Abstract
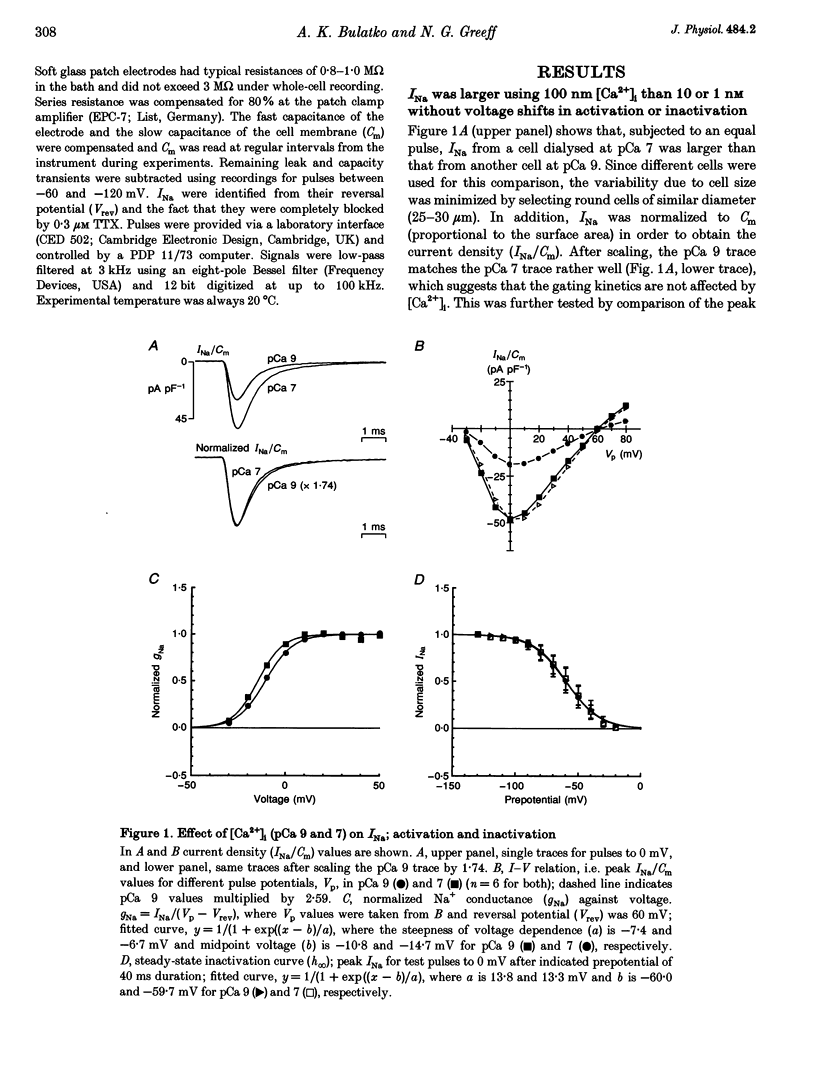
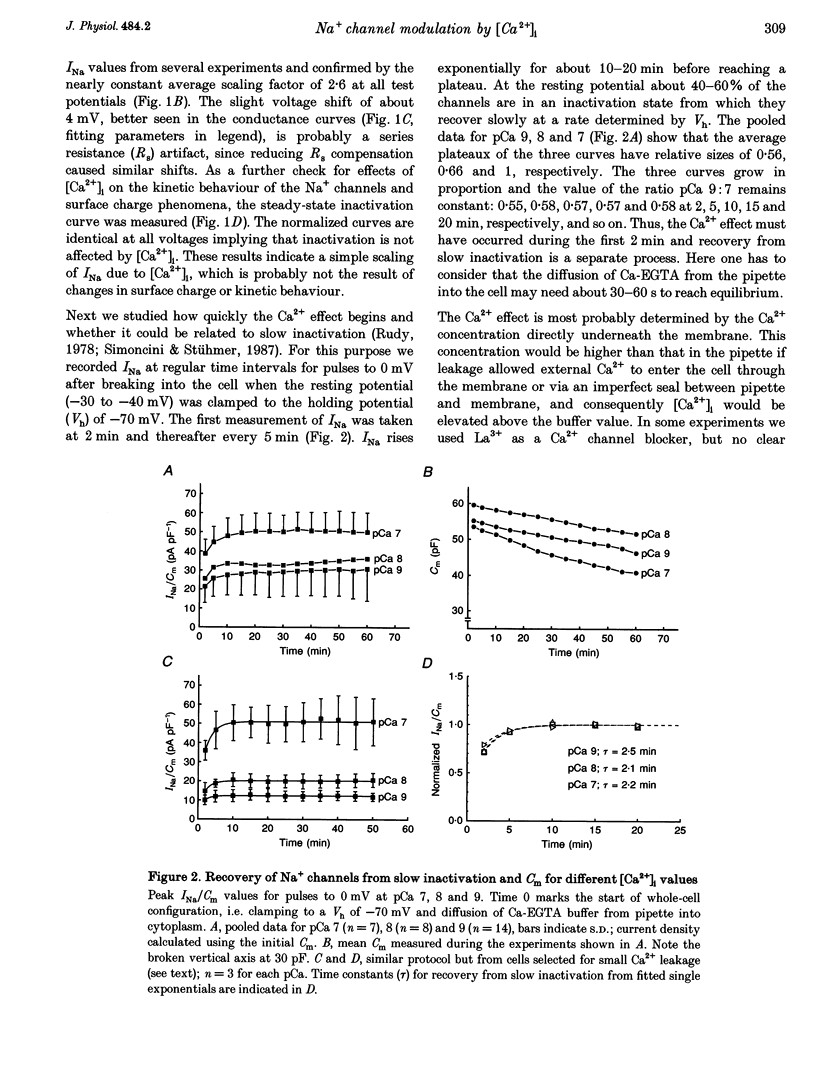
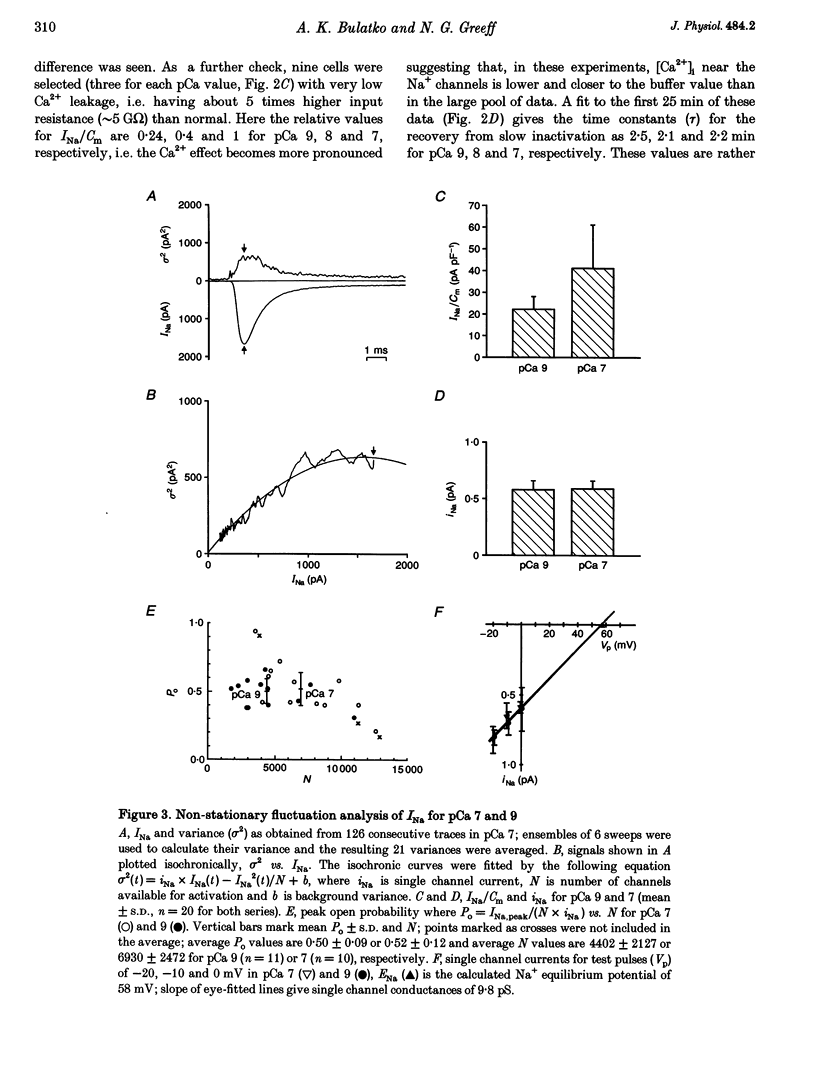
1. Whole-cell sodium currents (INa) were measured in mouse neuroblastoma cells (N1E-115) at different [Ca2+]i values using appropriate Ca-EGTA buffers in the pipettes. 2. INa was found to be larger at pCa 7 than at pCa 8 or 9 with a ratio of 1:0.65 or 0.55, respectively. The steady-state inactivation (h infinity curve) was independent of [Ca2+]i, thus excluding surface charge effects as a cause of the Ca2+ effect. 3. Recovery of INa from slow inactivation after changing from resting (-30 to -40 mV) to holding potential (-70 mV) occurred in a similar way at all pCa values. The Ca2+ effect appears to be independent of slow inactivation and to occur within the first 2 min of pipette buffer-cytoplasm equilibration. 4. The cell membrane capacitance (Cm) was independent of [Ca2+]i, thus excluding exo- or endocytosis of sodium channel-containing membrane as a cause of the Ca2+ effect. 5. Non-stationary fluctuation analysis was used to determine simultaneously the single channel current (iNa) and the size of INa. At pCa values of 7 and 9, iNa was identical, i.e. 0.59 and 0.58 pA, while INa/Cm differed, i.e. 41.1 and 22.2 pA pF-1, respectively. The peak open probability at 0 mV was about 0.5 for both pCa values indicating that [Ca2+]i controls the fraction of channels available for activation. 6. Since [Ca2+]i in other neurons varies between 30 and 100 nM in the resting and active state, respectively, the present data suggest a modulatory role for [Ca2+]i in neuronal excitability.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asaoka Y., Nakamura S., Yoshida K., Nishizuka Y. Protein kinase C, calcium and phospholipid degradation. Trends Biochem Sci. 1992 Oct;17(10):414–417. doi: 10.1016/0968-0004(92)90011-w. [DOI] [PubMed] [Google Scholar]
- BAKER P. F., HODGKIN A. L., SHAW T. I. Replacement of the axoplasm of giant nerve fibres with artificial solutions. J Physiol. 1962 Nov;164:330–354. doi: 10.1113/jphysiol.1962.sp007025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekkers J. M., Greeff N. G., Keynes R. D. The conductance and density of sodium channels in the cut-open squid giant axon. J Physiol. 1986 Aug;377:463–486. doi: 10.1113/jphysiol.1986.sp016198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall W. A. Cellular and molecular biology of voltage-gated sodium channels. Physiol Rev. 1992 Oct;72(4 Suppl):S15–S48. doi: 10.1152/physrev.1992.72.suppl_4.S15. [DOI] [PubMed] [Google Scholar]
- Cohen P. Signal integration at the level of protein kinases, protein phosphatases and their substrates. Trends Biochem Sci. 1992 Oct;17(10):408–413. doi: 10.1016/0968-0004(92)90010-7. [DOI] [PubMed] [Google Scholar]
- Kennedy M. B. Regulation of neuronal function by calcium. Trends Neurosci. 1989 Nov;12(11):417–420. doi: 10.1016/0166-2236(89)90089-1. [DOI] [PubMed] [Google Scholar]
- Knöpfel T., Gähwiler B. H. Activity-induced elevations of intracellular calcium concentration in pyramidal and nonpyramidal cells of the CA3 region of rat hippocampal slice cultures. J Neurophysiol. 1992 Sep;68(3):961–963. doi: 10.1152/jn.1992.68.3.961. [DOI] [PubMed] [Google Scholar]
- Marty A. The physiological role of calcium-dependent channels. Trends Neurosci. 1989 Nov;12(11):420–424. doi: 10.1016/0166-2236(89)90090-8. [DOI] [PubMed] [Google Scholar]
- Ohsako S., Deguchi T. Receptor-mediated regulation of calcium mobilization and cyclic GMP synthesis in neuroblastoma cells. Biochem Biophys Res Commun. 1984 Jul 18;122(1):333–339. doi: 10.1016/0006-291x(84)90479-0. [DOI] [PubMed] [Google Scholar]
- Rudy B. Slow inactivation of the sodium conductance in squid giant axons. Pronase resistance. J Physiol. 1978 Oct;283:1–21. doi: 10.1113/jphysiol.1978.sp012485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigworth F. J. The variance of sodium current fluctuations at the node of Ranvier. J Physiol. 1980 Oct;307:97–129. doi: 10.1113/jphysiol.1980.sp013426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simoncini L., Stühmer W. Slow sodium channel inactivation in rat fast-twitch muscle. J Physiol. 1987 Feb;383:327–337. doi: 10.1113/jphysiol.1987.sp016411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatsumi H., Katayama Y. Regulation of the intracellular free calcium concentration in acutely dissociated neurones from rat nucleus basalis. J Physiol. 1993 May;464:165–181. doi: 10.1113/jphysiol.1993.sp019628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer S. A., Miller R. J. Regulation of the intracellular free calcium concentration in single rat dorsal root ganglion neurones in vitro. J Physiol. 1990 Jun;425:85–115. doi: 10.1113/jphysiol.1990.sp018094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tohse N., Kameyama M., Irisawa H. Intracellular Ca2+ and protein kinase C modulate K+ current in guinea pig heart cells. Am J Physiol. 1987 Nov;253(5 Pt 2):H1321–H1324. doi: 10.1152/ajpheart.1987.253.5.H1321. [DOI] [PubMed] [Google Scholar]


