Abstract
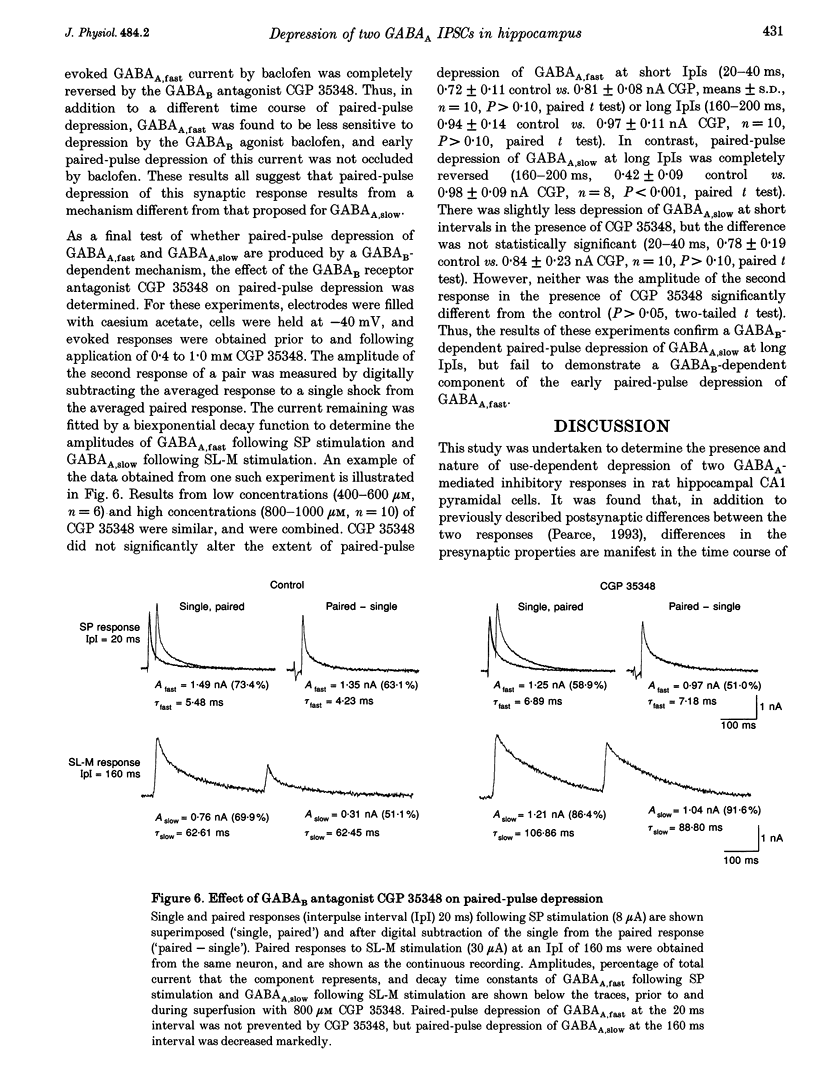
1. The mechanisms involved in the use-dependent depression of GABAA,fast and GABAA,slow, two GABAA-mediated IPSCs in the rat hippocampal slice preparation, were investigated by observing the effects of paired-pulse depression and of baclofen and CGP 35348 on monosynaptic inhibitory currents recorded from CA1 pyramidal neurons. 2. The second of a pair of evoked responses that consisted of both inhibitory components was depressed and decayed more rapidly compared to the first at an interpulse interval (IpI) of 200 ms. This effect was due to a decrease in the amplitude of GABAA,slow, with no effect on the time constant of decay or on the amplitude or time constant of GABAA,fast. 3. The time course of paired-pulse depression of both components at IpIs ranging from 5 to 2560 ms was compared. GABAA,slow was depressed maximally by 55% at IpIs of 80-160 ms. GABAA,fast was depressed maximally by 38% at 5 ms, and recovered exponentially with a time constant of 130 ms. 4. GABAA,slow was more sensitive than GABAA,fast to depression by baclofen. GABAA,slow was susceptible to complete block, with an ED50 of approximately 200 nM for (+/-)-baclofen and 100 nM for the active enantiomer, (R)-(+)-baclofen. GABAA,fast was blocked by only 50% by the highest concentrations of baclofen tested (10-100 microM (R)-(+)-baclofen), with an ED50 of approximately 2 microM for (+/-)-baclofen and 1 microM for (R)-(+)-baclofen. Paired-pulse depression of GABAA,fast was not occluded by 10 or 100 microM (R)-(+)-baclofen. 5. The GABAB antagonist CGP 35348 (0.4-1 mM), prevented paired-pulse depression of GABAA,slow at IpIs of 160 to 200 ms, and reversed the depression of GABAA,fast by baclofen, but had no effect on paired-pulse depression of GABAA,fast at IpIs of 20 to 40 ms. 6. It is concluded that use-dependent depression of GABAA,slow, but not GABAA,fast, is mediated by a presynaptic GABAB receptor. It is speculated that use-dependent depression of GABAA,fast, which occurs only over a much faster time scale, may be due to rapid postsynaptic GABAA receptor desensitization.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonanno G., Raiteri M. gamma-Aminobutyric acid (GABA) autoreceptors in rat cerebral cortex and spinal cord represent pharmacologically distinct subtypes of the GABAB receptor. J Pharmacol Exp Ther. 1993 May;265(2):765–770. [PubMed] [Google Scholar]
- Buhl E. H., Halasy K., Somogyi P. Diverse sources of hippocampal unitary inhibitory postsynaptic potentials and the number of synaptic release sites. Nature. 1994 Apr 28;368(6474):823–828. doi: 10.1038/368823a0. [DOI] [PubMed] [Google Scholar]
- Cohen G. A., Doze V. A., Madison D. V. Opioid inhibition of GABA release from presynaptic terminals of rat hippocampal interneurons. Neuron. 1992 Aug;9(2):325–335. doi: 10.1016/0896-6273(92)90171-9. [DOI] [PubMed] [Google Scholar]
- Davies C. H., Collingridge G. L. The physiological regulation of synaptic inhibition by GABAB autoreceptors in rat hippocampus. J Physiol. 1993 Dec;472:245–265. doi: 10.1113/jphysiol.1993.sp019945. [DOI] [PMC free article] [PubMed] [Google Scholar]
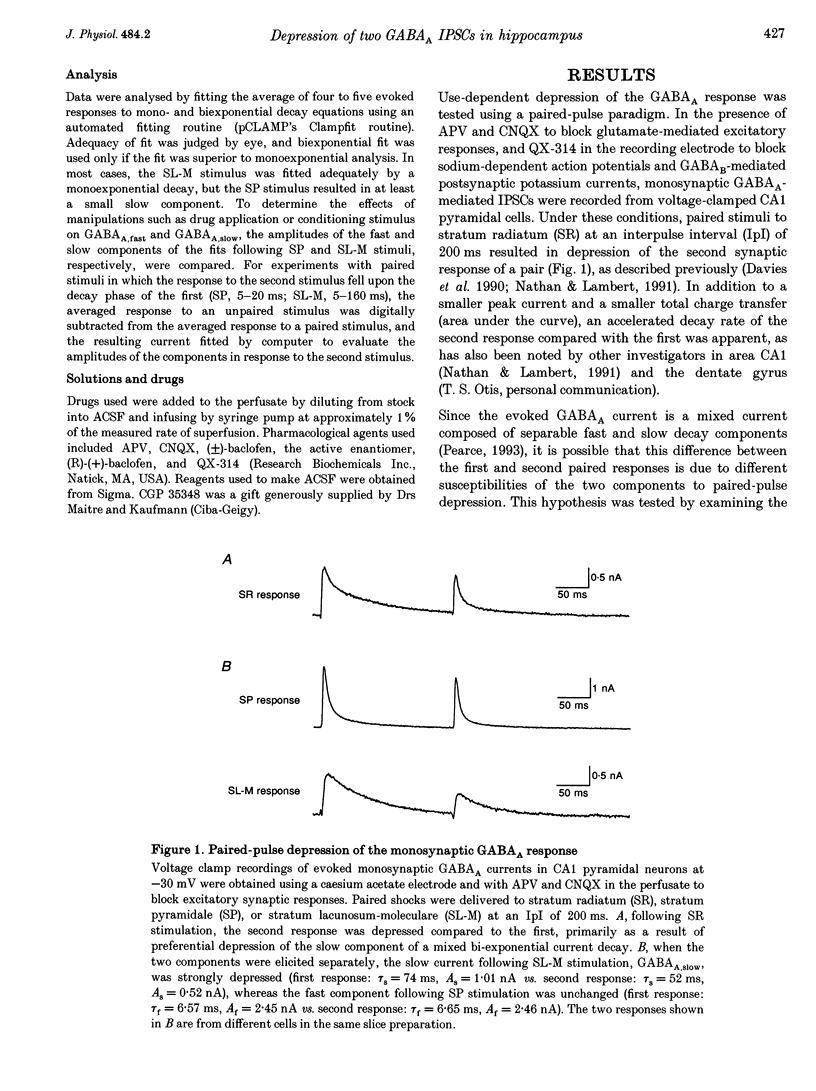
- Davies C. H., Davies S. N., Collingridge G. L. Paired-pulse depression of monosynaptic GABA-mediated inhibitory postsynaptic responses in rat hippocampus. J Physiol. 1990 May;424:513–531. doi: 10.1113/jphysiol.1990.sp018080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies C. H., Starkey S. J., Pozza M. F., Collingridge G. L. GABA autoreceptors regulate the induction of LTP. Nature. 1991 Feb 14;349(6310):609–611. doi: 10.1038/349609a0. [DOI] [PubMed] [Google Scholar]
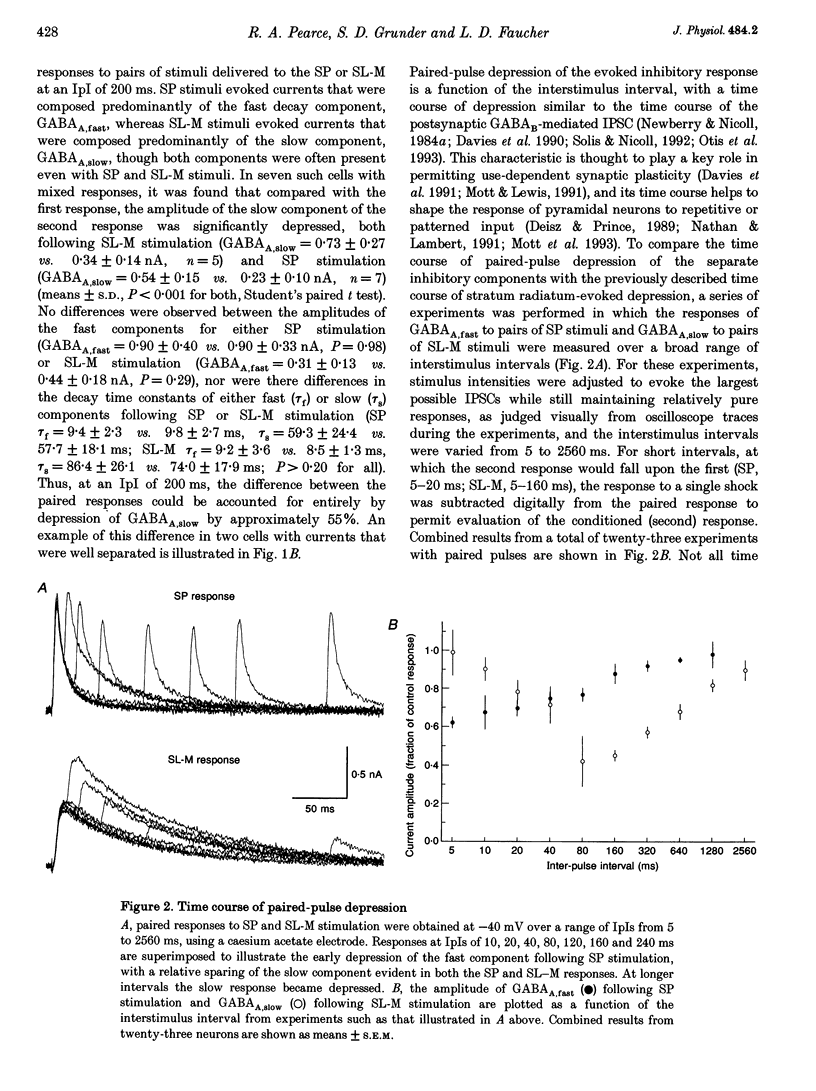
- Deisz R. A., Prince D. A. Frequency-dependent depression of inhibition in guinea-pig neocortex in vitro by GABAB receptor feed-back on GABA release. J Physiol. 1989 May;412:513–541. doi: 10.1113/jphysiol.1989.sp017629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frosch M. P., Lipton S. A., Dichter M. A. Desensitization of GABA-activated currents and channels in cultured cortical neurons. J Neurosci. 1992 Aug;12(8):3042–3053. doi: 10.1523/JNEUROSCI.12-08-03042.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison N. L. On the presynaptic action of baclofen at inhibitory synapses between cultured rat hippocampal neurones. J Physiol. 1990 Mar;422:433–446. doi: 10.1113/jphysiol.1990.sp017993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacson J. S., Solís J. M., Nicoll R. A. Local and diffuse synaptic actions of GABA in the hippocampus. Neuron. 1993 Feb;10(2):165–175. doi: 10.1016/0896-6273(93)90308-e. [DOI] [PubMed] [Google Scholar]
- Ishizuka N., Weber J., Amaral D. G. Organization of intrahippocampal projections originating from CA3 pyramidal cells in the rat. J Comp Neurol. 1990 May 22;295(4):580–623. doi: 10.1002/cne.902950407. [DOI] [PubMed] [Google Scholar]
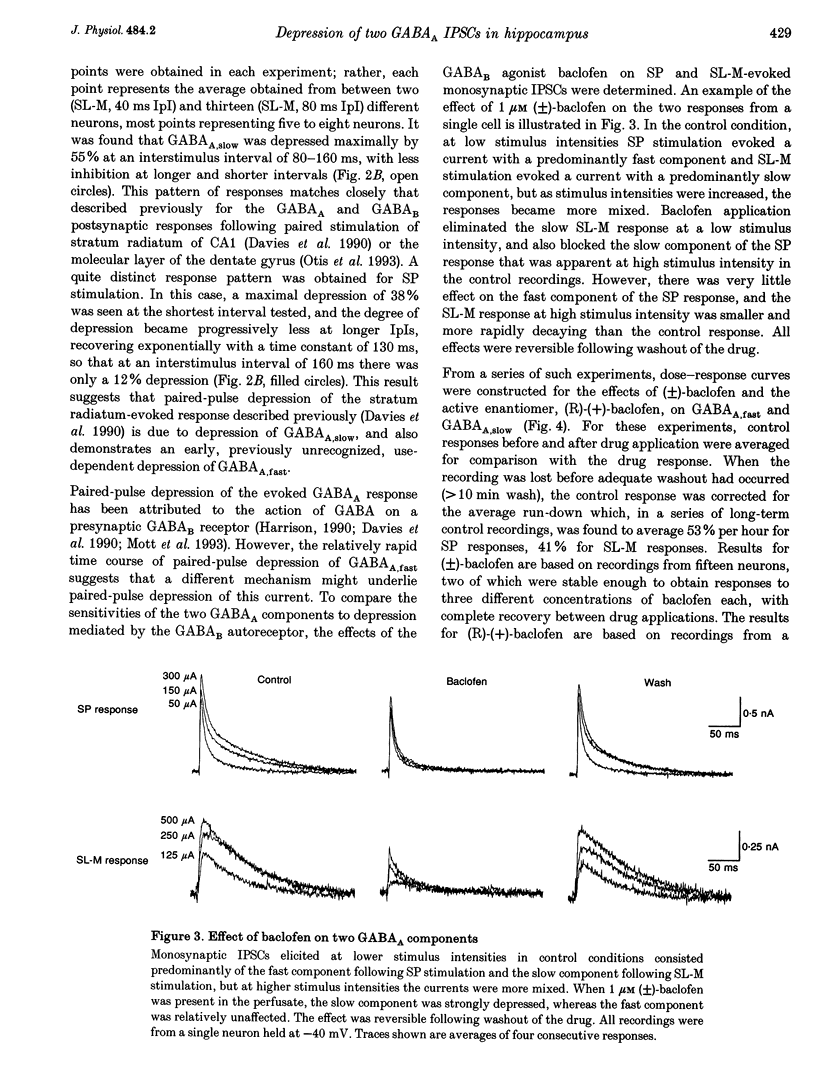
- Lacaille J. C., Schwartzkroin P. A. Stratum lacunosum-moleculare interneurons of hippocampal CA1 region. I. Intracellular response characteristics, synaptic responses, and morphology. J Neurosci. 1988 Apr;8(4):1400–1410. doi: 10.1523/JNEUROSCI.08-04-01400.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb T. D., Pugh E. N., Jr G-protein cascades: gain and kinetics. Trends Neurosci. 1992 Aug;15(8):291–298. doi: 10.1016/0166-2236(92)90079-n. [DOI] [PubMed] [Google Scholar]
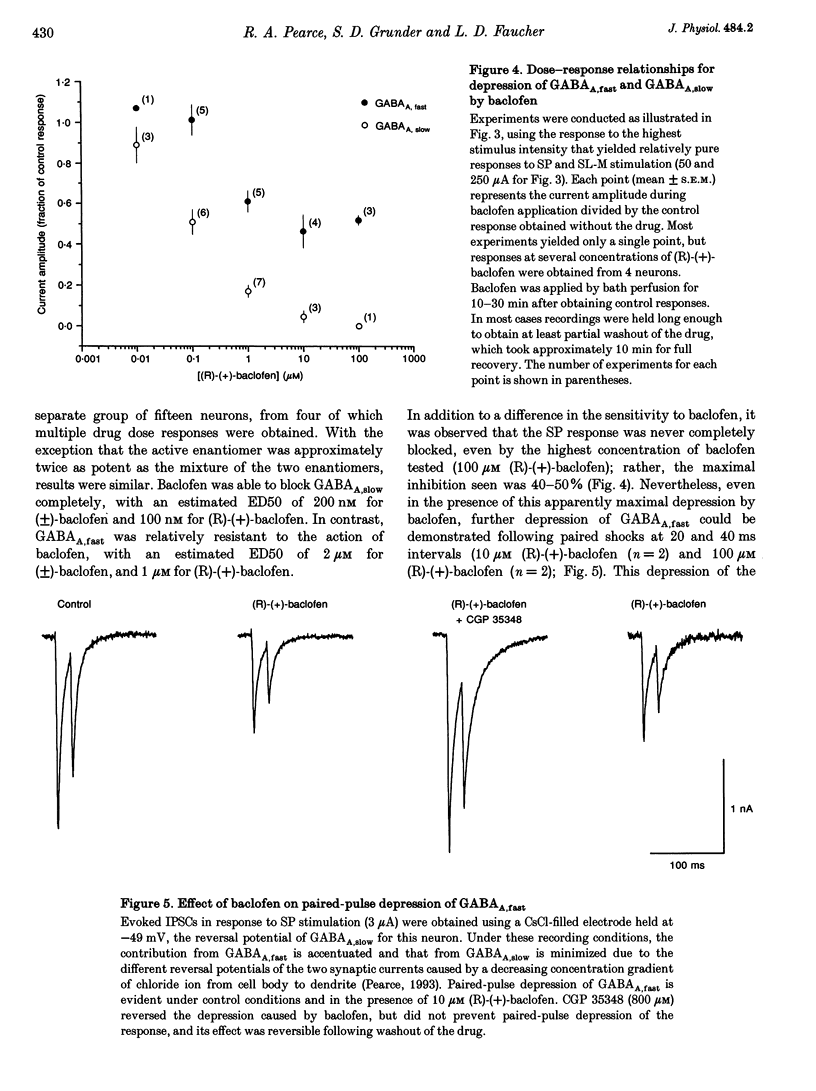
- Lambert N. A., Harrison N. L., Teyler T. J. Baclofen-induced disinhibition in area CA1 of rat hippocampus is resistant to extracellular Ba2+. Brain Res. 1991 May 3;547(2):349–352. doi: 10.1016/0006-8993(91)90985-5. [DOI] [PubMed] [Google Scholar]
- Lambert N. A., Wilson W. A. Heterogeneity in presynaptic regulation of GABA release from hippocampal inhibitory neurons. Neuron. 1993 Dec;11(6):1057–1067. doi: 10.1016/0896-6273(93)90219-h. [DOI] [PubMed] [Google Scholar]
- Lambert N. A., Wilson W. A. Temporally distinct mechanisms of use-dependent depression at inhibitory synapses in the rat hippocampus in vitro. J Neurophysiol. 1994 Jul;72(1):121–130. doi: 10.1152/jn.1994.72.1.121. [DOI] [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L. The action of N-methyl-D-aspartic acid on mouse spinal neurones in culture. J Physiol. 1985 Apr;361:65–90. doi: 10.1113/jphysiol.1985.sp015633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mott D. D., Lewis D. V. Facilitation of the induction of long-term potentiation by GABAB receptors. Science. 1991 Jun 21;252(5013):1718–1720. doi: 10.1126/science.1675489. [DOI] [PubMed] [Google Scholar]
- Mott D. D., Xie C. W., Wilson W. A., Swartzwelder H. S., Lewis D. V. GABAB autoreceptors mediate activity-dependent disinhibition and enhance signal transmission in the dentate gyrus. J Neurophysiol. 1993 Mar;69(3):674–691. doi: 10.1152/jn.1993.69.3.674. [DOI] [PubMed] [Google Scholar]
- Nathan T., Jensen M. S., Lambert J. D. The slow inhibitory postsynaptic potential in rat hippocampal CA1 neurones is blocked by intracellular injection of QX-314. Neurosci Lett. 1990 Mar 14;110(3):309–313. doi: 10.1016/0304-3940(90)90865-7. [DOI] [PubMed] [Google Scholar]
- Nathan T., Lambert J. D. Depression of the fast IPSP underlies paired-pulse facilitation in area CA1 of the rat hippocampus. J Neurophysiol. 1991 Nov;66(5):1704–1715. doi: 10.1152/jn.1991.66.5.1704. [DOI] [PubMed] [Google Scholar]
- Newberry N. R., Nicoll R. A. A bicuculline-resistant inhibitory post-synaptic potential in rat hippocampal pyramidal cells in vitro. J Physiol. 1984 Mar;348:239–254. doi: 10.1113/jphysiol.1984.sp015107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newberry N. R., Nicoll R. A. Direct hyperpolarizing action of baclofen on hippocampal pyramidal cells. 1984 Mar 29-Apr 4Nature. 308(5958):450–452. doi: 10.1038/308450a0. [DOI] [PubMed] [Google Scholar]
- Otis T. S., De Koninck Y., Mody I. Characterization of synaptically elicited GABAB responses using patch-clamp recordings in rat hippocampal slices. J Physiol. 1993 Apr;463:391–407. doi: 10.1113/jphysiol.1993.sp019600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otis T. S., Mody I. Differential activation of GABAA and GABAB receptors by spontaneously released transmitter. J Neurophysiol. 1992 Jan;67(1):227–235. doi: 10.1152/jn.1992.67.1.227. [DOI] [PubMed] [Google Scholar]
- Otis T. S., Mody I. Modulation of decay kinetics and frequency of GABAA receptor-mediated spontaneous inhibitory postsynaptic currents in hippocampal neurons. Neuroscience. 1992 Jul;49(1):13–32. doi: 10.1016/0306-4522(92)90073-b. [DOI] [PubMed] [Google Scholar]
- Pearce R. A. Physiological evidence for two distinct GABAA responses in rat hippocampus. Neuron. 1993 Feb;10(2):189–200. doi: 10.1016/0896-6273(93)90310-n. [DOI] [PubMed] [Google Scholar]
- Pfrieger F. W., Gottmann K., Lux H. D. Kinetics of GABAB receptor-mediated inhibition of calcium currents and excitatory synaptic transmission in hippocampal neurons in vitro. Neuron. 1994 Jan;12(1):97–107. doi: 10.1016/0896-6273(94)90155-4. [DOI] [PubMed] [Google Scholar]
- Puia G., Costa E., Vicini S. Functional diversity of GABA-activated Cl- currents in Purkinje versus granule neurons in rat cerebellar slices. Neuron. 1994 Jan;12(1):117–126. doi: 10.1016/0896-6273(94)90157-0. [DOI] [PubMed] [Google Scholar]
- Scanziani M., Capogna M., Gähwiler B. H., Thompson S. M. Presynaptic inhibition of miniature excitatory synaptic currents by baclofen and adenosine in the hippocampus. Neuron. 1992 Nov;9(5):919–927. doi: 10.1016/0896-6273(92)90244-8. [DOI] [PubMed] [Google Scholar]
- Scholz K. P., Miller R. J. GABAB receptor-mediated inhibition of Ca2+ currents and synaptic transmission in cultured rat hippocampal neurones. J Physiol. 1991 Dec;444:669–686. doi: 10.1113/jphysiol.1991.sp018900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal M. A subset of local interneurons generate slow inhibitory postsynaptic potentials in hippocampal neurons. Brain Res. 1990 Mar 12;511(1):163–164. doi: 10.1016/0006-8993(90)90236-5. [DOI] [PubMed] [Google Scholar]
- Somogyi P., Nunzi M. G., Gorio A., Smith A. D. A new type of specific interneuron in the monkey hippocampus forming synapses exclusively with the axon initial segments of pyramidal cells. Brain Res. 1983 Jan 17;259(1):137–142. doi: 10.1016/0006-8993(83)91076-4. [DOI] [PubMed] [Google Scholar]
- Thompson S. M., Capogna M., Scanziani M. Presynaptic inhibition in the hippocampus. Trends Neurosci. 1993 Jun;16(6):222–227. doi: 10.1016/0166-2236(93)90160-n. [DOI] [PubMed] [Google Scholar]
- Tomasulo R. A., Ramirez J. J., Steward O. Synaptic inhibition regulates associative interactions between afferents during the induction of long-term potentiation and depression. Proc Natl Acad Sci U S A. 1993 Dec 15;90(24):11578–11582. doi: 10.1073/pnas.90.24.11578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trussell L. O., Zhang S., Raman I. M. Desensitization of AMPA receptors upon multiquantal neurotransmitter release. Neuron. 1993 Jun;10(6):1185–1196. doi: 10.1016/0896-6273(93)90066-z. [DOI] [PubMed] [Google Scholar]
- Williams S., Lacaille J. C. GABAB receptor-mediated inhibitory postsynaptic potentials evoked by electrical stimulation and by glutamate stimulation of interneurons in stratum lacunosum-moleculare in hippocampal CA1 pyramidal cells in vitro. Synapse. 1992 Jul;11(3):249–258. doi: 10.1002/syn.890110309. [DOI] [PubMed] [Google Scholar]
- Zhang S. J., Jackson M. B. GABA-activated chloride channels in secretory nerve endings. Science. 1993 Jan 22;259(5094):531–534. doi: 10.1126/science.8380942. [DOI] [PubMed] [Google Scholar]


