al. (2015) |
| Male SD rats | MCAO-induced brain damage | In vivo and in vitro | p-p38↓, p-JNK↓, p-ERK1/2↑, iNOS↓, TNF-α↓, IL-1β↓ | Cerebral ischemia | Brain | Chen et al. (2020) |
| BV-2 cells | LPS induced BV-2 cells | p-JNK↓, p-p38 MAPKs↓ | ||||
| SD rats | MCAO-induced brain damage | In vivo | ACE↓, AT1R↓, Ang II↓, TNF-α↓, IL-6↓, IL-1β ↓ | Cerebral ischemia | Brain | Wang et al. (2016a) |
| Male Wistar rats | BCCAO-induced brain damage | In vivo | Glu↓, Asp↓, Gly↓, GABA↓, Tau↓, Ca 2+-ATPase↑ | Cerebral ischemia | Brain | Tang et al. (2014) |
| Na +, K +-ATPase↑ | ||||||
| SD rats | — | In vitro | p-VASP↑ | Hypoxia | Coronary artery | Chen et al. (2015) |
| SD rats, | Rats with cerebral I/R treatment | In vivo | P-VASP↑, PKG↑ | Hypoxia | Brai | Du et al. (2015) |
| HBMECs | In vitro | |||||
| Male SD rats, BV-2 cell | MCAO-induced brain damage | In vivo and in vitro | cyclin B1↑, cyclin B1↑, cyclin D1↑, NT-3↑ | Cerebral ischemia | Brain | Fang et al. (2016) |
| IGF-1↑, AP-2↑, PSD-95↑ | ||||||
| Male SD rats | — | In vivo | eNOS↑, p-Erk5↑, KLF2↑ | Subarachnoid hemorrhage | Brain | Li et al. (2016) |
| Male SD rats | LPS-induced behavioral deficits | In vivo | ROS↓NLRP3, caspase-1, IL-1β↓ | Depression | Brain | Bian et al. (2020) |
| Wistar rats | — | In vivo | IL-1β↓, IL-6↓, TNF-α↓, SOD↑, MAO↓ | Depression | Brain | Guo et al. (2013) |
| Male C57BL/6 mice | Depression-like behaviors | In vivo | TNFα↓, IL-1β↓, IL-6↓, iNOS↓, IL-4↑, BDNF↑ | Depression | Brain | Lu et al. (2021) |
| Primary astrocytes | induced by LPS | In vitro | ||||
| Male C57BL/6 mice | — | In vivo | GABAA Rα1↓, GABAAγ2↓, mEPSC↓ | Anxiety disorders | Brain | Guo et al. (2021) |
| MCF-7 cells | — | In vitro | Aggregation of beta-amyloid↓ | Alzheimer's disease | Brain | Zhu et al. (2009) |
| Male APPswe/PS1dE9 mice | — | In vivo and in vitro | Aβ aggregation↑, soluble Aβ oligomers↓, Aβ42↓, Aβ40↓ | Alzheimer's disease | Brain | Zhang et al. (2020) |
| C57BL/6 mice | ||||||
| BV-2 cells | — | In vitro | NO↓, TNFα↓, IL-1β↓, ROS↓, iNOS↓, TNFα↓, IL-1β↓ | Alzheimer's disease | Brain | Lu et al. (2021) |
| SH-SY5Y cells | NF-κB↓, JNK↓, p38↓, IFN-γ↓, STAT1α↓ | |||||
| HT22 cell | — | In vivo and in vitro | Lactate dehydrogenase↓, lactate dehydrogenase↓, ROS↓, Aβ1‑42↑ | Alzheimer's disease | Brain | Chiba et al. (2012) |
| Balb/c male mice | p-Tau↓, ROS↓, Bcl-2↑, Bcl-xL↑, Bax↓, cleaved caspase-3↓ | |||||
| APP/PS1 transgenic mice | — | In vivo and in vitro | Aβ plaque↓, TNF-α↓, IL-6↓ | Alzheimer's disease | Brain | Zeng et al. (2018) |
| WT mice, H-SY5Y cell line | ||||||
| Male SD rats | Permanent bilateral | In vivo | Aβ (1-40) ↓, Aβ (1–42) ↓, Iba1↓ | Vascular dementia | Brain | Shin et al. (2018) |
| Common carotid artery occlusion | Alzheimer's disease | |||||
| Male Wistar rats | In vivo | nAChR↑, nAChR α4↑, α7↑ | Cognitive disorder | Brain | Guo et al. (2011b) | |
| APP/PS1 transgenic mice | — | In vivo | SCFAs, IL-1β↓ | Alzheimer's disease | Brain | Zhang et al., (2022c) |
| BALB/cmale mice | — | In vivo | MDA↓, SOD↑, IL-1β↓, IL-6↓, HO-1↓, NQO1↑, Nrf2↓ | Alcohol induced brain injury | Brain | Zhang et al. (2022d) |
| BV-2 cells | — | In vitro | NF-κB-p65↓, TNF-α↓, IL-1β↓, IL-6↓, NO↓, TNF-α↓, IL-1β↓ | neuroinflammation | Brain | You et al. (2018) |
| IL-6↓, iNOS↓, IκB↓, IKKβ↓, p38↓, JNK↓, AKT↓ | ||||||
| Male albino Wistar rats | — | In vivo | MDA↓, SOD↑, GSH↑, AChE↓, NF-κB↓, TNFα↓, IL-6↓ | Cognitive disorder | Brain | Baluchnejadmojarad et al. (2018) |
| Nrf2↑, beclin-1↓, LC3 II↓, mTOR↓, P62↓ | ||||||
| Male C57BL/6 mice | — | In vivo and in vitro | nestin↑, Tuj-1↑, ERK1/2↑ | Cognitive disorder | Brain | Wang et al. (2017) |
| Neural stem cells | ||||||
| Male Wistar albino rats | Metanil yellow induced | In vivo | GFAP↓, cleaved caspase-3↓, MDA↓, SOD↑, GSH↑ | Gliosis | Brain | Tawfeek et al. (2021) |
| Male SD rats | — | In vivo | TLR4↓, NF- κ B p65↓, TNF- α↓, IL-1 β↓, IL-18↓ | Hypertension | Brain | Chen et al. (2013) |
| Bax↓, cleaved-caspase-3 p17↓, Mcl1↑ | ||||||
| Male SD rats | — | In vivo | CTn-T↓, CTn-I↓, AST↓, LDH↓, SOD↑, CAT↑GSH↑ | Myocardial infarction | Heart | Huang et al. (2018) |
| Caspase3↓, Caspase9↓, cytochrome C↓, NGAL↓, NFκB↓, IL-1β↓ | ||||||
| P53↓, IL-6↓, Bcl2↑, MDA↓, iNOS↓, Bax↓ | ||||||
| Male Wistar rats | — | In vivo | FN1↓, TGFβ1↓, CFs↓, p38-MAPK↓, ERK1/2↓ | Cardiac fibrosis | Heart | Pan et al. (2011) |
| Male SD rats | — | In vivo | LVWI↓, RVWI↓, type I and type III collagen↓, MVD↑, CD31↑ | Cardiac fibrosis | Heart | Zhou et al. (2014) |
| α-sma ↓, Jagged1↑, Notch 1↑, Hes1↑ | ||||||
| C57BL/6 mice | — | In vitro and in vivo | CaMKII↓, β-MHC↑, ANP↑ | Cardiac hypertrophy | Heart | Pan et al. (2010) |
| Cardiac myocytes | ||||||
| SD rats, H9c2 cells | — | In vitro and in vivo | NLRP3↓, mTORC1↓, p-Akt↑, Casp-1↓, IL-1β↓ | Myocardial I/R injury | Heart | Xu et al. (2020) |
| Rats, endothelial cell | — | In vitro and in vivo | P-JAK↓, P-STAT3↓ | Myocardial I/R injury | Heart | Lin et al. (2014) |
| HCMECs | — | In vitro | JAK2↓, p-JAK2↓, STAT3↓, p-STAT3↓ | Myocardial ischemia | Heart | Chen et al. (2019) |
| H9c2 cells | — | In vitro | JAK/STAT3↑, Bcl2↑, VEGF↑, MMP2↑, MMP9↑, TNFα↓ | Myocardial I/R injury | Heart | Wang et al. (2016b) |
| IL-8↓, CK ↓, NO↑, ROS↓, SOD ↑, MDA ↓, STAT3↑, Bcl2↑ | ||||||
| VEGF↑, MMP2↑, MMP9↑, IL-1β↓, IL-6↓ | ||||||
| HCMECs | — | In vitro | EIF6↓, HSPD1↑, CCT6A↑ | Anoxia | Heart | Shi et al. (2015) |
| HCMECs, SD rats | MIR model | In vitro and in vivo | PKG-I↑, PKG-Iα↑ | Myocardial I/R injury | Heart | Li et al. (2015) |
| Male SD rats | LPS induced lung injury | In vivo | ROS↓, SOD↓, IL-1β↑, IL−18↑, IL−6↑, IL−4↑, IL−10↑, MDA↓ | Lung injury | Lung | Fan et al. (2022) |
| Mice | Injected with a dose of LPS | In vivo | TNF-α↓, iNOS↓, c-Fos↓, iNOS↓, NF-kappaB↓, IkBa↓, GSH↑ | Lung injury | Lung | Tan et al.(2010) |
| Male albino rats | Rat Model of Bilateral | In vivo | iNOS↑, Bax↑, Bcl2↓, COX2↓ | Posterior limb I/R injury | Lung | Ibrahim et al. (2019) |
| Hind Limb I/R | ||||||
| HBE-16 cell | In vitro | MUC5AC↓, p-PKC↓, ERK1/2↓ | Airway mucus secretion | Lung | Jiang et al. (2011b) | |
| HBE-16 cell, Male SD rats | In vivo and in vitro | MUC5AC↓, PKC↓, ERK1/2↓ | Airway mucus secretion | Lung | Jiang et al. (2011a) | |
| Male BALB/c mice | BLM-induced | In vivo and in vitro | p-p65/p65 ratio↓, IκBα↓NLRP3↓, caspase-1↓, caspase-11↓, IL-1β↓ | Pulmonary fibrosis | Lung | Peng et al. (2020) |
| A549 cell, RLE-6TN cell | IL-18↓, fibronectin↓, vimentin↓, N-cadherin↓, MMP-2↓, MMP-9↓ | |||||
| HK-2 cells, Wistar rats | — | In vitro and in vivo | HO-1↑, SCr↓, BUN↓, KIM-1↓, ROS↓ | I/R injury | kidney | Dai et al. (2022) |
| HK-2 cells | — | In vitro | NGAL↓, Kim-1↓, cystatin C↓, IL-18↓, NLRP3↓, CCN1↑ | Hyperuricemia | kidney | Li et al. (2020a) |
| Male C57BL/6 mice | In vivo | |||||
| Male C57BL/6 mice | — | In vivo | BUN↑, CRE↑, TNF-α↓, IL-6↓, Cleaved caspase-3↓ | Chemotherapy toxicity | kidney | Sun et al. (2019) |
| Cleaved PARP↓, p53↓, Bax/Bcl-2↓, LC3-II/LC3-I↑, Atg7↑ | ||||||
| p62↓JNK↓, ERK↓, p38↓, stat3↓ | ||||||
| HepG2 cells | Acid-treated HepG2 cells | In vivo | XBP1↓, SREBP-1c↓, IRE1α↓ | NAFLD | Liver | Zhang et al. (2022e) |
| C57/BL6 mice | HFD-induced | In vitro | ||||
| HepG2 cells | — | In vitro | CD36↓, Fasn↓, ACC↓, AKT↑, mTOR↓, n-SREBP-1c↓ | Hepatocyte lipid metabolism | Liver | Han and Wang (2018) |
| Male C57BL/6 mice | In vivo | |||||
| Mice | HFD-induced | In vivo and in vitro | SREBP-1c↓, mTOR↓ | Hepatocyte lipid metabolism | Liver | Luan et al. (2020) |
| HepG2 cells | PA-treated HepG2 cells | |||||
| Male C57BL/6 mice | HFD induced mice | In vivo and in vitro | PPARγ↑, PGC-1α↑, Nrf2↑, HO-1↑, GST↑, NQO1↑ | NAFLD | Liver | Zhang et al. (2018) |
| HepG2 cells | Oleic acid induced cells | NF-κB↓, Keap1↓ | ||||
| SD rats | HFD-induced | In vivo | Nrf2, HO-1, and PI3K, and AKT ↑HO-1, NQO1, and Nrf2↑ | NAFLD | Liver | Fan et al. (2017) |
| subjected to chronic stress | ||||||
| BALB/c mice | CCl4-induced liver injury | In vivo | AST↓, ALT↓, TBIL↓, IL-1β↓, TNF-α↓, CYP2E1↓, IκBα/NF-κB↓ | Hepatotoxicity | Liver | Miao et al. (2021) |
| S180 tumor-bearing mic | DB-induced liver injury | In vivo | MPO↑, IκB↓, NF-κB p65↓, TNF-α↓, IL-6↓, IFN-γ↓, MDA↓, GPx↑ | Hepatotoxicity | Liver | Niu et al. (2015) |
| Male ICR mice | Induced by concanavalin A | In vivo | ALT↓, AST↓, TNF-α↓, iNOS↓, c-Fos↓, c-Jun↓, iNOS↓, IkappaB↑ | Hepatitis autoimmune | Liver | Tan et al. (2007) |
| Male Wistar rats | Se-treated rats | In vivo | MDA↑, GSH-Px↑, TR↑ | Hepatotoxicity | Liver | Eltayeb et al. (2004) |
| HL-7702 | Under hypoxic condition | In vitro | ROS↓, MDA↓, SOD↑, bcl-2↑, Keap1↓ Nrf2↑, HO-1↑, NQO1↑, Nrf2↓ | I/R injury | Liver | Wu and Jia (2019) |
| Chondrocytes | — | In vitro | MMP1↓, MMP13↓, ADAMTS-5↓, Wnt3a↓, Frizzled7↓ | Osteoarthritis | Bones and joints | Liu et al. (2020) |
| C57BL/6 male mice | In vivo | Collagen II↑, Aggrecan↑ | ||||
| C57BL/6 mice | — | In vivo and in vitro | MMP-13↓, ADAMTS-5↓, COX-2↓, iNOS↓, IL-6↓, | Osteoarthritis | Bones and joints | Luo et al. (2020) |
| Chondrocytes | TNF-α↓, PGE2↓, IL-1β↓, NF-κB↑, Nrf2↑ | |||||
| BL6/C57 male mice | — | In vivo and in vitro | TNF-α↓, IL-1β↓, iNOS↓, MMP13↓, ADAMTS-5↓ | Osteoarthritis | Bones and joints | Wang et al. (2019) |
| Chondrocyte cells | COX-2↓, IL-6↓, NO↓ | |||||
| SW1353 cells | — | In vitro | IL-6↓, AKT↓, mTOR↓, p-mTOR↓, CH25H↓ | Osteoarthritis | Bones and joints | Ju et al. (2021) |
| CYP7B1↓, ABCA1↑, APOA-1↑ | ||||||
| Human Enucleus | — | In vivo and in vitro | ROS↓, NF-κB↓, MAPK↓, TNF-α↓, NLRP3↓ | Intervertebral | Bones and joints | Wang et al. (2022b) |
| Pulposus Cells | disc degeneration | |||||
| Male SD rats | ||||||
| SD rats | — | In vivo and in vitro | ATG5↑, Rab8a↑, PI3K↑, PTEN↑, Akt↑ | Intervertebral | Bones and joints | Hu et al. (2022) |
| Nucleus pulposus cells | disc degeneration | |||||
| Male DBA/1J mice | collagen‑induced arthritis | In vivo | IL‑1β↓, IL‑6↓, TNF‑α↓, Caspase‑3/-9↓Bax/Bcl‑2↓TLR4↓NF‑κB↓ | Arthritis | Bones and joints | Zhang et al., (2017) |
| Raw264.7 cell line | — | In vivo and in vitro | RANKL↓, MAPK↓, NF-κB↓, JNK1/2↓, p38↓, ERK1/2↓, IκBα↓ | Arthritis | Bones and joints | Zhao et al. (2016) |
| C57BL/6 male mice | ||||||
| Osteoblasts female SD rats | — | In vivo and in vitro | ALP secretion↑, intracellular calcium ion influx↑ | Osteoporosis | Bones and joints | Wang et al. (2018) |
| calcium deposition↑, CXCR4↑, p65↑ | ||||||
| 3T3-L1 cells | In vitro | PPARγ↓ C/EBPα↓ | Obesity | Metabolic disease | Lu et al. (2013) | |
| db/db mice db/m+ mice | — | In vivo | Nrf2↑, HO-1↑, IL-1β↓, IL-2↓, IL- 4↑ | Diabetic Nephropathy | Diabetic complication | Liu et al. (2019b) |
| C57BL/6 male mice | — | In vitro and in vivo | laudin-1↑, claudin-19↑, NFκB↓, TNF-α↓, p-ERK 1/2↓, Nrf2↑ | Diabetic retinopathy | Diabetic complication | Mei et al. (2019) |
| WT mice and HRECs | ||||||
| Human retinal endothelial cells | — | In vitro | VEGF↓, p-ERK↓, p-FAK↓, p-Src↓ | Diabetic retinopathy | Diabetic | Long et al. (2019) |
| Rats | In vivo | complication | ||||
| Male Wistar rats | — | In vivo | MDA↓, ROS↓, Bcl-2↑, BAX↓, VEGF↑ | Testicular Damages | Diabetic | Long et al. (2015) |
| complication | ||||||
| Male SD rats | — | In vivo | blood glucose↓, TC↓, TG↓ LDL↓, HDL↑, LDH1↓, CK↓, | Diabetic cardiomyopathy | Diabetic | Su et al. (2022) |
| Beclin-1↑, LC3-II↑ | complication | |||||
| Male Swiss mice | HFD-induced | In vivo | CK-MB↓, Troponin↓, BNP↓, SOD↑, CAT↑, GPx↑, GST↑, Nrf2↓ | Diabetic cardiomyopathy | Diabetic | Huo et al. (2021) |
| Nqo-1↓, Ho-1↓, Tlr4↓, Myd88↓, Nf-κb↓, IL-6↓, TNf-α↓, IKKβ↑ | complication | |||||
| Cyt-c↓, Parp 1↓, bcl-2↑, caspase-3↓, caspase-9↓, Bax↓ | ||||||
| HUVECs | — | In vitro | Bcl-2↓, Bax↓ Cyt c↓, ROS↑, SOD↑, SOD2↑, LC3 II↑, Beclin 1↑ | Diabetic | Diabetic | Xi et al. (2021) |
| Atg 5↑, PINK1↑, Parkin↑, Mitofusin2↑ | cardiomyopathy | complicatio | ||||
| Male C57/B6 mice | — | In vivo | NLRP3↓, NF-κB↓, p-AKT↓, Nrf2↓, HO-1↓, EF↓, LVVd↓ | Diabetic | Diabetic | Xu et al. (2021) |
| CK↓, Col I↓, TGF-β1↓, SOD↑, CAT↑, GSH-Px↑, MDA↓ | cardiomyopathy | complication | ||||
| ROS↓, IL-1β↓, IFN-γ↓, IL-6↓, MCP-1↓, TNF-α↓, IL-18↓ | ||||||
| LDH↓, cTnI↓ | ||||||
| LO2 | Induced by Hcy | In vivo and in vitro | Hcy↓, TG↓, CHO↓, LDL↓, ALT↓, AST↓, insulin↓, CBS↓, CSE↓ | Diabetic | Diabetic | Wang et al. (2020) |
| Male SD rats | High-fat induced | MTHFR↓, folic acid↑, VitB6↑, VitB12↑ | Liver Injury | complication | ||
| PC-9, H1975, Hela cells | — | In vitro | p-ERK1/2↑ ERK1/2↓, p-AKT↓, AKT↓, LC3-II↓ | Lung cancer | Cancer | Sun et al. (2018a) |
| HepG2, Beas-2B cells | p-ERK1/2↑, p-AKT↓ | |||||
| A549 cells | — | In vitro | G0/G1↓, AKT↓, mTOR↓, BCL-XL↓, STAT3↓, p-STAT3↓, 4EBP1↑ | Lung cancer | Cancer | Cao et al. (2019) |
| A549 cells | — | In vitro | ROS↑, caspase-3↑, TGF-β1↓ | Lung cancer | Cancer | Zhang et al. (2021) |
| HepG2 and MHCC97-H cells | — | In vitro | EMT↓, p-JAK2↓, p-STAT3↓ E‐cadherin↑, snail↓, vimentin↓ | Liver cancer | Cancer | Liu et al. (2019a) |
| HepG2 and SK-Hep1 cells | — | In vitro | STAT3↓, Girdin↓, AKT↓ | Liver cancer | Cancer | Ke et al. (2017) |
| Male BALB/c nude mice | In vivo | |||||
| C57BL/6 male mice | CAC caused by AOM/DSS | In vivo and in vitro | NF-κB↓, SHH↓, Ptch1↓, Smo↓, Gli1↓ | Colorectal cancer | Cancer | Zeng et al. (2022) |
| SW480 cells | ||||||
| SW620, HCT116, LOVO | — | In vitro and in vivo | ephrinb2↓, ephB6↓, ephA1↓ | Colorectal cancer | Cancer | Zhu et al. (2017) |
| HT29 cells and Balb/c nude mice | ||||||
| HT-29 CSC cells | — | In vitro and in vivo | Lgr5↓, c-Myc↓, CK20↓, Nanog↓, Gli1↓, CD133↓, Lgr5↓ | Colorectal cancer | Cancer | Lei et al. (2020) |
| Nude mice | Gli1↓, Ptch1↓, c-Myc↓, Ki-67↓, CK20↓ | |||||
| HCT116 p53+/+ (wild-type) | — | In vitro | caspase-6↑ | Colorectal cancer | Cancer | Chan et al. (2009) |
| p53-/- (knockout) cells | ||||||
| Male C57BL/6 mice | Induced by azoxymethane and | In vivo | Wnt/β-catenin↓, TNF-α↓, IL-6↓, Bax↑ | Colorectal cancer | Cancer | Zeng et al. (2021) |
| HT-29 cells | dextran sulfate sodium | In vitro | Bcl-2↓, GSK-3β↓, cyclin D1↓ | |||
| Mice | — | In vivo | p38 MAPK↓, TNFR2+Tregs↓, CD8+T↑ | Colorectal cancer | Cancer | Chen et al. (2022) |
| WEHI-13VAR and CT26 cell | In vitro | |||||
| HCT‑116 cells | — | In vitro | Bcl‑2↓, Bax↑, caspase‑3↑, p-p53↑ | Colorectal cancer | Cancer | Yang et al. (2017) |
| AGS | — | In vitro and in vivo | LDH↓, G0-G1↑S↓, G2-M↓, SOD↑, GSH↑, CAT↑, MDA↓, 8-OHdG↓ | Gastric cancer | Cancer | Sun and Meng (2022) |
| MGC-803 and AGS | — | In vitro | PI3K↓, PTEN↑ | Gastric cancer | Cancer | Li et al. (2021) |
| SAS cells | — | In vitro and in vivo | MMP-2 and -9↓, integrin αvβ6↓, c-JUN↓ | Oral squamous cell carcinoma | Cancer | Li et al. (2013) |
| Athymic Balb/ca nude mice | ||||||
| SAS and HSC-4 cells | — | In vitro | E-cadherin↑, αvβ6 integrin↓ | Oral squamous cell carcinoma | Cancer | Li et al. (2010) |
| C57BL/6J mice | — | In vivo | retinal thinning and reduced visual behavioral deficits | Glaucoma | Eye | Pang and Clark (2020) |
| Female C57BL/6 mice | LPS-primed macrophages | In vivo and in vitro | NLRP3↓, IL-1β↓, caspase-1 ↓ | Sepsis | Multiple Organ | Liu et al. (2017) |
2.1 Cerebrovascular diseases
2.1.1 Cerebral ischemia/reperfusion injury
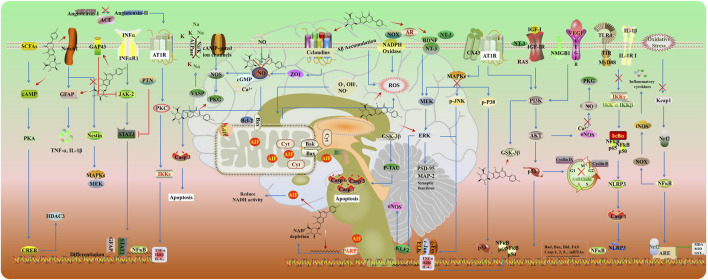
Cerebral ischemia/reperfusion injury (CIRI) refers to brain damage that occurs when blood supply is restored to the brain following a period of ischemia. The primary treatment involves timely thrombolytic therapy or surgical intervention (Zhang et al., 2024). However, reperfusion can potentially promote secondary cell death and exacerbate brain injury, leading to cerebral ischemia/reperfusion injury (Zheng et al., 2023). In the initial stage of ischemia, insufficient blood flow during cerebral ischemia results in an inadequate supply of glucose, and oxygen, low ATP, and excessive glutamate excitatory toxicity (Huang et al., 2023). Consequently, excessive release of glutamate leads to calcium (Ca2+) overload and further triggers the generation of free radicals and nitric oxide (NO), initiating a cascade of detrimental processes. These include mitochondrial dysfunction and DNA damage, which collectively promote oxidative stress and neurotoxicity. Upon reperfusion, the accumulation of reactive oxygen species (ROS) and inflammatory cells, such as neutrophils, exacerbates the ischemic damage. These pathological processes are associated with oxidative stress, destruction of the blood-brain barrier, inflammation, apoptosis, and ionic imbalance (Candelario-Jalil et al., 2022; Oyefeso et al., 2021). The signal transduction pathways involved in CIRI are summarized in Figure 3. Therefore, it is imperative to explore novel drugs that target the underlying pathological progression of CIRI, to enhance neurological recovery and prognosis in patients. The action mechanism of SCU protected against CIRI mainly includes anti-apoptosis, anti-oxidative stress, anti-inflammatory, and regulation of the ion channel.
FIGURE 3.
Potential mechanisms and targets of cerebral protection effect.
Increasing evidence suggests that cerebrovascular disease is closely linked to multiple forms of programmed cell death (PCD), such as apoptosis, autophagy, pyroptosis, and ferroptosis. Consequently, the targeted inhibition of these PCD pathways plays a critical role in mitigating the severity of cerebrovascular diseases and improving neurological outcomes (Zhang et al., 2022a). NO and tumor necrosis factor (TNF-α) activate intrinsic and extrinsic pathways of apoptosis in CIRI. Emerging evidence indicates that apoptosis involves the synthesis of new proteins (Gong et al., 2017). Poly (ADP-ribose) polymerase (PARP) is a DNA-binding protein that utilizes nicotinamide adenine dinucleotide (NAD) as a substrate and is activated by extensive DNA damage (Chatterjee et al., 2022). PARP-1 produces long and branched poly-ADP ribose (PAR) polymers. PAR production and translocation to the cytosol induces a cascade of events, including PAR binding to mitochondrial apoptosis-inducing factor (AIF), AIF translocation to the cytosol, AIF binding to macrophage migration inhibitory factor (MIF), co-translocation of AIF-MIF complex to the nucleus, and large-scale DNA fragmentation by MIF nuclease activity. These steps result in subsequent cell death (Yang et al., 2024a).
In a rat model of middle cerebral artery occlusion (MCAO), SCU reduced the infarct volume and ameliorated the neurological deficit by inhibiting PARP overactivation and AIF translocation from the mitochondria to the nucleus following CIRI (Zhu et al., 2009). Moreover, SCU plays a neuroprotective role by reducing microglial neuroinflammation and apoptosis mediated by the activated PI3K/AKT/GSK3β/NF-κB signaling pathway in LPS-induced BV2 cell (Duan et al., 2024). In oxygen-glucose deprivation and reperfusion-induced HT22 cell injury, SCU pretreatment could improve mitochondrial dysfunction and inhibit apoptosis by stimulating mitophagy (Yang et al., 2024c).
Neonatal hypoxic-ischaemic encephalopathy (HIE) is a major cause of neonatal mortality due to its devastating impact on neonatal brain development (Greco et al., 2020). Increasing evidence indicates that HIE can lead to acute cerebral reperfusion injury, edema, increased intracranial pressure, impaired autoregulation, and hemorrhage, which are known as important pathologies of later neurodevelopmental impairments (Cao et al., 2020). Growth-associated protein 43 (GAP43), a nervous tissue-specific cytoplasmic protein, plays a crucial role in neurite outgrowth during axon development and regeneration. Inhibiting GAP43 expression exerts adverse effects on axon outgrowth (Dan et al., 2022). SCU treatment could improve cell viability, and ameliorate cell apoptosis and long-term neurological deficits after HI injury via upregulating GAP43 expression and inhibiting JAK/STAT3 signaling in oxygen-glucose deprivation-induced primary cortical neurons (Niu et al., 2021).
Reactive oxygen and nitrogen species (ROS/RNS) are continuously produced from internal metabolism and external exposures in mammalian systems. ROS/RNS in physiological amounts serve as mediators and regulators, ensuring proper cellular functions: growth, proliferation, differentiation, and apoptosis. However, the imbalance between the continuous production of reactive oxygen species and their elimination as a result of enzymatic and non-enzymatic neutralization reactions and the action of exogenous antioxidants causes oxidative stress, which leads to brain damage after a stroke and permanent or reversible neurological deficits (Pawluk et al., 2024). Due to hypoxia, there is a deficit of ATP, a decrease in energy, an influx of calcium, and, as a result, mitochondrial failure. Excitotoxicity and ROS/RNS activity stimulate nerve cells, mainly microglia and astrocytes, to secrete inflammatory markers. Increased activity of pro-inflammatory cytokines generates ROS, which are responsible for protein oxidation, peroxidation of polyunsaturated fatty acids, and disruption of redox homeostasis, ultimately leading to cell death (Wierońska et al., 2021). In the CIRI rat model, SCU is an efficient radical scavenger against ROS and RNS, including hydroxyl radical, superoxide anion radical, and hydrogen peroxide (Zhang Y. et al., 2022).
Nuclear factor erythroid 2-related factor 2 (Nrf2) is a vital transcription factor that regulates antioxidant defense and detoxification enzymes, including NAD(P)H quinone oxidoreductase, heme oxygenase-1 (HO-1), and glutathione S-transferases (GSTs) (He et al., 2020). SCU showed antioxidant activity by promoting Nrf2 nuclear translocation, upregulating HO-1 expression, increasing superoxide dismutase (SOD) activity, and inhibiting ROS generation in OGD/R-induced HT22 cells. Furthermore, SCU reduced infarct volume and blood-brain barrier (BBB) permeability, improved sensorimotor functions and depressive behaviors, and alleviated oxidative stress and neuroinflammation by activating PI3K/Akt/Nrf2 signaling in CIRI rats (Zhang Y. et al., 2022).
Aldose reductase (AR) is a key protein in the polysaccharide pathway of sugar metabolism that regulates the intracellular redox balance and maintains cellular osmotic pressure and oxidative stress (Sardelli et al., 2023). In the CIRI rat model, SCU remediated oxidative stress injury by activating AR- NADPH oxidase (NOX) (Deng et al., 2022).
NO is an important signaling molecule that plays a key role in the central nervous system (CNS) (Iova et al., 2023). During ischemia, NO reacts with ROS, resulting in the formation of reactive radicals. Chen Y. J. et al. (2021) demonstrated that SCU has neuroprotective properties by activating NO synthase (NOS) and protein kinase G (PKG). Moreover, SCU pretreatment could ameliorate the neurological deficit and reduce the permeability of the BBB by upregulation of eNOS expression and downregulation of VEGF, bFGF, and iNOS expression after CIRI (Wang et al., 2021). The loss of connexin 43 (CX43), a gap junction protein in astrocytes, can exacerbate neuronal injury in cerebral ischemia (Yin et al., 2018). Sun J. B. et al. (2018) suggested SCU alleviates brain ischemic injury by regulating the expression of NOX2 and CX43.
Homocysteine (Hcy) is an important risk factor for stroke, whose overexpression reduces the ability of tight junction (TJ) proteins and alters the basement membrane, destroying the BBB. Exosomes have been shown to accelerate functional recovery and neurovascular plasticity following ischemia by modulating TJ proteins (Huang et al., 2022). SCU-treated exosomes could enhance cell viability of homocysteine-induced rat brain microvascular endothelial cells by increasing the expression of NO, claudin 5, occludin, and zonula occludens-1 (ZO-1) and decreasing the expression of LDH and ROS (Zhong et al., 2019).
Inflammation is a key factor in the pathogenesis of ischemic stroke (Chen J. et al., 2021). Anti-inflammatory therapy is a potential therapeutic strategy for post-CIRI. Accumulating evidence has shown that SCU exerts neuroprotective effects by modulating multiple inflammatory signaling. Neuroinflammation contributes to the progression of cerebral ischemia/reperfusion (I/R) damage. The Notch pathway plays a vital role in activated microglia in response to hypoxic brain injury through its transactivation of NF-κB and subsequent cytokine release. In the CIRI rat model and LPS-induced BV-2 cells, SCU attenuated microglia-mediated neuroinflammation by inhibiting the Notch/NF-κB pathway (Xie et al., 2019; Yuan et al., 2015). Moreover, the NF-κB signaling pathway is positively controlled by MAPK which is another regulator that controls the production and release of pro-inflammatory factors in response to cerebral ischemic injury (Xie et al., 2019). SCU could protect the brain against neuroinflammatory injuries by inhibiting the MAPK/NF-κB signaling in CIRI rats (Zhang Y. et al., 2022).
Angiotensin-converting enzyme (ACE) of the renin-angiotensin system plays an important role in stroke (Abdel-Fattah et al., 2018). ACE, which converts angiotensin I (Ang I) into angiotensin II (Ang II), is closely linked to brain edema, inflammatory response, and neuronal apoptosis following ischemic stroke. Ang II, after binding to the Ang II type 1 receptor (AT1R), can induce ischemic injury by causing local cerebrovascular vasoconstriction and dysfunction. SCU decreased neurological deficit score, infarct area, and cell apoptosis in CIRI rats by inhibiting the Ang II/AT1R pathway in a dose-dependent manner (Wang W. et al., 2016).
During the CIRI, the disruption of blood deprives cells of energy and disturbs the ionic homeostasis of the cells. Brain edema is a typical syndrome in ischemic cerebrovascular disease, partly resulting from the dysfunction of Na+ and K+-ATPase in the cell membrane. Glutamate receptor-mediated ionic imbalance and neurotoxicity have been well-established in cerebral ischemia (Olivares-Bañuelos et al., 2019). SCU could attenuate neuronal cell damage and reduce brain edema by regulating the levels of glutamic acid, aspartic acid, and gamma-aminobutyric acid (GABA). Additionally, SCU increased the activities of Ca2+-ATPase and Na+, K+-ATPase (Tang et al., 2014).
Accumulating evidence indicates that PKG dysfunction is related to CIRI. Vasodilator-stimulated phosphoprotein (VASP), an important PKG-I substrate and actin regulatory protein, serves as a critical indicator of PKG-I activity and downstream ion channels in intact cells (Xu et al., 2023). SCU enhanced endothelium-dependent relaxation in isolated basilar arteries and counteracted vascular endothelium dysfunction in hypoxia-reoxygenation-induced human brain microvascular endothelial cells (HBMECs) by increasing the expression of VASP (Du et al., 2015). Moreover, SCU promotes the production of neurotrophic factors and accelerates neuronal integrity and synaptic plasticity of microglia by upregulation of the expression of brain-derived neurotrophic factor (BDNF), such as neurotrophin 3 (NT-3), insulin-like growth factor-I (IGF-I), microtubule-associated protein-2 (MAP-2) and postsynaptic density protein-95 (PSD-95) (Fang et al., 2016).
2.1.2 Subarachnoid hemorrhage and behavioral deficits
Subarachnoid hemorrhage (SAH) is a neurological disease with high morbidity and mortality. Dysfunction of eNOS plays an indispensable role in vasospasm post-SAH (Gao et al., 2022). Kruppel-like factor 2 (KLF2) is a key regulator of eNOS, affecting vascular tone, inflammation, and cell migration. Extracellular-regulated kinase 5 (Erk5) modulates KLF2 and eNOS (Angolano et al., 2021). SCU improved SAH by increasing the expression of eNOS in the intima of the cerebral arteries and enhanced the levels of p-Erk5 and KLF2 (Li et al., 2016).
Depression is a complex mental disorder linked to inflammatory reactions and microglial activation and affects approximately 350 million people worldwide (Wang H. et al., 2022; Xia et al., 2023). Activation of the Nod-like receptor pyrin-containing pyrin domain 3 (NLRP3) inflammasome in microglia leads to caspase-1 activation and subsequent production of bioactive IL-1β from pro-IL-1β. ROS promotes tissue inflammation and immune response via the NLRP3 pathway. SCU ameliorated LPS-induced depressive-like behaviors by inhibiting ROS generation and decreasing the expression of NLRP3, caspase-1, and IL-1β (Bian et al., 2020). Another prevalent psychiatric symptom is anxiety, which significantly impacts daily life and is related to glutamate neurotransmission. GABA, an inhibitory neurotransmitter, counteracts glutamate’s excitatory effects. SCU exhibited protective effects against anxiety-like behavior by downregulating glutamatergic receptors and abrogating GABAA Rα1 and GABAA γ2 in the prefrontal cortex (Guo et al., 2021).
2.1.3 Neurodegenerative disease
Alzheimer’s disease (AD) is a prevalent neurodegenerative condition characterized by amyloid formation, neurofibrillary degeneration, and synaptic loss. β-amyloid peptide (Aβ), derived from amyloid precursor protein (APP) cleavage by β- and γ-secretases, is central to cognitive dysfunction in AD (Jucker and Walker, 2023). SCU has demonstrated inhibitory effects on the aggregation of Aβ, reducing high toxic soluble Aβ 42 and Aβ 40 levels while elevating less toxic amyloid plaques in the cortex (Hu et al., 2018; Shin et al., 2018; Zeng et al., 2018; Zhang et al., 2020). Oxidative stress and inflammation contribute to AD progression by increasing the aggregation of Aβ (Zeng et al., 2018; Zhang S. et al., 2022; Zhang T. et al., 2022). SCU improved cognitive impairments in AD mice by upregulating the expression of Aβ-42 deposition and phosphorylated-Tau in the hippocampus of AD mice. Additionally, SCU enhanced SOD and GSH levels while reducing the levels of inflammatory factors such as iNOS, TNF-α, IL-1β, and IL-6 by inhibiting NF-κB signaling (Baluchnejadmojarad et al., 2018; Guo L. L. et al., 2011; Hu et al., 2018; Wang et al., 2017; You et al., 2018).
Neuronal nicotinic acetylcholine receptors (nAChRs) are recognized as therapeutic targets for improving cognitive function and retarding neurodegeneration in AD (Hoskin et al., 2019). SCU alleviated behavioral deficits by increasing the expression of α4 and α7 nAChR subunit and restoring the activities of acetylcholinesterase (AChE) and butyrylcholinesterase (BuChE) in AD mice (Guo L. L. et al., 2011). Additionally, the potential neuroprotection mechanism of SCU is partly attributed to the inhibition of p38 MAPK signaling (Wang et al., 2017; You et al., 2018).
2.2 Cardioprotective effects
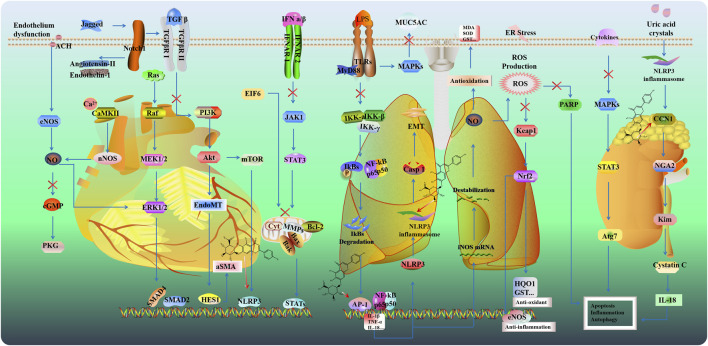
Hypertension is a major risk factor for cardiovascular events, such as ischemic stroke and cerebral hemorrhage, primarily due to its role in inflammation-mediated target organ damage. SCU reduced inflammatory responses in renal artery constriction-induced hypertension rat model by suppressing TLR4/NF-κB signaling and apoptotic markers like Bax and cleaved-caspase-3 (Mehta et al., 2014). Additionally, SCU demonstrated vasodilatory effects in isolated blood vessels by relaxing the thoracic and abdominal aortas in an endothelium-dependent manner (Chen Y. J. et al., 2021). Cardiovascular disease (CVD) poses a serious threat to patients’ physical and mental health, as well as their quality of life, due to its high morbidity and mortality rates. SCU offers multiple cardiovascular benefits, including anti-myocardial fibrosis, protection of vascular endothelial function, reduction of myocardial injury, and cardiac function. In cardiovascular diseases, SCU exhibits cardioprotective effects attributed to its actions against oxidative stress, inflammation, apoptosis, and fibrosis. These mechanisms contribute to its therapeutic benefits in mitigating cardiovascular morbidity and mortality (Figure 4).
FIGURE 4.
Potential mechanisms and targets of cardioprotection, lung and kidney protection.
2.2.1 Myocardial infarction
Myocardial infarction (MI) is related to the imbalance between coronary blood supply and myocardial demand, leading to cardiac remodeling and chronic heart failure. Loss of cardiomyocytes during either the acute or chronic stage of MI directly contributes to contractile dysfunction. Circulating apoptotic markers soluble TNF receptor 1 (sTNFR1) and sTNFR2 were found to be associated with myocardial infarct size and left ventricular insufficiency in patients with ST-segment elevation myocardial infarction (STEMI), suggesting that apoptosis may be a key determinant of the extent of I/R injury (Zhang et al., 2022b). SCU improved the impaired cardiac function of infarct rats and decreased interstitial fibrosis by downregulating pro-apoptotic markers such as Bax, caspase-3, caspase-9, and p53, while upregulating the anti-apoptotic protein Bcl-2 in an isoprenaline-induced rat model of MI (Huang et al., 2018; Rodríguez et al., 2002).
Fibrosis, another hallmark of post-MI remodeling, contributes significantly to the progression of ventricular function. Transforming growth factor-β1 (TGFβ1) is an indispensable molecule in cardiac fibrosis (Li et al., 2018). Activation of Notch signaling could restrain TGF-β induced EndoMT and myocardial fibrosis (Dong et al., 2023). SCU significantly improved cardiac function by inhibiting interstitial fibrosis, and the mechanisms may involve the suppression of pro-fibrotic cytokine TGFβ1 expression, inhibition of p38 MAPK and ERK1/2 phosphorylation, and activation of Notch signaling (Pan et al., 2011; Zhou et al., 2014). Intracellular calcium overload is involved in the pathogenesis of cardiac hypertrophy following MI. SCU exerts anti-hypertrophic effects by inhibiting the calcineurin-NFAT and CaMKII pathways (Rostas and Skelding, 2023).
2.2.2 Myocardial ischemia-reperfusion injury
Myocardial ischemia-reperfusion injury (MIRI), occurs during the restoration of blood flow to the ischemic myocardium and exacerbates cardiac dysfunction. SCU exerted a role in inhibiting NLRP3 activation and thus attenuating the inflammatory response by increasing AKT phosphorylation, and inhibiting mTORC1 activity in experiments in which acute myocardial I/R injury induced H9c2 damage. Moreover, SCU exerted cardioprotective effects in the experiments on I/R-injured H9c2 through the JAK/STAT3 signal pathway (Chen et al., 2019; Shen et al., 2021; Xu et al., 2020). The eNOS-cGMP-PKG pathway is considered a target for attenuating IR injury (James et al., 2023). In an experimental model of MIRI in rats, SCU restored endothelium-dependent vasodilation by increasing PKG-Iα levels, and pVASP protein and further improving the responsiveness of coronary artery rings to acetylcholine (Li et al., 2015).
2.3 Pulmonary protective effects
Acute lung injury (ALI) is a severe pulmonary disease characterized by pulmonary edema and increased alveolar permeability. Excessive lung inflammation heightened neutrophil infiltration, increased microvascular permeability, interstitial edema, thickened alveolar walls, and impaired gas exchange, all of which contribute to significant respiratory dysfunction (Sharawi et al., 2024). Mitochondrial dysfunction aggravates the deterioration of lung function by promoting excess ROS (Figure 4). In the LPS-induced ALI model of rats, SCU pretreatment reversed the high levels of ROS and MDA while increasing the levels of SOD and GSH via the inhibition of the JNK/c-jun/Phospho-c-jun/cleaved caspase three signaling pathway (Fan et al., 2022). Additionally, SCU decreased the expression of inflammatory cytokines such as IL-1β, IL-18, IL-6, and TNF-α in bronchoalveolar lavage fluid via inhibiting activator protein 1 (AP-1) and NF-κB signaling (Ibrahim et al., 2019).
Chronic airway diseases are characterized by persistent mucus hypersecretion and inflammation, leading to respiratory dysfunction. In human neutrophil elastase-induced rats and cell models, SCU treatment inhibited mucus hypersecretion in a concentration-dependent manner via inhibition of the expression of mucin 5AC and the phosphorylation of PKC and ERK1/2 (Jiang et al., 2011a). In another study, SCU suppressed inflammation and inflammatory cell infiltration into the lungs and attenuated airway hyperresponsiveness and airway remodeling in ovalbumin-challenged asthmatic mice. Moreover, SCU prevented the TGF-β-induced migration and EMT in 16HBE cells. The potential mechanism is related to the inactivation of the Smad/MAPK and NF-κB/NLRP3 pathways (Li et al., 2024; Peng et al., 2020).
2.4 Kidney and liver protective effects
Kidney injury (AKI) results in high morbidity and mortality among inpatients, while effective treatment and intervention are still absent. Inflammatory response, apoptosis, and oxidative stress play key roles in the pathogenesis of kidney injury (Li et al., 2024) (Figure 4). SCU protected renal tubular function against renal ischemia-reperfusion injury and increased the expression of antioxidant enzymes (SOD, CAT, HO-by activating the Nrf2/ARE signaling pathway (Dai et al., 2022). NLRP3 could be activated by uric acid crystals and increased IL-1β. SCU dose-dependently alleviated the renal injury, and apoptosis by downregulating the expression of NLRP3, IL-1β, NGAL, Kim-1, cystatin C, and IL-18 and increasing anti-apoptosis CCN1 in hyperuricemic nephropathy mice (Ding et al., 2022; Li G. et al., 2020). Additionally, SCU protected against cisplatin-induced renal injury by inhibiting MAPK pathways, reducing the Bax/Bcl-2 ratio, and suppressing cleaved caspase-3 and PARP cleavage and Atg7-dependent autophagy (Zhang X. et al., 2022).
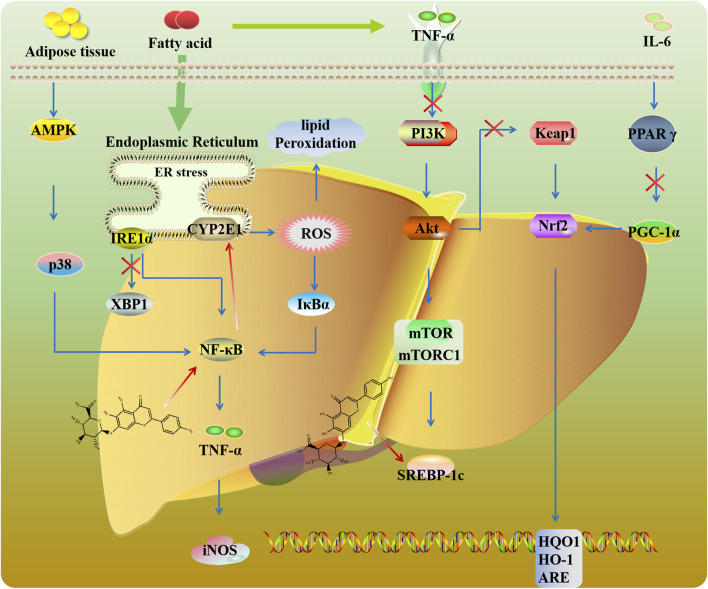
Nonalcoholic fatty liver disease (NAFLD) is the most prevalent liver disease, characterized by the presence of steatosis in more than 5% of hepatocytes with little or no alcohol intake. Endoplasmic reticulum (ER) stress is related to the progression of NAFLD. Sun et al. (2019) found that SCU mitigated hepatic lipid accumulation by inhibiting inositol-requiring enzyme 1α (IRE1α)/X-box-binding protein 1 (XBP1) signaling and further suppressing ER stress (Figure 5). Hepatic lipid accumulation activates PI3K/AKT/mTOR signaling (Han and Wang, 2018). mTORC1 could promote sterol-regulatory element binding protein (SREBP)-dependent lipogenesis. In high-fat diet (HFD) mice, SCU could ameliorate insulin resistance via mTOR/SREBP-dependent pathway (Han and Wang, 2018; Luan et al., 2020). Peroxisome proliferator-activated receptor gamma (PPARγ) plays a meaningful role in adipocyte differentiation and inflammation. As a transcriptional coactivator of PPARγ, peroxisome proliferator-activated receptor gamma coactivator-1 alpha (PGC-1α) is involved in mitochondria generation. It has been reported that PPARγ binds to the Nrf2 promoter and regulates the expression of antioxidant genes (Li L. et al., 2020). SCU exerts hypolipidemic, antioxidative, and liver protective by regulating the PPARγ/PGC-1α-Nrf2 signaling pathway (Zhang et al., 2018).
FIGURE 5.
Potential mechanisms and targets of liver protection.
Toxicity to hepatocytes caused by various insults including drugs is a common cause of chronic liver failure. CYP2E1 biotransforms toxins like carbon tetrachloride (CCl4) into hepatotoxins, exacerbating liver injury. SCU improved lipid metabolism and bile acid homeostasis by regulating CYP2E1 and NF-κB signaling in mice exposed to CCl4 (Miao et al., 2021). Moreover, SCU could alleviate poisoning-induced liver injury like selenium, concanavalin A, and diosbulbin B by regulating the NF-κB-TNF-α-iNOS pathway (Eltayeb et al., 2004; Niu et al., 2015; Tan et al., 2007). Liver I/R injury is a common complication after liver transplantation, stroke, and trauma (Liang et al., 2022). SCU protects the liver against oxidative stress by mediating Keap1/Nrf2/ARE signaling in I/R-induced hepatocytes (Wu and Jia, 2019).
2.5 Orthopedic diseases
Osteoarthritis (OA) is a chronic inflammatory joint disease. It is driven by an imbalance between anabolic and catabolic cartilage such as MMP1 and MMP13. SCU inhibited IL-1β-mediated inflammation in chondrocytes, reducing MMP-13, ADAMTS-5, COX-2, and iNOS via NF-κB and Nrf2 pathway (Liu et al., 2020; Luo et al., 2020; Wang et al., 2019). SCU also affects cholesterol metabolism in OA cells by modulating the CH25H/CYP7B1/RAR-related orphan receptor α axis (Ju et al., 2021; Luo et al., 2020; Wang et al., 2019) (Figure 6).
FIGURE 6.
Potential mechanisms and targets of SCU against bone and joint damage.
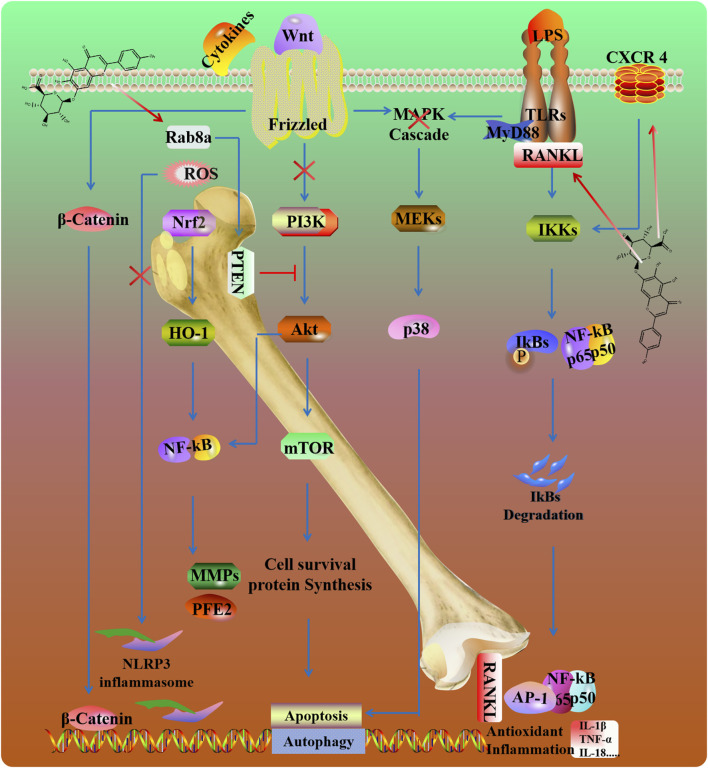
Intervertebral disc degeneration (IVDD) is the most widespread cause of disc herniation. Inflammatory responses, mitochondrial dysfunction, and extracellular matrix degradation are the main etiologies of this disease (Chao-Yang et al., 2021). In a rat needle puncture model, SCU attenuated the inflammatory reaction and retained the production of major intervertebral disc components. Mechanistically, SCU reduced the amount of ROS and alleviated mitochondrial damage by inhibiting NLRP3/NF-κB/MAPK signaling in TNF-α induced human primary nucleus pulposus cells (Wang Z. et al., 2022). Moreover, SCU enhanced autophagy, upregulated the expression of Rab8a and promoted the release of exosomes through the inactivation of PTEN/PI3K/Akt pathway in nucleus pulposus cells (Hu et al., 2022).
Arthritis, characterized by synovitis and hypertrophic synovium (swelling), can be improved by inhibiting inflammation and oxidative stress (Zhang et al., 2017). SCU inhibited RANKL-mediated MAPKs and NF-κB signaling pathways to counter osteoclastogenesis (Zhao et al., 2016). Osteoporosis is characterized by low bone mass and micro-architectural deterioration of bone tissue. C-X-C chemokine receptor type 4 (CXCR4) participates in immune responses and bone remodeling by modulating mesenchymal stem cells and osteoclast precursors' proliferation, maturation, and migration (Liu et al., 2024). SCU improves osteoblast function by increasing the expression of CXCR4 and inhibiting the NF-κB signaling pathway (Wang et al., 2018).
2.6 Metabolic diseases
Obesity is characterized by excessive fat deposition. SREBP family, CCAAT-enhancer binding protein (C/EBP) family and other adipogenic transcription factors are involved in the generation of adipogenesis. Emerging evidence reveals that PPARγ acts cooperatively with C/EBPα to mediate adipocyte differentiation (Benchamana et al., 2019). In 3T3-L1 preadipocytes, SCU could attenuate fat cell differentiation by upregulating PPARα and downregulating PPARγ and C/EBP (Li et al., 2009).
Diabetic nephropathy is one of the most frequent and severe complications of diabetes mellitus (DM) and is associated with increased morbidity and mortality in diabetic patients. Oxidative stress, angiotensin II (Ang-II), and inflammatory processes are recently considered to play an important role in the development and progression of DN (Jin et al., 2023). In DN mice, SCU could ameliorates proteinuria, glomerular expansion, mesangial matrix accumulation, renal fibrosis, and podocyte injury by inhibiting TGF-β and as well as its interaction with the extracellular signal-regulated kinase (Erk) and Wnt/β-catenin pathways (Huang et al., 2024).
Diabetic retinopathy (DR) is another serious microvascular complication of DM and is the leading cause of visual loss in the elderly, with a prevalence of 34.6% (93 million) in adults aged 40 years and over (Yue et al., 2022). Network pharmacology demonstrated that SCU can effectively protect retina ganglion cells from pyroptosis in DR, and underlying mechanisms are involved in the inhibition of caspase-1, GSDMD, NLRP3, IL-1β and IL-18 (Li N. et al., 2023). The loss of blood-retinal barrier (BRB) integrity leads to ischemic retinal. Claudin-1 and claudin-19 are critical for maintaining BRB integrity. In high glucose and hypoxia-induced human retinal endothelial cells, SCU attenuated HREC proliferation, migration, and tube formation. Meanwhile, SCU decreased neovascularization and resistive index in the retina of diabetic rats. The mechanism of SCU appears to the inhibition the expression of the crosstalk of NLRP3, VEGF, p-ERK, p-FAK, and p-Src and promote the levels of claudin-1, and claudin-19 (Long et al., 2019; Mei et al., 2019; Yang et al., 2024b).
Diabetic cardiomyopathy is a major complication of diabetes and the prominent features are cardiac hypertrophy and fibrosis, which is closely related to autophagy or apoptosis of cardiomyocytes (Lezoualc’h et al., 2023). In the high-fat and high-sugar diet-induced DCM model, SCU alleviated myocardial damage in a dose-dependent manner by promoting the expression of Beclin-1 and LC3-II and decreasing caspase-3, caspase-8, Bax, and other apoptosis-related factors in diabetic cardiomyopathy (Huo et al., 2021; Su et al., 2022). Additionally, SCU reversed high-glucose-induced inflammatory and oxidation stress by inhibiting the NLRP3/NF-κB pathway and enhancing the AKT/Nrf2/HO-1 pathway (Xi et al., 2021; Xu et al., 2021).
Increased advanced glycation end products and free fatty acids lead to diabetic liver injuries (Kumar et al., 2021; Stefan and Cusi, 2022). In a T2DM animal model and homocysteine-induced hepatocyte line LO2, SCU improved liver function, enhanced the clearance of homocysteine and ameliorated hepatic injury. Furthermore, SCU suppressed the secretion of IL-1, IL-6, and TNF-α and reduced hepatocyte apoptosis (Fan et al., 2023; Wang et al., 2020).
2.7 Cancer
2.7.1 Lung cancer
Lung cancer is the second most prevalent and the deadliest cancer worldwide. Non-small cell lung cancer (NSCLC) accounts for approximately 85% of lung cancer and the five-year survival rate remains <15% (Miao et al., 2024). Acquired resistance of cisplatin has been a major obstacle for the clinical application. Drug-induced apoptosis and autophagy can sensitize cancer cells to chemotherapy (Lou et al., 2021). SCU enhanced cisplatin-induced autophagy by suppressing the c-met/AKT signaling and apoptosis via enhancing ERK/P53 signaling and further reversing cisplatin resistance (Sun C. Y. et al., 2018). The pro-apoptosis and autophagy efficacy of SCU was also confirmed by another study, which demonstrated that SCU could inhibit the proliferation of A549 cells, induce G0/G1 phase arrest, apoptosis, and autophagy via AKT/mTOR/4EBP1 and ERK1/2/STAT3 pathways (Cao et al., 2019). Moreover, SCU improved the radiosensitivity of non-small cell lung cancer cells to 125I seeds by downregulating the AKT/mTOR pathway in vivo and vitro in a concentration and time-dependent manner (Cao et al., 2019; Zhang et al., 2021).
2.7.2 Liver cancer
Hepatocellular carcinoma (HCC) is the sixth most common malignancy and the fourth leading cause of cancer-related death worldwide (Brown et al., 2023). JAK/STAT signaling pathway has been documented to arbitrate the transcription pathways of several cytokines in human malignancies, including HCC (Liao et al., 2024). STAT3 is a crucial regulatory molecule in cancer immunity (Kang et al., 2021). Girders of actin filaments (Girdin) are related to poor prognosis of HCC. In HepG2 and MHCC97-H cells, SCU potentially suppresses invasiveness by inhibition of the EMT process, which could be attributed to the downregulation of the JAK2/STAT3/Girdin/Akt pathway (Ke et al., 2017; Liu K. et al., 2019). Immunogenic cell death of cancer cells may induce adaptive immunity against tumors, thereby providing great potential for treating HCC. Li L. et al. (2023) produced an aminoethyl anisamide-targeted polyethylene glycol-modified poly (lactide-co-glycolide) (PLGA-PEG-AEAA) for encapsulating SCU. PLGA-PEG-AEAA.SCU achieved anti-HCC efficacy due to the reversal of immunosuppressive tumor microenvironment, significantly prolonging the survival of orthotopic HCC mice, without inducing toxicity. Recently, Isochlorate dehydrogenase one can limit glycolysis in hepatocellular carcinoma (HCC) cells to activate the tumor immune microenvironment. SCU showed significant anti-hepatoma effects by inhibiting glycolysis, recruiting immune cells into the tumor microenvironment, and blocking PD-L1 expression in transplanted tumor models. In hypoxia induced HepG2 and Huh7 cell, SCU inhibited glycolysis by regulating the IDH1–α-KG–HIF1a signaling axis (Cui et al., 2024).
2.7.3 Colorectal cancer
Colorectal cancer (CRC) is one of the heterogeneous diseases with high morbidity and mortality worldwide. Increasing evidence suggests that Hedgehog signaling plays a pivotal role in the initiation, development, and metastasis of CRC (Geyer and Gerling, 2021). SCU suppressed the proliferation, migration, and colony formation by inhibiting the Hedgehog, Wnt/β-catenin, NF-κB and ephrinb2/VEGF signaling (; Lei et al., 2020; Zeng et al., 2021; Zeng et al., 2022). Regulation of CD4+, Foxp3+ regulatory T cells (Tregs) is emerging as a potential therapeutic target in CRC. SCU has shown promising effects in reducing the number of tumor-infiltrating TNFR2-positive Tregs and increasing the infiltration of IFN γ-induced CD8+ T cells. This shift in the immune environment favors anti-tumor activity and can potentially hinder tumor growth and spread (Chen et al., 2022; Yang et al., 2017). Pyruvate kinase isoenzyme M2 is overexpressed in cancer cells and associated with cancer development. SCU resensitizes oxaliplatin-resistant CRC cells to oxaliplatin treatment through inhibition of PKM2 and reduction of the glycometabolism rate and the production of ATP (Sun et al., 2021).
2.7.4 Gastric cancer and oral squamous cell carcinoma
Gastric cancer is one of the most common malignancies with high mortality, especially in East Asia (Guan et al., 2023). SCU improved enzymatic and non-enzymatic antioxidant profiles and reversed inflammation in N-methyl-N′-nitro-N-nitrosoguanidine induced gastric carcinogenesis model (Sun and Meng, 2022). PTEN is frequently mutated in gastric cancer and is regarded as a tumor suppressor. The mechanistic study supported that SCU silenced PI3K by up-regulating PTEN, thus dampening tumor progression in nude mice (Li et al., 2021). Additionally, SCU suppressed gastric cancer cell proliferation and promoted apoptosis by inhibition of the Wnt/β-catenin pathway in a dose-independent manner (Wang et al., 2023). Oral squamous cell carcinoma (OSCC) is ranked as the sixth most common cancer worldwide, with approximately 900,000 cases and more than 400,000 cases of incidence and mortality rate (Badwelan et al., 2023). SCU inhibited the proliferation and induced apoptosis by reducing the expression of transcription factor AP-1, MMP-2, MMP-9, and integrin αvβ6 in the HSC-4 and SAS human OSCC cells (Li et al., 2013; Li et al., 2010).
2.8 Sepsis
Sepsis and septic shock are severe systemic inflammatory responses to infection, that result in physiologic organ system dysfunction (Marshall and Leligdowicz, 2022). The NLRP3/caspase-1/IL-1 axis plays a critical role in the innate immune system and the progression of inflammation. SCU suppressed NLRP3 inflammasome activation in LPS-induced macrophages by enhancing PKA signaling (Liu et al., 2017). Moreover, SCU protects against LPS-provoked AKI by restraining inflammation and oxidative stress. The mechanism appears to regulate Nrf2/PPAR-γ/PGC-1α/NF-κB/TLR4 signaling (Liu et al., 2022; Shahmohammadi et al., 2023).
2.9 Toxicity-reducing and efficacy-enhancing
Doxorubicin (DOX), an anthracycline antineoplastic agent, is limited in clinical due to cardiotoxicity. The reduction of oxidative stress, mitochondrial dysfunction, DNA damage, apoptosis, and autophagy has been shown to confer significant protection against doxorubicin (DOX)-induced cardiotoxicity in vivo (Kong et al., 2022). SCU attenuation of DOX-induced oxidative stress, DNA damage, mitochondrial dysfunction, apoptosis, and autophagy in H9C2 cells, cardiomyocytes, cardiac fibroblast cells, and human umbilical vein endothelial cells and in rats (Sun et al., 2021; Sun et al., 2023; Tang et al., 2019; Zhou et al., 2022). The pharmacokinetic and tissue distribution study suggested that SCU reduced the concentration of DOX in heart tissues through its antioxidant activity (Sun et al., 2017).
3 Conclusion and future perspectives
The pathological development of chronic diseases is intricate due to the multiple signaling pathways involved in these dynamic interactions. The current treatment is still unsatisfactory due to the single or a few molecular targets of the targeted agents. Additionally, these treatments can cause serious side effects, known as “on-target” or “off-target” effects. SCU is a flavonoid that exerts a variety of pharmacological and biological activities, including anti-inflammatory, antioxidant, apoptosis-regulating, and vasodilating properties. However, current research lacks specificity and depth in elucidating how these targets and pathways interconnect within the broader context of each disease. While the referenced review focuses on the anti-inflammatory mechanisms of SCU, our work provides a broader scope, covering its role not only in inflammation but also in cardiovascular diseases, neuroprotection, and ischemia/reperfusion injury. Additionally, we explore novel findings regarding the role of vasodilation and apoptosis regulation, particularly in the context of ischemic stroke and myocardial infarction.
Through the collection of the published articles, most experimental results are preliminary results from cells and rats. The deficiency of positive control leads to a lack of reference for the clinical application of SCU. Despite the promising therapeutic potential of SCU, identifying the key molecular targets of SCU is challenging, and more pharmacological mechanisms of action need to be further explored. Moreover, SCU, when used in combination with other drugs, can enhance therapeutic efficacy, presenting promising potential for future applications.
While the studies provide compelling evidence of SCU’s broad pharmacological effects, including its antioxidant, anti-inflammatory, and cardioprotective properties, several limitations exist in the current body of research. First, many of the studies are preclinical, primarily using cell and animal models, which may not accurately reflect human physiology. The clinical relevance remains uncertain until more human trials are conducted. Second, some studies lack detailed dose-response analyses, which makes it difficult to determine the optimal therapeutic dosage for SCU and raises concerns about potential toxicity or side effects at higher concentrations. Third, poor bioavailability significantly impacts its therapeutic potential. Few studies address effective delivery systems leaving a gap in the practical application of SCU as a therapeutic agent. Future research should focus on advanced drug delivery systems, such as nanoformulations. Co-crystallization and nanoformulation technologies offer an innovative approach to developing combination therapies involving SCU. These technologies improve the therapeutic effectiveness and address key challenges associated with SCU, such as poor stability, low water solubility, limited oral bioavailability, and a short half-life in vivo.
While preclinical studies provide compelling evidence of SCU’s therapeutic potential, there is a lack of robust clinical data. Large-scale, randomized controlled trials are needed to validate its efficacy and safety in human populations. Future clinical trials should focus on establishing optimal dosages, long-term safety profiles, and potential drug interactions when used in combination with other therapeutic agents. Although the pharmacological effects of SCU have been well-documented, more detailed mechanistic studies are needed to explore molecular pathways, including its interactions with the NF-κB, PI3K/Akt, and MAPK signaling pathways. This will be helpful in better elucidating its therapeutic mechanisms and identifying potential biomarkers in various chronic diseases. This could help to identify new therapeutic targets and potential biomarkers for various diseases. Additionally, SCU has shown potential in combination with other drugs, which may enhance its therapeutic effects through synergistic mechanisms. Future studies should explore the efficacy of SCU in combination with other standard therapies, focusing on its potential to reduce drug resistance or adverse side effects. Beyond its well-studied effects on cardiovascular, cancer, diabetes, and neurodegenerative diseases, SCU’s anti-inflammatory and antioxidant properties make it a potential candidate for managing other chronic diseases driven by oxidative stress and inflammation. Future research could expand the application of SCU to a wider range of chronic diseases.
In summary, SCU has demonstrated significant potential as a therapeutic agent across a broad spectrum of chronic diseases, including cardiovascular, cerebrovascular, diabetes, organ injury, metabolic disorders, and neurodegenerative disorders. Antioxidant, anti-inflammatory, anti-apoptotic, and vasodilatory activities underscore its therapeutic versatility. SCU demonstrates the ability to modulate multiple signaling pathways in the treatment of chronic diseases, highlighting its promising future applications in preclinical models. Despite the encouraging results in preclinical models, SCU faces several challenges, such as poor bioavailability and limited clinical data, which hinder its broader application in clinical. To fully realize the potential of SCU as a widely applicable drug, more systematic and comprehensive studies are needed to accelerate its development for clinical use.
Funding Statement
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was financially supported by grants from National Natural Science Foundation of China (No. 82030120) and the National Key Research and Development Program of China (No. 2019YFC1710000).
Author contributions
SN: Writing–review and editing, Writing–original draft, Funding acquisition. SZ: Writing–review and editing, Writing–original draft, Resources. RW: Writing–review and editing, Writing–original draft, Resources. YZ: Writing–review and editing. YW: Writing–original draft. XW: Writing–review and editing, Writing–original draft, Funding acquisition. MZ: Writing–review and editing, Writing–original draft, Funding acquisition. PH: Writing–review and editing, Writing–original draft, Supervision.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- Abdel-Fattah M. M., Messiha B. A. S., Mansour A. M. (2018). Modulation of brain ACE and ACE2 may be a promising protective strategy against cerebral ischemia/reperfusion injury: an experimental trial in rats. Naunyn Schmiedeb. Arch. Pharmacol. 391, 1003–1020. 10.1007/s00210-018-1523-3 [DOI] [PubMed] [Google Scholar]
- Angolano C., Kaczmarek E., Essayagh S., Daniel S., Choi L. Y., Tung B., et al. (2021). A20/TNFAIP3 increases ENOS expression in an ERK5/KLF2-dependent manner to support endothelial cell health in the face of inflammation. Front. Cardiovasc Med. 8, 651230. 10.3389/fcvm.2021.651230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badwelan M., Muaddi H., Ahmed A., Lee K. T., Tran S. D. (2023). Oral squamous cell carcinoma and concomitant primary tumors, what do we know? A review of the literature. Curr. Oncol. 30, 3721–3734. 10.3390/curroncol30040283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baluchnejadmojarad T., Zeinali H., Roghani M. (2018). Scutellarin alleviates lipopolysaccharide-induced cognitive deficits in the rat: insights into underlying mechanisms. Int. Immunopharmacol. 54, 311–319. 10.1016/j.intimp.2017.11.033 [DOI] [PubMed] [Google Scholar]
- Benchamana A., Mori H., MacDougald O. A., Soodvilai S. (2019). Regulation of adipocyte differentiation and metabolism by lansoprazole. Life Sci. 239, 116897. 10.1016/j.lfs.2019.116897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian H. T., Wang G. H., Huang J. J., Liang L., Xiao L., Wang H. L. (2020). Scutellarin protects against lipopolysaccharide-induced behavioral deficits by inhibiting neuroinflammation and microglia activation in rats. Int. Immunopharmacol. 88, 106943. 10.1016/j.intimp.2020.106943 [DOI] [PubMed] [Google Scholar]
- Brown Z. J., Tsilimigras D. I., Ruff S. M., Mohseni A., Kamel I. R., Cloyd J. M., et al. (2023). Management of hepatocellular carcinoma: a review. JAMA Surg. 158, 410–420. 10.1001/jamasurg.2022.7989 [DOI] [PubMed] [Google Scholar]
- Candelario-Jalil E., Dijkhuizen R. M., Magnus T. (2022). Neuroinflammation, stroke, blood-brain barrier dysfunction, and imaging modalities. Stroke 53, 1473–1486. 10.1161/STROKEAHA.122.036946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao P., Liu B., Du F., Li D., Wang Y., Yan X., et al. (2019). Scutellarin suppresses proliferation and promotes apoptosis in A549 lung adenocarcinoma cells via AKT/mTOR/4EBP1 and STAT3 pathways. Thorac. Cancer 10, 492–500. 10.1111/1759-7714.12962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y., Liu H., Zhang J., Dong Y. (2020). Circular RNA cZNF292 silence alleviates OGD/R-induced injury through up-regulation of miR-22 in rat neural stem cells (NSCs). Artif. Cells Nanomed Biotechnol. 48, 594–601. 10.1080/21691401.2020.1725536 [DOI] [PubMed] [Google Scholar]
- Chao-Yang G., Peng C., Hai-Hong Z. (2021). Roles of NLRP3 inflammasome in intervertebral disc degeneration. Osteoarthr. Cartil. 29, 793–801. 10.1016/j.joca.2021.02.204 [DOI] [PubMed] [Google Scholar]
- Chatterjee S., Dhal A. K., Paul S., Sinha S., Das B., Dash S. R., et al. (2022). Combination of talazoparib and olaparib enhanced the curcumin-mediated apoptosis in oral cancer cells by PARP-1 trapping. J. Cancer Res. Clin. Oncol. 148, 3521–3535. 10.1007/s00432-022-04269-7 [DOI] [PubMed] [Google Scholar]
- Chen C., Weng Z.-y., Wang Y.-l., Zheng C.-b., Li Y., Yang J., et al. (2019). Scutellarin protects human cardiac microvascular endothelial cells with hypoxia-reoxygenation injury via JAK2/STAT3 signal pathway. Chin. Herb. Med. 11, 103–107. 10.1016/j.chmed.2018.09.004 [DOI] [Google Scholar]
- Chen H. L., Jia W. J., Li H. E., Han H., Li F., Zhang X. L., et al. (2020). Scutellarin exerts anti-inflammatory effects in activated microglia/brain macrophage in cerebral ischemia and in activated BV-2 microglia through regulation of MAPKs signaling pathway. Neuromolecular Med. 22, 264–277. 10.1007/s12017-019-08582-2 [DOI] [PubMed] [Google Scholar]
- Chen J., Dong X., Cheng X., Zhu Q., Zhang J., Li Q., et al. (2021a). Ogt controls neural stem/progenitor cell pool and adult neurogenesis through modulating Notch signaling. Cell Rep. 34, 108905. 10.1016/j.celrep.2021.108905 [DOI] [PubMed] [Google Scholar]
- Chen S., Li R., Chen Y., Chou C. K., Zhang Z., Yang Y., et al. (2022). Scutellarin enhances anti-tumor immune responses by reducing TNFR2-expressing CD4(+)Foxp3(+) regulatory T cells. Biomed. Pharmacother. 151, 113187. 10.1016/j.biopha.2022.113187 [DOI] [PubMed] [Google Scholar]
- Chen X., Shi X., Zhang X., Lei H., Long S., Su H., et al. (2013). Scutellarin attenuates hypertension-induced expression of brain Toll-like receptor 4/nuclear factor kappa B. Mediat. Inflamm. 2013, 432623. 10.1155/2013/432623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y. J., Chen C., Li M. Y., Li Q. Q., Zhang X. J., Huang R., et al. (2021b). Scutellarin reduces cerebral ischemia reperfusion injury involving in vascular endothelium protection and PKG signal. Nat. Prod. Bioprospect 11, 659–670. 10.1007/s13659-021-00322-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y. J., Wang L., Zhou G. Y., Yu X. L., Zhang Y. H., Hu N., et al. (2015). Scutellarin attenuates endothelium-dependent aasodilation impairment induced by hypoxia reoxygenation, through regulating the PKG signaling pathway in rat coronary artery. Chin. J. Nat. Med. 13, 264–273. 10.1016/S1875-5364(15)30013-3 [DOI] [PubMed] [Google Scholar]
- Chiba S., Numakawa T., Ninomiya M., Richards M. C., Wakabayashi C., Kunugi H. (2012). Chronic restraint stress causes anxiety- and depression-like behaviors, downregulates glucocorticoid receptor expression, and attenuates glutamate release induced by brain-derived neurotrophic factor in the prefrontal cortex. Prog. Neuropsychopharmacol. Biol. Psychiatry 39, 112–119. 10.1016/j.pnpbp.2012.05.018 [DOI] [PubMed] [Google Scholar]
- Cui Z., Li C., Liu W., Sun M., Deng S., Cao J., et al. (2024). Scutellarin activates IDH1 to exert antitumor effects in hepatocellular carcinoma progression. Cell Death Dis. 15, 267. 10.1038/s41419-024-06625-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai J., Li C., Zhao L., Guan C., Yang C., Zhang N., et al. (2022). Scutellarin protects the kidney from ischemia/reperfusion injury by targeting Nrf2. Nephrol. Carlt. 27, 690–700. 10.1111/nep.14069 [DOI] [PubMed] [Google Scholar]
- Dan Q. Q., Ma Z., Tan Y. X., Visar B., Chen L. (2022). AQP4 knockout promotes neurite outgrowth via upregulating GAP43 expression in infant rats with hypoxic-ischemic brain injury. Ibrain 8, 324–337. 10.1002/ibra.12062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng M., Sun J., Peng L., Huang Y., Jiang W., Wu S., et al. (2022). Scutellarin acts on the AR-NOX axis to remediate oxidative stress injury in a mouse model of cerebral ischemia/reperfusion injury. Phytomedicine 103, 154214. 10.1016/j.phymed.2022.154214 [DOI] [PubMed] [Google Scholar]
- Ding Z., Zhao J., Wang X., Li W., Chen C., Yong C., et al. (2022). Total extract of Abelmoschus manihot L. alleviates uric acid-induced renal tubular epithelial injury via inhibition of caspase-8/caspase-3/NLRP3/GSDME signaling. Front. Pharmacol. 13, 907980. 10.3389/fphar.2022.907980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y., Wang B., Du M., Zhu B., Cui K., Li K., et al. (2023). Targeting epsins to inhibit fibroblast growth factor signaling while potentiating transforming growth factor-β signaling constrains endothelial-to-mesenchymal transition in atherosclerosis. Circulation 147, 669–685. 10.1161/CIRCULATIONAHA.122.063075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du X., Chen C., Zhang M., Cai D., Sun J., Yang J., et al. (2015). Scutellarin reduces endothelium dysfunction through the PKG-I pathway. Evid. Based Complement. Altern. Med. 2015, 430271. 10.1155/2015/430271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan Z., Chen H., Miao W., He J., Xu D., Qi Z., et al. (2024). Scutellarin alleviates microglia-mediated neuroinflammation and apoptosis after ischemic stroke through the PI3K/AKT/GSK3β signaling pathway. J. Cell Commun. Signal 18, e12023. 10.1002/ccs3.12023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eltayeb A. A., Liu Q., Gan L., Liu H., Xu H. (2004). Antagonistic effect of scutellarin on the toxicity of selenium in rat livers. Biol. Trace Elem. Res. 98, 253–264. 10.1385/bter:98:3:253 [DOI] [PubMed] [Google Scholar]
- Fan D., Wang D., Zhu L. (2022). Protective role of scutellarin on LPS induced - acute lung injury and regulation of apoptosis, oxidative stress and reduction of mitochondrial dysfunction. Saudi J. Biol. Sci. 29, 371–378. 10.1016/j.sjbs.2021.08.105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan H., Lin P., Kang Q., Zhao Z. L., Wang J., Cheng J. Y. (2021). Metabolism and pharmacological mechanisms of active ingredients in Erigeron breviscapus. Curr. Drug Metab. 22, 24–39. 10.2174/1389200221666201217093255 [DOI] [PubMed] [Google Scholar]
- Fan H., Ma X., Lin P., Kang Q., Zhao Z., Wang L., et al. (2017). Scutellarin prevents nonalcoholic fatty liver disease (NAFLD) and hyperlipidemia via PI3K/AKT-Dependent activation of nuclear factor (Erythroid-Derived 2)-like 2 (Nrf2) in rats. Med. Sci. Monit. 23, 5599–5612. 10.12659/msm.907530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan X., Wang Y., Li X., Zhong T., Cheng C., Zhang Y. (2023). Scutellarin alleviates liver injury in type 2 diabetic mellitus by suppressing hepatocyte apoptosis in vitro and in vivo . Chin. Herb. Med. 15, 542–548. 10.1016/j.chmed.2023.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang M., Yuan Y., Lu J., Li H. E., Zhao M., Ling E. A., et al. (2016). Scutellarin promotes microglia-mediated astrogliosis coupled with improved behavioral function in cerebral ischemia. Neurochem. Int. 97, 154–171. 10.1016/j.neuint.2016.04.007 [DOI] [PubMed] [Google Scholar]
- Fang M., Yuan Y., Rangarajan P., Lu J., Wu Y., Wang H., et al. (2015). Scutellarin regulates microglia-mediated TNC1 astrocytic reaction and astrogliosis in cerebral ischemia in the adult rats. BMC Neurosci. 16, 84. 10.1186/s12868-015-0219-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao S. Q., Shi J. J., Xue W., Miao S. H., Li T., Gao C. C., et al. (2022). Endothelial NOX4 aggravates eNOS uncoupling by decreasing dihydrofolate reductase after subarachnoid hemorrhage. Free Radic. Biol. Med. 193, 499–510. 10.1016/j.freeradbiomed.2022.10.318 [DOI] [PubMed] [Google Scholar]
- Geyer N., Gerling M. (2021). Hedgehog signaling in colorectal cancer: all in the stroma? Int. J. Mol. Sci. 22, 1025. 10.3390/ijms22031025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong L., Tang Y., An R., Lin M., Chen L., Du J. (2017). RTN1-C mediates cerebral ischemia/reperfusion injury via ER stress and mitochondria-associated apoptosis pathways. Cell Death Dis. 8, e3080. 10.1038/cddis.2017.465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greco P., Nencini G., Piva I., Scioscia M., Volta C. A., Spadaro S., et al. (2020). Pathophysiology of hypoxic-ischemic encephalopathy: a review of the past and a view on the future. Acta Neurol. Belg 120, 277–288. 10.1007/s13760-020-01308-3 [DOI] [PubMed] [Google Scholar]
- Guan W. L., He Y., Xu R. H. (2023). Gastric cancer treatment: recent progress and future perspectives. J. Hematol. Oncol. 16, 57. 10.1186/s13045-023-01451-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H., Hu L. M., Wang S. X., Wang Y. L., Shi F., Li H., et al. (2011a). Neuroprotective effects of scutellarin against hypoxic-ischemic-induced cerebral injury via augmentation of antioxidant defense capacity. Chin. J. Physiol. 54, 399–405. 10.4077/CJP.2011.AMM059 [DOI] [PubMed] [Google Scholar]
- Guo L. L., Guan Z. Z., Huang Y., Wang Y. L., Shi J. S. (2013). The neurotoxicity of β-amyloid peptide toward rat brain is associated with enhanced oxidative stress, inflammation and apoptosis, all of which can be attenuated by scutellarin. Exp. Toxicol. Pathol. 65, 579–584. 10.1016/j.etp.2012.05.003 [DOI] [PubMed] [Google Scholar]
- Guo L. L., Guan Z. Z., Wang Y. L. (2011b). Scutellarin protects against Aβ-induced learning and memory deficits in rats: involvement of nicotinic acetylcholine receptors and cholinesterase. Acta Pharmacol. Sin. 32, 1446–1453. 10.1038/aps.2011.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y. Y., Zhou Y., Li Y. J., Liu A., Yue J., Liu Q. Q., et al. (2021). Scutellarin ameliorates the stress-induced anxiety-like behaviors in mice by regulating neurotransmitters. Phytother. Res. 35, 3936–3944. 10.1002/ptr.7106 [DOI] [PubMed] [Google Scholar]
- Han J., Wang Y. (2018). mTORC1 signaling in hepatic lipid metabolism. Protein Cell 9, 145–151. 10.1007/s13238-017-0409-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He F., Ru X., Wen T. (2020). NRF2, a transcription factor for stress response and beyond. Int. J. Mol. Sci. 21, 4777. 10.3390/ijms21134777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoskin J. L., Al-Hasan Y., Sabbagh M. N. (2019). Nicotinic acetylcholine receptor agonists for the treatment of alzheimer's dementia: an update. Nicotine Tob. Res. 21, 370–376. 10.1093/ntr/nty116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S. Q., Zou Y. P., Jiang Y. Q., Zhang Q. C., Cheng H. X., Wang H. R., et al. (2022). Scutellarin-mediated autophagy activates exosome release of rat nucleus pulposus cells by positively regulating Rab8a via the PI3K/PTEN/Akt pathway. Cell Biol. Int. 46, 1588–1603. 10.1002/cbin.11838 [DOI] [PubMed] [Google Scholar]
- Hu X., Teng S., He J., Sun X., Du M., Kou L., et al. (2018). Pharmacological basis for application of scutellarin in Alzheimer's disease: antioxidation and antiapoptosis. Mol. Med. Rep. 18, 4289–4296. 10.3892/mmr.2018.9482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X. M., Zhou M. M., Hu X. M., Zeng F. D. (2005). Neuroprotective effects of scutellarin on rat neuronal damage induced by cerebral ischemia/reperfusion. Acta Pharmacol. Sin. 26, 1454–1459. 10.1111/j.1745-7254.2005.00239.x [DOI] [PubMed] [Google Scholar]
- Huang A. J., Grady D. (2022). Menopausal hormone therapy for prevention of chronic conditions: when is enough, enough? Jama 328, 1712–1713. 10.1001/jama.2022.19098 [DOI] [PubMed] [Google Scholar]
- Huang B., Han R., Tan H., Zhu W., Li Y., Jiang F., et al. (2024). Scutellarin ameliorates diabetic nephropathy via TGF-β1 signaling pathway. Nat. Prod. Bioprospect 14, 25. 10.1007/s13659-024-00446-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H., Geng Q., Yao H., Shen Z., Wu Z., Miao X., et al. (2018). Protective effect of scutellarin on myocardial infarction induced by isoprenaline in rats. Iran. J. Basic Med. Sci. 21, 267–276. 10.22038/ijbms.2018.26110.6415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H., Oo T. T., Apaijai N., Chattipakorn N., Chattipakorn S. C. (2023). An updated review of mitochondrial transplantation as a potential therapeutic strategy against cerebral ischemia and cerebral ischemia/reperfusion injury. Mol. Neurobiol. 60, 1865–1883. 10.1007/s12035-022-03200-y [DOI] [PubMed] [Google Scholar]
- Huang L. Y., Song J. X., Cai H., Wang P. P., Yin Q. L., Zhang Y. D., et al. (2022). Healthy serum-derived exosomes improve neurological outcomes and protect blood-brain barrier by inhibiting endothelial cell apoptosis and reversing autophagy-mediated tight junction protein reduction in rat stroke model. Front. Cell Neurosci. 16, 841544. 10.3389/fncel.2022.841544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huo Y., Mijiti A., Cai R., Gao Z., Aini M., Mijiti A., et al. (2021). Scutellarin alleviates type 2 diabetes (HFD/low dose STZ)-induced cardiac injury through modulation of oxidative stress, inflammation, apoptosis and fibrosis in mice. Hum. Exp. Toxicol. 40, S460–s474. 10.1177/09603271211045948 [DOI] [PubMed] [Google Scholar]
- Ibrahim M. A. A., Elwan W. M., Elgendy H. A. (2019). Role of scutellarin in ameliorating lung injury in a rat model of bilateral hind limb ischemia-reperfusion. Anat. Rec. Hob. 302, 2070–2081. 10.1002/ar.24175 [DOI] [PubMed] [Google Scholar]
- Iova O. M., Marin G. E., Lazar I., Stanescu I., Dogaru G., Nicula C. A., et al. (2023). Nitric oxide/nitric oxide synthase system in the pathogenesis of neurodegenerative disorders-an overview. Antioxidants (Basel) 12, 753. 10.3390/antiox12030753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- James A. S., Eteng O. E., Dosumu O. A., Moses C. A., Ogbonna C. U., Adeleye O. A., et al. (2023). Morin augmented myocardial eNOS/cGMP/PKG signaling pathway and abated oxidative and inflammo-apoptotic responses in diethyl phthalate and bisphenol-S Co-exposed male albino rats. Inflammation 46, 175–189. 10.1007/s10753-022-01720-2 [DOI] [PubMed] [Google Scholar]
- Jiang D. P., Li Q., Yang J., Perelman J. M., Kolosov V. P., Zhou X. D. (2011a). Scutellarin attenuates human-neutrophil-elastase-induced mucus production by inhibiting the PKC-ERK signaling pathway in vitro and in vivo . Am. J. Chin. Med. 39, 1193–1206. 10.1142/S0192415X11009494 [DOI] [PubMed] [Google Scholar]
- Jiang D. P., Perelman J. M., Kolosov V. P., Zhou X. D. (2011b). Effects of scutellarin on MUC5AC mucin production induced by human neutrophil elastase or interleukin 13 on airway epithelial cells. J. Korean Med. Sci. 26, 778–784. 10.3346/jkms.2011.26.6.778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Q., Liu T., Qiao Y., Liu D., Yang L., Mao H., et al. (2023). Oxidative stress and inflammation in diabetic nephropathy: role of polyphenols. Front. Immunol. 14, 1185317. 10.3389/fimmu.2023.1185317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju S. H., Tan L. R., Liu P. W., Tan Y. L., Zhang Y. T., Li X. H., et al. (2021). Scutellarin regulates osteoarthritis in vitro by inhibiting the PI3K/AKT/mTOR signaling pathway. Mol. Med. Rep. 23, 83. 10.3892/mmr.2020.11722 [DOI] [PubMed] [Google Scholar]
- Jucker M., Walker L. C. (2023). Alzheimer's disease: from immunotherapy to immunoprevention. Cell 186, 4260–4270. 10.1016/j.cell.2023.08.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang F. B., Wang L., Jia H. C., Li D., Li H. J., Zhang Y. G., et al. (2021). Correction to: B7-H3 promotes aggression and invasion of hepatocellular carcinoma by targeting epithelial-to-mesenchymal transition via JAK2/STAT3/Slug signaling pathway. Cancer Cell Int. 21, 570. 10.1186/s12935-021-02216-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke Y., Bao T., Wu X., Tang H., Wang Y., Ge J., et al. (2017). Scutellarin suppresses migration and invasion of human hepatocellular carcinoma by inhibiting the STAT3/Girdin/Akt activity. Biochem. Biophys. Res. Commun. 483, 509–515. 10.1016/j.bbrc.2016.12.114 [DOI] [PubMed] [Google Scholar]
- Kong C. Y., Guo Z., Song P., Zhang X., Yuan Y. P., Teng T., et al. (2022). Underlying the mechanisms of doxorubicin-induced acute cardiotoxicity: oxidative stress and cell death. Int. J. Biol. Sci. 18, 760–770. 10.7150/ijbs.65258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar V., Xin X., Ma J., Tan C., Osna N., Mahato R. I. (2021). Therapeutic targets, novel drugs, and delivery systems for diabetes associated NAFLD and liver fibrosis. Adv. Drug Deliv. Rev. 176, 113888. 10.1016/j.addr.2021.113888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei N., Xiong S. H., Tan L., He M., Zhang M., Sun Q., et al. (2020). Inhibition of scutellarin on differentiation of colonic cancer stem cells via hedgehog signaling pathway. Zhongguo Zhong Yao Za Zhi 45, 1676–1683. 10.19540/j.cnki.cjcmm.20200108.401 [DOI] [PubMed] [Google Scholar]
- Lezoualc'h F., Badimon L., Baker H., Bernard M., Czibik G., de Boer R. A., et al. (2023). Diabetic cardiomyopathy: the need for adjusting experimental models to meet clinical reality. Cardiovasc Res. 119, 1130–1145. 10.1093/cvr/cvac152 [DOI] [PubMed] [Google Scholar]
- Li F., Wang S., Niu M. (2021). Scutellarin inhibits the growth and EMT of gastric cancer cells through regulating PTEN/PI3K pathway. Biol. Pharm. Bull. 44, 780–788. 10.1248/bpb.b20-00822 [DOI] [PubMed] [Google Scholar]
- Li G., Guan C., Xu L., Wang L., Yang C., Zhao L., et al. (2020a). Scutellarin ameliorates renal injury via increasing CCN1 expression and suppressing NLRP3 inflammasome activation in hyperuricemic mice. Front. Pharmacol. 11, 584942. 10.3389/fphar.2020.584942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Huang D., Gao Z., Chen Y., Zhang L., Zheng J. (2013). Scutellarin inhibits the growth and invasion of human tongue squamous carcinoma through the inhibition of matrix metalloproteinase-2 and -9 and αvβ6 integrin. Int. J. Oncol. 42, 1674–1681. 10.3892/ijo.2013.1873 [DOI] [PubMed] [Google Scholar]
- Li H., Huang D., Gao Z., Lv Y., Zhang L., Cui H., et al. (2010). Scutellarin inhibits cell migration by regulating production of αvβ6 integrin and E-cadherin in human tongue cancer cells. Oncol. Rep. 24, 1153–1160. 10.3892/or_00000967 [DOI] [PubMed] [Google Scholar]
- Li L., Fu J., Liu D., Sun J., Hou Y., Chen C., et al. (2020b). Hepatocyte-specific Nrf2 deficiency mitigates high-fat diet-induced hepatic steatosis: involvement of reduced PPARγ expression. Redox Biol. 30, 101412. 10.1016/j.redox.2019.101412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Li L., Chen C., Yang J., Li J., Hu N., et al. (2015). Scutellarin's cardiovascular endothelium protective mechanism: important role of PKG-iα. PLoS One 10, e0139570. 10.1371/journal.pone.0139570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Zou Y., Wang L., Yang L., Li Y., Liao A., et al. (2023a). Nanodelivery of scutellarin induces immunogenic cell death for treating hepatocellular carcinoma. Int. J. Pharm. 642, 123114. 10.1016/j.ijpharm.2023.123114 [DOI] [PubMed] [Google Scholar]
- Li M., Jia D., Li J., Li Y., Wang Y., Wang Y., et al. (2024). Scutellarin alleviates ovalbumin-induced airway remodeling in mice and TGF-β-induced pro-fibrotic phenotype in human bronchial epithelial cells via MAPK and smad2/3 signaling pathways. Inflammation 47, 853–873. 10.1007/s10753-023-01947-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N., Guo X. L., Xu M., Chen J. L., Wang Y. F., Jie S., et al. (2023b). Network pharmacology mechanism of Scutellarin to inhibit RGC pyroptosis in diabetic retinopathy. Sci. Rep. 13, 6504. 10.1038/s41598-023-33665-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Chen Y., Zhang X., Zuo S., Ge H., Chen Y., et al. (2016). Scutellarin attenuates vasospasm through the Erk5-KLF2-eNOS pathway after subarachnoid hemorrhage in rats. J. Clin. Neurosci. 34, 264–270. 10.1016/j.jocn.2016.09.028 [DOI] [PubMed] [Google Scholar]
- Li Q., Wu J. H., Guo D. J., Cheng H. L., Chen S. L., Chan S. W. (2009). Suppression of diet-induced hypercholesterolemia by scutellarin in rats. Planta Med. 75, 1203–1208. 10.1055/s-0029-1185539 [DOI] [PubMed] [Google Scholar]
- Li Y., Lui K. O., Zhou B. (2018). Reassessing endothelial-to-mesenchymal transition in cardiovascular diseases. Nat. Rev. Cardiol. 15, 445–456. 10.1038/s41569-018-0023-y [DOI] [PubMed] [Google Scholar]
- Liang C., Takahashi K., Furuya K., Ohkohchi N., Oda T. (2022). Dualistic role of platelets in living donor liver transplantation: are they harmful? World J. Gastroenterol. 28, 897–908. 10.3748/wjg.v28.i9.897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao C., Zhao M., Jiang X., Sun W., Zeng Q., Cai C., et al. (2024). Obovatol inhibits proliferation, invasion and immune escape of hepatocellular carcinoma cells through modulating the JAK/STST3/PD-L1 pathway. Int. Immunopharmacol. 141, 112775. 10.1016/j.intimp.2024.112775 [DOI] [PubMed] [Google Scholar]
- Lin L., Lei W., Chen C., Yue D., Donghua C., Youlan W., et al. (2014). GW25-e0617 scutellarin attenuates myocardial ischemia reperfusion injury by inhibiting JAK2/STAT3 pathway. J. Am. Coll. Cardiol. 64, C28–C29. 10.1016/j.jacc.2014.06.138 [DOI] [Google Scholar]
- Liu F., Li L., Lu W., Ding Z., Huang W., Li Y. T., et al. (2020). Scutellarin ameliorates cartilage degeneration in osteoarthritis by inhibiting the Wnt/β-catenin and MAPK signaling pathways. Int. Immunopharmacol. 78, 105954. 10.1016/j.intimp.2019.105954 [DOI] [PubMed] [Google Scholar]
- Liu H., Song P., Zhang H., Zhou F., Ji N., Wang M., et al. (2024). Synthetic biology-based bacterial extracellular vesicles displaying BMP-2 and CXCR4 to ameliorate osteoporosis. J. Extracell. Vesicles 13, e12429. 10.1002/jev2.12429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H., Yang X., Tang R., Liu J., Xu H. (2005). Effect of scutellarin on nitric oxide production in early stages of neuron damage induced by hydrogen peroxide. Pharmacol. Res. 51, 205–210. 10.1016/j.phrs.2004.09.001 [DOI] [PubMed] [Google Scholar]
- Liu K., Tian T., Zheng Y., Zhou L., Dai C., Wang M., et al. (2019a). Scutellarin inhibits proliferation and invasion of hepatocellular carcinoma cells via down-regulation of JAK2/STAT3 pathway. J. Cell Mol. Med. 23, 3040–3044. 10.1111/jcmm.14169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Qin Z., Guan C., Xu L., Dai J., Yang C., et al. (2022). Scutellarin alleviates lipopolysaccharide-induced renal injury via mediating cysteine-rich protein 61-connective tissue growth factor-nephroblastoma overexpressed gene 1 expression to inhibit nuclear factor-κB signaling pathway. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 34, 400–406. 10.3760/cma.j.cn121430-20210401-00767 [DOI] [PubMed] [Google Scholar]
- Liu Y., Jing Y. Y., Zeng C. Y., Li C. G., Xu L. H., Yan L., et al. (2017). Scutellarin suppresses NLRP3 inflammasome activation in macrophages and protects mice against bacterial sepsis. Front. Pharmacol. 8, 975. 10.3389/fphar.2017.00975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Wang J., Zhang X., Wang L., Hao T., Cheng Y., et al. (2019b). Scutellarin exerts hypoglycemic and renal protective effects in db/db mice via the Nrf2/HO-1 signaling pathway. Oxid. Med. Cell Longev. 2019, 1354345. 10.1155/2019/1354345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long L., Li Y., Yu S., Li X., Hu Y., Long T., et al. (2019). Scutellarin prevents angiogenesis in diabetic retinopathy by downregulating VEGF/ERK/FAK/src pathway signaling. J. Diabetes Res. 2019, 4875421. 10.1155/2019/4875421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long L., Wang J., Lu X., Xu Y., Zheng S., Luo C., et al. (2015). Protective effects of scutellarin on type II diabetes mellitus-induced testicular damages related to reactive oxygen species/Bcl-2/Bax and reactive oxygen species/microcirculation/staving pathway in diabetic rat. J. Diabetes Res. 2015, 252530. 10.1155/2015/252530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou J. S., Zhao L. P., Huang Z. H., Chen X. Y., Xu J. T., Tai W. C., et al. (2021). Ginkgetin derived from Ginkgo biloba leaves enhances the therapeutic effect of cisplatin via ferroptosis-mediated disruption of the Nrf2/HO-1 axis in EGFR wild-type non-small-cell lung cancer. Phytomedicine 80, 153370. 10.1016/j.phymed.2020.153370 [DOI] [PubMed] [Google Scholar]
- Lu K., Han M., Ting H. L., Liu Z., Zhang D. (2013). Scutellarin from Scutellaria baicalensis suppresses adipogenesis by upregulating PPARα in 3T3-L1 cells. J. Nat. Prod. 76, 672–678. 10.1021/np300889y [DOI] [PubMed] [Google Scholar]
- Lu L., Yang L. K., Yue J., Wang X. S., Qi J. Y., Yang F., et al. (2021). Scutellarin alleviates depression-like behaviors induced by LPS in mice partially through inhibition of astrocyte-mediated neuroinflammation. Neurosci. Lett. 765, 136284. 10.1016/j.neulet.2021.136284 [DOI] [PubMed] [Google Scholar]
- Luan H., Huo Z., Zhao Z., Zhang S., Huang Y., Shen Y., et al. (2020). Scutellarin, a modulator of mTOR, attenuates hepatic insulin resistance by regulating hepatocyte lipid metabolism via SREBP-1c suppression. Phytother. Res. 34, 1455–1466. 10.1002/ptr.6582 [DOI] [PubMed] [Google Scholar]
- Luo Z., Hu Z., Bian Y., Su W., Li X., Li S., et al. (2020). Scutellarin attenuates the IL-1β-induced inflammation in mouse chondrocytes and prevents osteoarthritic progression. Front. Pharmacol. 11, 107. 10.3389/fphar.2020.00107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma H., Yue G. G., Lee J. K., Gao S., Yuen K. K., Cheng W., et al. (2024). Scutellarin, a flavonoid compound from Scutellaria barbata, suppresses growth of breast cancer stem cells in vitro and in tumor-bearing mice. Phytomedicine 128, 155418. 10.1016/j.phymed.2024.155418 [DOI] [PubMed] [Google Scholar]
- Marshall J. C., Leligdowicz A. (2022). Gaps and opportunities in sepsis translational research. EBioMedicine 86, 104387. 10.1016/j.ebiom.2022.104387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta J. L., Ding Z., Liu S., Wang X., Khaidakov M. (2014). Hypertension, TLR4 activation in brain and cardiac hypertrophy. Cardiovasc Res. 103, 3–4. 10.1093/cvr/cvu128 [DOI] [PubMed] [Google Scholar]
- Mei X., Zhang T., Ouyang H., Lu B., Wang Z., Ji L. (2019). Scutellarin alleviates blood-retina-barrier oxidative stress injury initiated by activated microglia cells during the development of diabetic retinopathy. Biochem. Pharmacol. 159, 82–95. 10.1016/j.bcp.2018.11.011 [DOI] [PubMed] [Google Scholar]
- Miao D., Zhao J., Han Y., Zhou J., Li X., Zhang T., et al. (2024). Management of locally advanced non-small cell lung cancer: state of the art and future directions. Cancer Commun. (Lond) 44, 23–46. 10.1002/cac2.12505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao Z., Lai Y., Zhao Y., Chen L., Zhou J., Li C., et al. (2021). Protective property of scutellarin against liver injury induced by carbon tetrachloride in mice. Front. Pharmacol. 12, 710692. 10.3389/fphar.2021.710692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu C., Sheng Y., Yang R., Lu B., Bai Q., Ji L., et al. (2015). Scutellarin protects against the liver injury induced by diosbulbin B in mice and its mechanism. J. Ethnopharmacol. 164, 301–308. 10.1016/j.jep.2015.02.031 [DOI] [PubMed] [Google Scholar]
- Niu R. Z., Xiong L. L., Zhou H. L., Xue L. L., Xia Q. J., Ma Z., et al. (2021). Scutellarin ameliorates neonatal hypoxic-ischemic encephalopathy associated with GAP43-dependent signaling pathway. Chin. Med. 16, 105. 10.1186/s13020-021-00517-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivares-Bañuelos T. N., Chí-Castañeda D., Ortega A. (2019). Glutamate transporters: gene expression regulation and signaling properties. Neuropharmacology 161, 107550. 10.1016/j.neuropharm.2019.02.032 [DOI] [PubMed] [Google Scholar]
- Oyefeso F. A., Muotri A. R., Wilson C. G., Pecaut M. J. (2021). Brain organoids: a promising model to assess oxidative stress-induced central nervous system damage. Dev. Neurobiol. 81, 653–670. 10.1002/dneu.22828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Z., Zhao W., Zhang X., Wang B., Wang J., Sun X., et al. (2011). Scutellarin alleviates interstitial fibrosis and cardiac dysfunction of infarct rats by inhibiting TGFβ1 expression and activation of p38-MAPK and ERK1/2. Br. J. Pharmacol. 162, 688–700. 10.1111/j.1476-5381.2010.01070.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Z. W., Zhang Y., Mei D. H., Zhang R., Wang J. H., Zhang X. Y., et al. (2010). Scutellarin exerts its anti-hypertrophic effects via suppressing the Ca2+-mediated calcineurin and CaMKII signaling pathways. Naunyn Schmiedeb. Arch. Pharmacol. 381, 137–145. 10.1007/s00210-009-0484-y [DOI] [PubMed] [Google Scholar]
- Pang I. H., Clark A. F. (2020). Inducible rodent models of glaucoma. Prog. Retin Eye Res. 75, 100799. 10.1016/j.preteyeres.2019.100799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawluk H., Tafelska-Kaczmarek A., Sopońska M., Porzych M., Modrzejewska M., Pawluk M., et al. (2024). The influence of oxidative stress markers in patients with ischemic stroke. Biomolecules 14, 1130. 10.3390/biom14091130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng L., Wen L., Shi Q. F., Gao F., Huang B., Meng J., et al. (2020). Scutellarin ameliorates pulmonary fibrosis through inhibiting NF-κB/NLRP3-mediated epithelial-mesenchymal transition and inflammation. Cell Death Dis. 11, 978. 10.1038/s41419-020-03178-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez M., Lucchesi B. R., Schaper J. (2002). Apoptosis in myocardial infarction. Ann. Med. 34, 470–479. 10.1080/078538902321012414 [DOI] [PubMed] [Google Scholar]
- Rostas J. A. P., Skelding K. A. (2023). Calcium/calmodulin-stimulated protein kinase II (CaMKII): different functional outcomes from activation, depending on the cellular microenvironment. Cells 12, 401. 10.3390/cells12030401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sardelli G., Scali V., Signore G., Balestri F., Cappiello M., Mura U., et al. (2023). Response of a human lens epithelial cell line to hyperglycemic and oxidative stress: the role of aldose reductase. Antioxidants (Basel) 12, 829. 10.3390/antiox12040829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahmohammadi A., Golchoobian R., Mirahmadi S. M., Rousta A. M., Ansari F., Sharayeli M., et al. (2023). Scutellarin alleviates lipopolysaccharide-provoked septic nephrotoxicity via attenuation of inflammatory and oxidative events and mitochondrial dysfunction. Immunopharmacol. Immunotoxicol. 45, 295–303. 10.1080/08923973.2022.2141644 [DOI] [PubMed] [Google Scholar]
- Sharawi Z. W., Ibrahim I. M., Abd-Alhameed E. K., Althagafy H. S., Jaber F. A., Harakeh S., et al. (2024). Baicalin and lung diseases. Naunyn Schmiedeb. Arch. Pharmacol. 397, 1405–1419. 10.1007/s00210-023-02704-1 [DOI] [PubMed] [Google Scholar]
- Shen S., He F., Cheng C., Xu B., Sheng J. (2021). Uric acid aggravates myocardial ischemia-reperfusion injury via ROS/NLRP3 pyroptosis pathway. Biomed. Pharmacother. 133, 110990. 10.1016/j.biopha.2020.110990 [DOI] [PubMed] [Google Scholar]
- Shi M., Liu Y., Feng L., Cui Y., Chen Y., Wang P., et al. (2015). Protective effects of scutellarin on human cardiac microvascular endothelial cells against hypoxia-reoxygenation injury and its possible target-related proteins. Evid. Based Complement. Altern. Med. 2015, 278014. 10.1155/2015/278014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin J. W., Kweon K. J., Kim D. K., Kim P., Jeon T. D., Maeng S., et al. (2018). Scutellarin ameliorates learning and memory deficit via suppressing β-amyloid formation and microglial activation in rats with chronic cerebral hypoperfusion. Am. J. Chin. Med. 46, 1203–1223. 10.1142/S0192415X18500635 [DOI] [PubMed] [Google Scholar]
- Stefan N., Cusi K. (2022). A global view of the interplay between non-alcoholic fatty liver disease and diabetes. Lancet Diabetes Endocrinol. 10, 284–296. 10.1016/S2213-8587(22)00003-1 [DOI] [PubMed] [Google Scholar]
- Su B., Li D., Xie J., Wang Y., Wu X., Li J., et al. (2023). Chronic disease in China: geographic and socioeconomic determinants among persons aged 60 and older. J. Am. Med. Dir. Assoc. 24, 206–212.e5. 10.1016/j.jamda.2022.10.002 [DOI] [PubMed] [Google Scholar]
- Su Y., Fan X., Li S., Li Z., Tian M., Li S. (2022). Scutellarin improves type 2 diabetic cardiomyopathy by regulating cardiomyocyte autophagy and apoptosis. Dis. Markers 2022, 3058354. 10.1155/2022/3058354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun C., Li C., Li X., Zhu Y., Su Z., Wang X., et al. (2018a). Scutellarin induces apoptosis and autophagy in NSCLC cells through ERK1/2 and AKT Signaling Pathways in vitro and in vivo . J. Cancer 9, 3247–3256. 10.7150/jca.25921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun C. Y., Nie J., Zheng Z. L., Zhao J., Wu L. M., Zhu Y., et al. (2019). Renoprotective effect of scutellarin on cisplatin-induced renal injury in mice: impact on inflammation, apoptosis, and autophagy. Biomed. Pharmacother. 112, 108647. 10.1016/j.biopha.2019.108647 [DOI] [PubMed] [Google Scholar]
- Sun C. Y., Zhu Y., Li X. F., Wang X. Q., Tang L. P., Su Z. Q., et al. (2018b). Scutellarin increases cisplatin-induced apoptosis and autophagy to overcome cisplatin resistance in non-small cell lung cancer via ERK/p53 and c-met/AKT signaling pathways. Front. Pharmacol. 9, 92. 10.3389/fphar.2018.00092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J., Meng M. (2022). Chemoprotective effect of scutellarin against gastric cancer in rats: an in vitro and in vivo study. J. Oleo Sci. 71, 1003–1012. 10.5650/jos.ess21399 [DOI] [PubMed] [Google Scholar]
- Sun J. B., Li Y., Cai Y. F., Huang Y., Liu S., Yeung P. K., et al. (2018c). Scutellarin protects oxygen/glucose-deprived astrocytes and reduces focal cerebral ischemic injury. Neural Regen. Res. 13, 1396–1407. 10.4103/1673-5374.235293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W., Ge Y., Cui J., Yu Y., Liu B. (2021). Scutellarin resensitizes oxaliplatin-resistant colorectal cancer cells to oxaliplatin treatment through inhibition of PKM2. Mol. Ther. Oncolytics 21, 87–97. 10.1016/j.omto.2021.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X., Zhou L., Han Y., Yang Q., Li X., Xin B., et al. (2023). Scutellarin attenuates doxorubicin-induced cardiotoxicity by inhibiting myocardial fibrosis, apoptosis and autophagy in rats. Chem. Biodivers. 20, e202200450. 10.1002/cbdv.202200450 [DOI] [PubMed] [Google Scholar]
- Sun X. P., Wan L. L., Yang Q. J., Huo Y., Han Y. L., Guo C. (2017). Scutellarin protects against doxorubicin-induced acute cardiotoxicity and regulates its accumulation in the heart. Arch. Pharm. Res. 40, 875–883. 10.1007/s12272-017-0907-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan Z. H., Yu L. H., Wei H. L., Liu G. T. (2007). The protective action of scutellarin against immunological liver injury induced by concanavalin A and its effect on pro-inflammatory cytokines in mice. J. Pharm. Pharmacol. 59, 115–121. 10.1211/jpp.59.1.0015 [DOI] [PubMed] [Google Scholar]
- Tan Z. H., Yu L. H., Wei H. L., Liu G. T. (2010). Scutellarin protects against lipopolysaccharide-induced acute lung injury via inhibition of NF-kappaB activation in mice. J. Asian Nat. Prod. Res. 12, 175–184. 10.1080/10286020903347906 [DOI] [PubMed] [Google Scholar]
- Tang H., Tang Y., Li N., Shi Q., Guo J., Shang E., et al. (2014). Neuroprotective effects of scutellarin and scutellarein on repeatedly cerebral ischemia-reperfusion in rats. Pharmacol. Biochem. Behav. 118, 51–59. 10.1016/j.pbb.2014.01.003 [DOI] [PubMed] [Google Scholar]
- Tang S. L., Gao Y. L., Hu W. Z. (2019). Scutellarin inhibits the metastasis and cisplatin resistance in glioma cells. Onco Targets Ther. 12, 587–598. 10.2147/OTT.S187426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tawfeek S. E., Shalaby A. M., Alabiad M. A., Albackoosh A. A. A., Albakoush K. M. M., Omira M. M. A. (2021). Metanil yellow promotes oxidative stress, astrogliosis, and apoptosis in the cerebellar cortex of adult male rat with possible protective effect of scutellarin: a histological and immunohistochemical study. Tissue Cell 73, 101624. 10.1016/j.tice.2021.101624 [DOI] [PubMed] [Google Scholar]
- Wang H., He Y., Sun Z., Ren S., Liu M., Wang G., et al. (2022a). Microglia in depression: an overview of microglia in the pathogenesis and treatment of depression. J. Neuroinflammation 19, 132. 10.1186/s12974-022-02492-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Zhao B., Yang S., Wang D., Xu H., Teng M. (2018). Scutellarin enhances osteoblast proliferation and function via NF-κB-mediated CXCR4 induction. Gene 676, 29–36. 10.1016/j.gene.2018.06.068 [DOI] [PubMed] [Google Scholar]
- Wang L., Yang J., Xiao X., Zheng C., Ming D. (2021). VEGF modulates the neural dynamics of hippocampal subregions in chronic global cerebral ischemia rats. Neuromolecular Med. 23, 416–427. 10.1007/s12017-020-08642-y [DOI] [PubMed] [Google Scholar]
- Wang W., Li J., Li F., Peng J., Xu M., Shangguan Y., et al. (2019). Scutellarin suppresses cartilage destruction in osteoarthritis mouse model by inhibiting the NF-κB and PI3K/AKT signaling pathways. Int. Immunopharmacol. 77, 105928. 10.1016/j.intimp.2019.105928 [DOI] [PubMed] [Google Scholar]
- Wang W., Ma X., Han J., Zhou M., Ren H., Pan Q., et al. (2016a). Neuroprotective effect of scutellarin on ischemic cerebral injury by down-regulating the expression of angiotensin-converting enzyme and AT1 receptor. PLoS One 11, e0146197. 10.1371/journal.pone.0146197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W. W., Han J. H., Wang L., Bao T. H. (2017). Scutellarin may alleviate cognitive deficits in a mouse model of hypoxia by promoting proliferation and neuronal differentiation of neural stem cells. Iran. J. Basic Med. Sci. 20, 272–279. 10.22038/IJBMS.2017.8355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Fan X., Fan B., Jiang K., Zhang H., Kang F., et al. (2020). Scutellarin reduce the homocysteine level and alleviate liver injury in type 2 diabetes model. Front. Pharmacol. 11, 538407. 10.3389/fphar.2020.538407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Liu X., Chen W., Guo X., Chen L., Wei Z., et al. (2023). Scutellarin suppressed proliferation and induced apoptosis in gastric cancer via wnt/β-catenin signaling pathway. Curr. Pharm. Des. 29, 368–378. 10.2174/1381612829666230130141931 [DOI] [PubMed] [Google Scholar]
- Wang Z., Yu J., Wu J., Qi F., Wang H., Wang Z., et al. (2016b). Scutellarin protects cardiomyocyte ischemia-reperfusion injury by reducing apoptosis and oxidative stress. Life Sci. 157, 200–207. 10.1016/j.lfs.2016.01.018 [DOI] [PubMed] [Google Scholar]
- Wang Z., Zhang P., Zhao Y., Yu F., Wang S., Liu K., et al. (2022b). Scutellarin protects against mitochondrial reactive oxygen species-dependent NLRP3 inflammasome activation to attenuate intervertebral disc degeneration. Front. Bioeng. Biotechnol. 10, 883118. 10.3389/fbioe.2022.883118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierońska J. M., Cieślik P., Kalinowski L. (2021). Nitric oxide-dependent pathways as critical factors in the consequences and recovery after brain ischemic hypoxia. Biomolecules 11, 1097. 10.3390/biom11081097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H., Jia L. (2019). Scutellarin attenuates hypoxia/reoxygenation injury in hepatocytes by inhibiting apoptosis and oxidative stress through regulating Keap1/Nrf2/ARE signaling. Biosci. Rep. 39. 10.1042/BSR20192501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi J., Rong Y., Zhao Z., Huang Y., Wang P., Luan H., et al. (2021). Scutellarin ameliorates high glucose-induced vascular endothelial cells injury by activating PINK1/Parkin-mediated mitophagy. J. Ethnopharmacol. 271, 113855. 10.1016/j.jep.2021.113855 [DOI] [PubMed] [Google Scholar]
- Xia C. Y., Guo Y. X., Lian W. W., Yan Y., Ma B. Z., Cheng Y. C., et al. (2023). The NLRP3 inflammasome in depression: potential mechanisms and therapies. Pharmacol. Res. 187, 106625. 10.1016/j.phrs.2022.106625 [DOI] [PubMed] [Google Scholar]
- Xie W., Zhu T., Dong X., Nan F., Meng X., Zhou P., et al. (2019). HMGB1-triggered inflammation inhibition of notoginseng leaf triterpenes against cerebral ischemia and reperfusion injury via MAPK and NF-κB signaling pathways. Biomolecules 9, 512. 10.3390/biom9100512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X., Wang F., Ge W., Meng X., Fan L., Zhang W., et al. (2023). Scutellarin attenuates oxidative stress and neuroinflammation in cerebral ischemia/reperfusion injury through PI3K/Akt-mediated Nrf2 signaling pathways. Eur. J. Pharmacol. 957, 175979. 10.1016/j.ejphar.2023.175979 [DOI] [PubMed] [Google Scholar]
- Xu D., Liu A., Liu Q., Zhang H., Tian M., Bian Y., et al. (2023). Cucurbitacin C suppresses the progression of pancreatic ductal adenocarcinoma via inhibition of the cGMP-PKG-VASP axis. Biochem. Pharmacol. 217, 115810. 10.1016/j.bcp.2023.115810 [DOI] [PubMed] [Google Scholar]
- Xu L., Chen R., Zhang X., Zhu Y., Ma X., Sun G., et al. (2021). Scutellarin protects against diabetic cardiomyopathy via inhibiting oxidative stress and inflammatory response in mice. Ann. Palliat. Med. 10, 2481–2493. 10.21037/apm-19-516 [DOI] [PubMed] [Google Scholar]
- Xu L. J., Chen R. C., Ma X. Y., Zhu Y., Sun G. B., Sun X. B. (2020). Scutellarin protects against myocardial ischemia-reperfusion injury by suppressing NLRP3 inflammasome activation. Phytomedicine 68, 153169. 10.1016/j.phymed.2020.153169 [DOI] [PubMed] [Google Scholar]
- Yang L., Guttman L., Dawson V. L., Dawson T. M. (2024a). Parthanatos: mechanisms, modulation, and therapeutic prospects in neurodegenerative disease and stroke. Biochem. Pharmacol. 228, 116174. 10.1016/j.bcp.2024.116174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L., Li Z., Fang J. (2024b). Scutellarin alleviates diabetic retinopathy via the suppression of nucleotide-binding oligomerization domain (NOD)-Like receptor pyrin domain containing protein 3 inflammasome activation. Curr. Eye Res. 49, 180–187. 10.1080/02713683.2023.2273777 [DOI] [PubMed] [Google Scholar]
- Yang L., Liu X., Chen S., Sun J., Tao Y., Ma L., et al. (2024c). Scutellarin ameliorates mitochondrial dysfunction and apoptosis in OGD/R-insulted HT22 cells through mitophagy induction. Biomed. Pharmacother. 179, 117340. 10.1016/j.biopha.2024.117340 [DOI] [PubMed] [Google Scholar]
- Yang N., Zhao Y., Wang Z., Liu Y., Zhang Y. (2017). Scutellarin suppresses growth and causes apoptosis of human colorectal cancer cells by regulating the p53 pathway. Mol. Med. Rep. 15, 929–935. 10.3892/mmr.2016.6081 [DOI] [PubMed] [Google Scholar]
- Yin X., Feng L., Ma D., Yin P., Wang X., Hou S., et al. (2018). Roles of astrocytic connexin-43, hemichannels, and gap junctions in oxygen-glucose deprivation/reperfusion injury induced neuroinflammation and the possible regulatory mechanisms of salvianolic acid B and carbenoxolone. J. Neuroinflammation 15, 97. 10.1186/s12974-018-1127-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- You P., Fu S., Yu K., Xia Y., Wu H., Yang Y., et al. (2018). Scutellarin suppresses neuroinflammation via the inhibition of the AKT/NF-κB and p38/JNK pathway in LPS-induced BV-2 microglial cells. Naunyn Schmiedeb. Arch. Pharmacol. 391, 743–751. 10.1007/s00210-018-1503-7 [DOI] [PubMed] [Google Scholar]
- Yuan T., Yang H. Y., Li Y. P., Shi Z. J., Zhou Z. Y., You Y. P., et al. (2024). Scutellarin inhibits inflammatory PANoptosis by diminishing mitochondrial ROS generation and blocking PANoptosome formation. Int. Immunopharmacol. 139, 112710. 10.1016/j.intimp.2024.112710 [DOI] [PubMed] [Google Scholar]
- Yuan Y., Rangarajan P., Kan E. M., Wu Y., Wu C., Ling E. A. (2015). Scutellarin regulates the Notch pathway and affects the migration and morphological transformation of activated microglia in experimentally induced cerebral ischemia in rats and in activated BV-2 microglia. J. Neuroinflammation 12, 11. 10.1186/s12974-014-0226-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue T., Shi Y., Luo S., Weng J., Wu Y., Zheng X. (2022). The role of inflammation in immune system of diabetic retinopathy: molecular mechanisms, pathogenetic role and therapeutic implications. Front. Immunol. 13, 1055087. 10.3389/fimmu.2022.1055087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng S., Chen L., Sun Q., Zhao H., Yang H., Ren S., et al. (2021). Scutellarin ameliorates colitis-associated colorectal cancer by suppressing Wnt/β-catenin signaling cascade. Eur. J. Pharmacol. 906, 174253. 10.1016/j.ejphar.2021.174253 [DOI] [PubMed] [Google Scholar]
- Zeng S., Tan L., Sun Q., Chen L., Zhao H., Liu M., et al. (2022). Suppression of colitis-associated colorectal cancer by scutellarin through inhibiting Hedgehog signaling pathway activity. Phytomedicine 98, 153972. 10.1016/j.phymed.2022.153972 [DOI] [PubMed] [Google Scholar]
- Zeng Y. Q., Cui Y. B., Gu J. H., Liang C., Zhou X. F. (2018). Scutellarin mitigates aβ-induced neurotoxicity and improves behavior impairments in AD mice. Molecules 23, 869. 10.3390/molecules23040869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G. Y., Chen W. Y., Li X. B., Ke H., Zhou X. L. (2021). Scutellarin-induced A549 cell apoptosis depends on activation of the transforming growth factor-β1/smad2/ROS/caspase-3 pathway. Open Life Sci. 16, 961–968. 10.1515/biol-2021-0085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H. F., Hu X. M., Wang L. X., Xu S. Q., Zeng F. D. (2009). Protective effects of scutellarin against cerebral ischemia in rats: evidence for inhibition of the apoptosis-inducing factor pathway. Planta Med. 75, 121–126. 10.1055/s-0028-1088368 [DOI] [PubMed] [Google Scholar]
- Zhang L., Sun S., Li W., Zhang W., Wang X., Yang S.-Y. (2017). Effect of Scutellarin inhibits collagen-induced arthritis through TLR4/NF-κB-mediated inflammation. Mol. Med. Rep. 16, 5555–5560. 10.3892/mmr.2017.7292 [DOI] [PubMed] [Google Scholar]
- Zhang M., Liu Q., Meng H., Duan H., Liu X., Wu J., et al. (2024). Ischemia-reperfusion injury: molecular mechanisms and therapeutic targets. Signal Transduct. Target Ther. 9, 12. 10.1038/s41392-023-01688-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Jia M., Wang Y., Wang Q., Wu J. (2022a). Cell death mechanisms in cerebral ischemia-reperfusion injury. Neurochem. Res. 47, 3525–3542. 10.1007/s11064-022-03697-8 [DOI] [PubMed] [Google Scholar]
- Zhang Q., Wang L., Wang S., Cheng H., Xu L., Pei G., et al. (2022b). Signaling pathways and targeted therapy for myocardial infarction. Signal Transduct. Target Ther. 7, 78. 10.1038/s41392-022-00925-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S., Wei D., Lv S., Wang L., An H., Shao W., et al. (2022c). Scutellarin modulates the microbiota-gut-brain Axis and improves cognitive impairment in APP/PS1 mice. J. Alzheimers Dis. 89, 955–975. 10.3233/JAD-220532 [DOI] [PubMed] [Google Scholar]
- Zhang S., Zhang J., Wei D., An H., Liu W., Lai Y., et al. (2020). Dengzhan Shengmai capsules and their active component scutellarin prevent cognitive decline in APP/PS1 mice by accelerating Aβ aggregation and reducing oligomers formation. Biomed. Pharmacother. 121, 109682. 10.1016/j.biopha.2019.109682 [DOI] [PubMed] [Google Scholar]
- Zhang T., Wang K., Fan H., Yang Q., Zhang X., Liu F., et al. (2022d). Ameliorative effect of scutellarin on acute alcohol brain injury in mice. J. Zhejiang Univ. Sci. B 23, 258–264. 10.1631/jzus.B2100763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Huo Z., Luan H., Huang Y., Shen Y., Sheng L., et al. (2022e). Scutellarin ameliorates hepatic lipid accumulation by enhancing autophagy and suppressing IRE1α/XBP1 pathway. Phytother. Res. 36, 433–447. 10.1002/ptr.7344 [DOI] [PubMed] [Google Scholar]
- Zhang X., Ji R., Sun H., Peng J., Ma X., Wang C., et al. (2018). Scutellarin ameliorates nonalcoholic fatty liver disease through the PPARγ/PGC-1α-Nrf2 pathway. Free Radic. Res. 52, 198–211. 10.1080/10715762.2017.1422602 [DOI] [PubMed] [Google Scholar]
- Zhang Y., Zhang Z., Wang J., Zhang X., Zhao J., Bai N., et al. (2022f). Scutellarin alleviates cerebral ischemia/reperfusion by suppressing oxidative stress and inflammatory responses via MAPK/NF-κB pathways in rats. Environ. Toxicol. 37, 2889–2896. 10.1002/tox.23645 [DOI] [PubMed] [Google Scholar]
- Zhao S., Sun Y., Li X., Wang J., Yan L., Zhang Z., et al. (2016). Scutellarin inhibits RANKL-mediated osteoclastogenesis and titanium particle-induced osteolysis via suppression of NF-κB and MAPK signaling pathway. Int. Immunopharmacol. 40, 458–465. 10.1016/j.intimp.2016.09.031 [DOI] [PubMed] [Google Scholar]
- Zheng T., Jiang T., Huang Z., Ma H., Wang M. (2023). Role of traditional Chinese medicine monomers in cerebral ischemia/reperfusion injury:a review of the mechanism. Front. Pharmacol. 14, 1220862. 10.3389/fphar.2023.1220862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong X., Luo C., Deng M., Zhao M. (2019). Scutellarin-treated exosomes increase claudin 5, occludin and ZO1 expression in rat brain microvascular endothelial cells. Exp. Ther. Med. 18, 33–40. 10.3892/etm.2019.7562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H., Chen X., Chen L., Zhou X., Zheng G., Zhang H., et al. (2014). Anti-fibrosis effect of scutellarin via inhibition of endothelial-mesenchymal transition on isoprenaline-induced myocardial fibrosis in rats. Molecules 19, 15611–15623. 10.3390/molecules191015611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L., Han Y., Yang Q., Xin B., Chi M., Huo Y., et al. (2022). Scutellarin attenuates doxorubicin-induced oxidative stress, DNA damage, mitochondrial dysfunction, apoptosis and autophagy in H9c2 cells, cardiac fibroblasts and HUVECs. Toxicol Vitro 82, 105366. 10.1016/j.tiv.2022.105366 [DOI] [PubMed] [Google Scholar]
- Zhu J. T., Choi R. C., Li J., Xie H. Q., Bi C. W., Cheung A. W., et al. (2009). Estrogenic and neuroprotective properties of scutellarin from Erigeron breviscapus: a drug against postmenopausal symptoms and Alzheimer's disease. Planta Med. 75, 1489–1493. 10.1055/s-0029-1185776 [DOI] [PubMed] [Google Scholar]