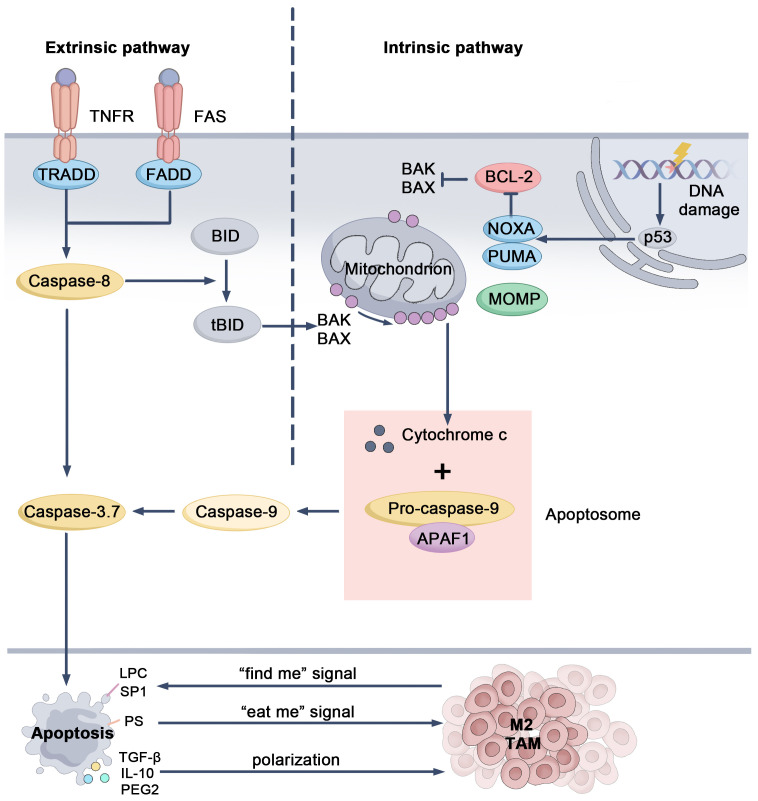
Figure 2.
Mechanistic pathways and regulatory targets of apoptosis. When DNA is damaged, the tumor suppressor protein p53 is activated, triggering the expression of pro-apoptotic genes like Noxa and Puma. These proteins inhibit the anti-apoptotic Bcl-2 family members, allowing Bax and Bak to oligomerize on the mitochondrial outer membrane. This causes MOMP, leading to the release of cytochrome c. Once released, cytochrome c combines with APAF-1 and pro-caspase-9, forming the apoptosome. The apoptosome activates caspase-9, which subsequently activates caspase-3 and caspase-7, ultimately driving the cell through apoptosis. In the extrinsic pathway, signals from death receptors trigger the activation of caspase-8. Caspase-8 can directly activate effector caspases or cleave BID into tBID, which then interacts with Bax and Bak, connecting this pathway to the intrinsic one. During apoptosis, tumor cells release “find-me” signals to recruit macrophages and express “eat-me” signals to facilitate phagocytosis. They also release immunosuppressive molecules that can shift macrophages towards an immunosuppressive TAM phenotype, contributing to the tumor’s immune evasion. BH3-interacting domain death agonist (BID), Bcl-2-associated X protein (Bax), Bcl-2 killer (Bak), Mitochondrial Outer Membrane Permeabilization (MOMP), Apoptotic protease activating factor 1 (APAF-1).