Abstract
The 5-fluorouracil (5-FU)-based chemotherapy regimen is a primary strategy for treating pancreatic cancer (PC). However, challenges related to 5-FU resistance persist. Investigating the mechanisms of 5-FU resistance and identifying a clinically viable therapeutic strategy are crucial for improving the prognosis of PC. Here, through clinical samples analysis, we found that the expression of 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMGCR), the rate-limiting enzyme in mevalonate metabolism, is negatively correlated with the efficacy of 5-FU treatment. There is a significant correlation between HMGCR and the pyroptosis marker gasdermin D (GSDMD), and the HMGCR inhibitor simvastatin can significantly inhibit the activation of pyroptosis signaling. The exogenous addition of geranylgeranyl pyrophosphate (GGPP), a key metabolite of the mevalonate pathway, can significantly reduce sensitivity to 5-FU, and simvastatin combined with 5-FU demonstrates a strong synergistic effect. Furthermore, in organoid models and genetically engineered mice with spontaneous PC, the combination of simvastatin and 5-FU significantly inhibits tumor growth. In conclusion, our study reveals the critical role of the mevalonate pathway in 5-FU resistance and proposes a clinically feasible combination therapy strategy.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12032-024-02557-5.
Keywords: 5-Fluorouracil, Mevalonate pathway, Pyroptosis, Pancreatic cancer, Chemoresistance
Introduction
Despite recent advances in pancreatic cancer (PC) treatment, it remains a significant challenge, with a 5-year survival rate of less than 10% [1, 2]. The National Comprehensive Cancer Network Pancreatic Cancer guidelines recommend that the modified FOLFIRINOX regimen will bring longer median survival. Among them, 5-fluorouracil (5-FU) is the cornerstone of the therapeutic protocol, and 5-FU-based FOLFIRINOX is recommended for patients with PC after surgery [3] 0.5-FU, a cell cycle-specific uracil analog, interacts with DNA,RNA, or both, leading to its accumulation in cells, and causing tumor cell damage and death [4]. Therefore, 5-FU plays an indispensable role in the treatment process of PC [5, 6]. However, due to the low stability of 5-FU in cells and the susceptibility to drug resistance, only a minority of patients benefit from FOLFIRINOX [7–9].Tumor metabolic reprogramming enhances tumor progression by disrupting the anti-tumor metabolic activity of 5-FU. Elevated glucose metabolism and increased enzyme expression diminish the drug's effectiveness [10, 11]. Additionally, the accumulation of lipid droplets and the overexpression of the nuclear protein sterol-regulatory element-binding protein-1 impede the anticancer effect of 5-FU [12, 13]. Consequently, further investigation into how tumor metabolic reprogramming regulates 5-FU chemotherapy resistance is crucial for enhancing the effectiveness of the FOLFIRINOX regimen.
The mevalonate pathway, a critical pathway for cholesterol synthesis, plays a significant role in tumor progression when abnormally activated [14]. Our study revealed that 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMGCR) exhibited elevated expression in the postoperative pathological tissues of PC patients undergoing the FOLFIRINOX regimen. Geranylgeranyl pyrophosphate (GGPP), a product of the mevalonate pathway, promotes the prenylation of GTPases. Prenylated GTPases play a key role in signal transduction, cell proliferation, cell plasticity, tumorigenesis, and cancer metastasis [15, 16]. Additionally, we identified that the accumulation of GGPP contributes to resistance against 5-FU. Consequently, targeting the mevalonate pathway could potentially enhance the efficacy of 5-FU by overcoming this resistance mechanism. Furthermore, recent studies have demonstrated that simvastatin has the capacity to trigger pyroptosis in tumor cells, thereby amplifying the anti-tumor effects of the treatment [17, 18]. This outcome was substantiated through our rigorous study, suggesting a promising approach to bolstering the effectiveness of 5-FU in combating PC.
The primary aim of this research was to investigate the correlation between the mevalonate pathway and resistance to 5-FU chemotherapy. Furthermore, the study aimed to shed light on the potential of combining simvastatin with 5-FU as a novel approach to enhance the limited effectiveness observed with the FOLFIRINOX regimen.
Materials and methods
Human PC clinical specimens
Fifty-six PC samples and 43 adjacent normal tissue samples were collected, along with relevant clinical data, to assess the association between HMGCR levels and 5-FU resistance. All patients, who received postoperative FOLFIRINOX chemotherapy, had not undergone preoperative chemotherapy or radiotherapy. The specimens were obtained in compliance with the guidelines approved by the Human Research Ethics Committee of Lanzhou University Second Hospital, with informed agreement from all participants.
Statistical analysis
SPSS 27.0 was used to perform all statistical analyses. The Shapiro–Wilk normality test was utilized to assess data normality. For data adhering to normal distribution, Student's t test was conducted for the comparison of means across groups, with results expressed as means ± standard deviation. The Chi-square test was employed for frequency comparison across groups. Nonparametric tests were applied to data that did not follow a normal distribution. Kaplan–Meier curves, in conjunction with the log-rank test, were used to conduct survival analysis. Multivariate Cox models were used to investigate the influence of HMGCR on prognosis. All analyses were two-tailed, with a P value below 0.05 regarded as significant in statistical terms. In vitro assays were conducted a minimum of three times, incorporating both biological and technical replicates.
Cell culture
PANC-1,MIA PaCa-2 (human PC cell lines, the Cell Bank of the Chinese Academy of Sciences) and Panc 02 (mouse PC cell line, Shanghai Fuheng Biotechnology Co., Ltd) were grown in Dulbecco’s modified Eagle medium with the addition of 10% fetal bovine serum (Panc 02,5%).
Genotype identification of mice
Three monogenic mouse strains, LSL-KrasG12D/+, LSL-Trp53R172H/+ and Pdx1-Cre, were crossbred to obtain progeny, and genomic DNA was extracted from their tails. The mice were genotyped through PCR amplification and subsequent sequencing of extracted genomic DNA.
Tumor subcutaneous transplantation model
In the allograft model using KPC mice, tumor tissues from these mice were cut into approximately 4 mm diameter pieces and implanted into the axillary region of 6-week-old female C57BL/6JGpt mice.
Progression and survival of KPC-derived allografts
5-FU (intraperitoneal injection, 20 mg/kg/day) and/or simvastatin (oral administration, 20 mg/kg/day) were administered to KPC-derived allografts when the tumor size reached approximately 150 mm3.Throughout the tumor progression studies, tumor diameters were monitored every 4 days during the treatment period. When the tumor volume of any mouse reached 1500 mm3, all mice were euthanized. The tumor tissues were then harvested, photographed, and preserved for subsequent analysis. For survival assays, tumor diameters were monitored daily during drug treatment. The ethical end point was defined as a tumor size nearing 1500 mm3, at which point the survival duration of each mouse was recorded.
Organoids studies
Tumor tissues from KPC mice were minced and incubated for 1–2 h at 37 °C in a digestion medium containing collagenase XI (Sigma-Aldrich, #C7657). After enzymatic digestion, the single-cell suspension was collected, resuspended in cell culture plates with Cultrex UltiMatrix Reduced Growth Factor Basement Membrane Extract (R&D Systems, #BME001-10), and cultured in organoid medium (STEMCELL Technologies, #06040).
Organoids’ viability and synergy assay
The viability of the KPC organoids was confirmed using the CellTiter-Glo® 3D Cell Viability Assay kit (Promega, #G9681). The three-dimensional synergy visualization model (LOEWE) and synergy score for 5-FU and simvastatin in KPC organoids were generated using Combenefit software and the SynergyFinder web application (version 3.0).
Additional materials and methods are provided in the Supplementary Materials.
Result
Abnormal activation of the mevalonate pathway promotes 5-FU resistance in PC
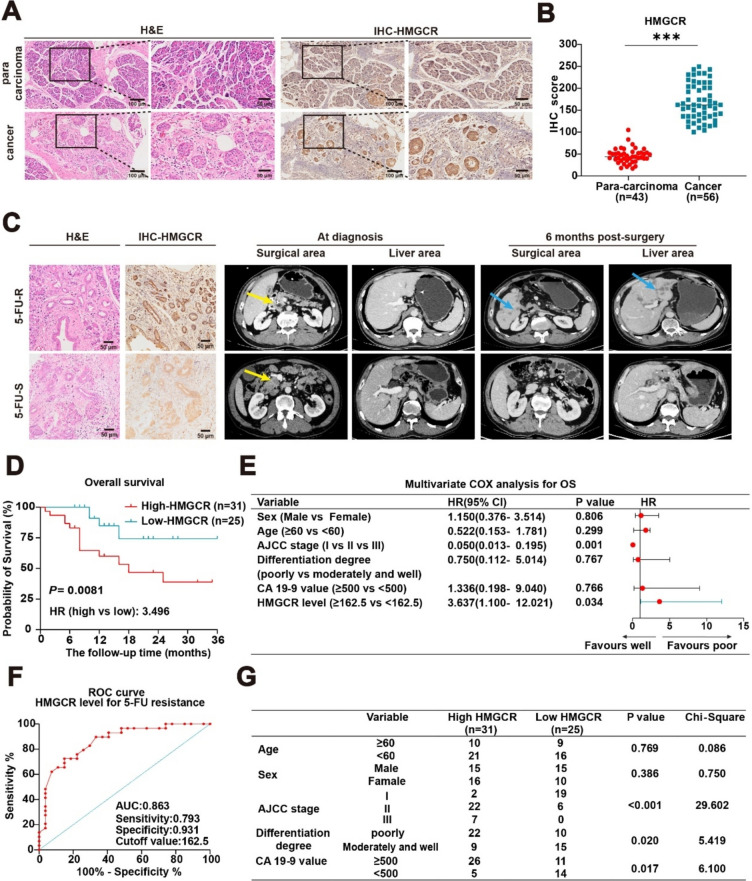
To investigate the role of the mevalonate pathway in conferring 5-FU chemoresistance in PC, we collected pathological samples from patients treated with FOLFIRINOX at the Lanzhou University Second Hospital between 2021 and 2023. These samples were subjected to H&E and IHC staining (Fig. 1A). Statistical analysis of these groups revealed significant differences in HMGCR levels between cancerous and adjacent non-cancerous tissues (Fig. 1B). Patients were categorized into 5-FU-sensitive (5-FU-S) and 5-FU-resistant (5-FU-R) groups based on imaging findings. Notably, HMGCR levels were markedly higher in the 5-FU-R group (Fig. 1C). Furthermore, patients treated with 5-FU were stratified using an optimal cutoff value, and Kaplan–Meier survival analysis demonstrated that elevated higher HMGCR levels were linked to worse survival outcomes (Fig. 1D). This finding aligns with the results from the multivariate Cox regression analysis (Fig. 1E). The receiver operating characteristic curve highlighted the high diagnostic value of HMGCR levels for predicting 5-FU resistance, with an area under the curve of 0.863 and an optimal cutoff value of 162.5 (Fig. 1F). Additionally, clinical data indicated a positive correlation between HMGCR levels and the American Joint Committee on Cancer (AJCC) staging (Fig. 1G). Collectively, these findings suggest a potential link between elevated HMGCR level and reduced 5-FU efficacy.
Fig. 1.
Abnormal activation of the mevalonate pathway promotes 5-FU resistance in PC. A-B Symbolic H&E staining and IHC staining of pathological tissues from PC patients, along with the IHC score. Results are presented as mean ± standard deviation. Student’s t test. ***P < 0.001. C Typical H&E and IHC staining of HMGCR and computed tomography scan in 5-FU-S and 5-FU-R patients. Yellow arrow, primary tumor; blue arrow, hepatic metastasis. D The correlation of OS and HMGCR expression level of patients treated with 5-FU. Kaplan–Meier analysis with log-rank test. HR, hazard ratio. E Multivariate Cox regression analysis of the prognosis in PC patients with FOLFIRINOX regimen, with 95% confidence intervals shown by the bars. F ROC curve illustrating the diagnostic accuracy of HMGCR expression levels in distinguishing 5-FU resistance among 56 patients with PC. G Analysis of the correlation between sex, age, AJCC staging, tumor differentiation, CA 19–9 value, and HMGCR levels in PC patients who have undergone treatment with the FOLFIRINOX regimen
Targeting the mevalonate pathway causes pyroptosis
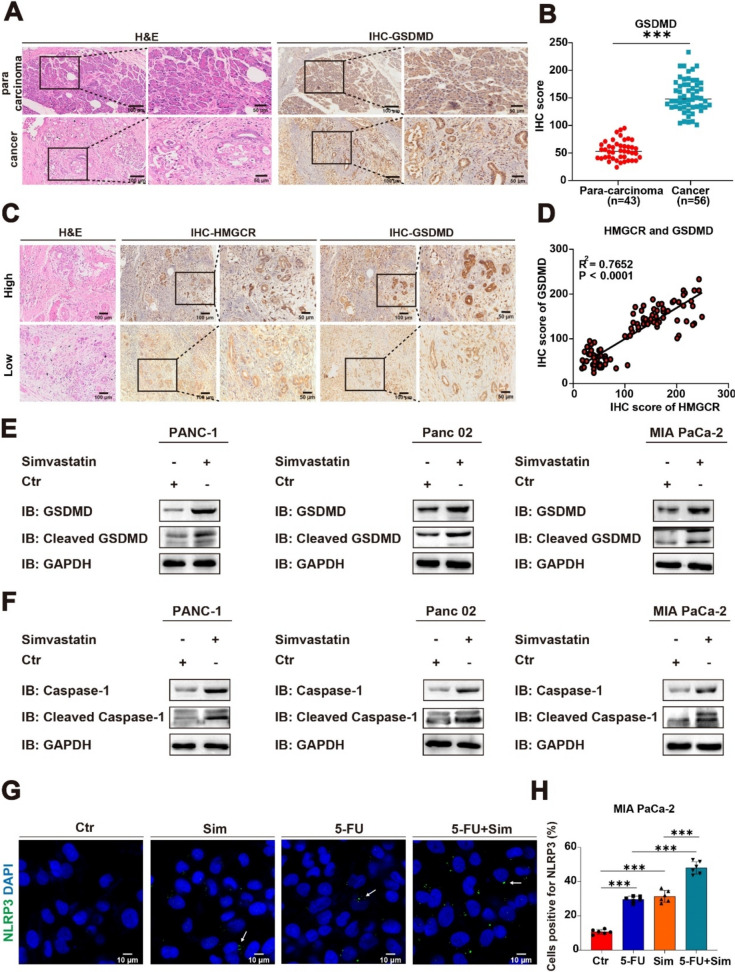
According to the study, simvastatin inhibits tumor progression by targeting the mevalonate pathway and inducing pyroptosis [17]. To explore the relationship between pyroptosis and the mevalonate pathway, we performed H&E and IHC staining of a marker protein of pyroptosis (gasdermin D, GSDMD) on clinical samples from PC patients and conducted statistical analysis (Fig. 2A-C). We noted a positive association between the levels of HMGCR and GSDMD (Fig. 2D). Next, we treated PANC-1, MIA PaCa-2 (human PC cell lines), and Panc 02 (mouse PC cell line) with simvastatin for 24 h. Then, we used immunoblotting to assess the expression of caspase-1 and GSDMD in PANC-1, Panc 02, and MIA PaCa-2 cells (Fig. 2E-F). To further verify this result, immunofluorescence (IF) staining was performed. We induced pyroptosis in MIA PaCa-2 cell line by monotherapy or in combination with simvastatin and 5-FU (simvastatin, 5 μM; 5-FU, 4 μM; 24 h). We found that the pyroptotic effect was significantly enhanced with the combination treatment (Fig. 2G-H). The results indicated that simvastatin treatment significantly promoted pyroptosis in tumor cells compared to the control group. Thus, simvastatin effectively inhibits tumor progression not only by down-regulating HMGCR, but also by inducing pyroptosis.
Fig. 2.
Targeting the mevalonate pathway causes pyroptosis. A-B Symbolic H&E staining and IHC staining of pathological tissues from PC patients, along with the IHC score. Results are presented as mean ± standard deviation. Student’s t test. ***P < 0.001. C Representative IHC staining images of high and low levels of HMGCR and GSDMD. D The protein level correlation between HMGCR and GSDMD in patients with PC from our cohort (n = 56) using linear regression analysis. E–F Immunoblotting assay showing the protein level of pyroptosis marker protein in control (Ctr) and treatment (simvastatin, 5 μM) of PANC-1, Panc 02, and MIA PaCa-2. G-H Representative IF staining images and quantification on the induction of pyroptosis in MIA PaCa-2 cells by simvastatin and 5-FU, either alone or in combination. White arrow, the inflammasome. Results are presented as mean ± standard deviation. Student’s t test. ***P < 0.001
Simvastatin combined with 5-FU is synergistic
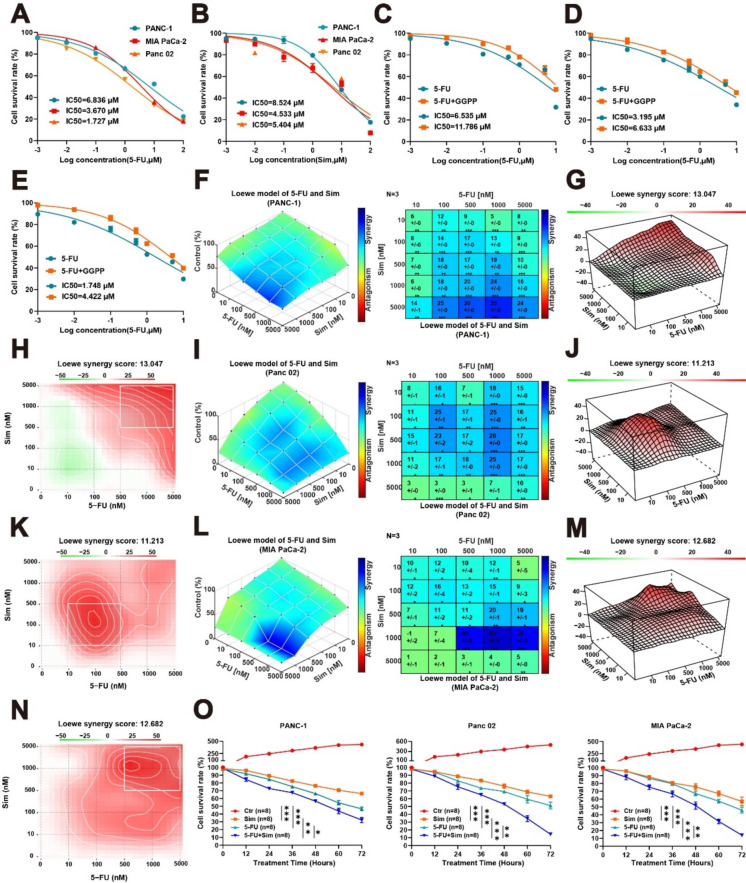
The anti-tumor effects of statins have been established, and treatment regimens that include combination chemotherapy drugs are already utilized in clinical settings [18]. Our study confirmed that the aberrant activation of the mevalonate pathway can inhibit the anti-tumor efficacy of 5-FU. We subsequently determined the concentrations required for 50% inhibition (IC50) of 5-FU and simvastatin for PANC-1, Panc 02, and MIA PaCa-2 and plotted the concentration–response curves (Fig. 3A-B). By exogenously supplementing the same concentration of GGPP, a downstream metabolite of mevalonate pathway, we observed a significant decrease in 5-FU sensitivity across all three cell lines following supplementation with GGPP. The results indicate that abnormal activation of the mevalonate pathway significantly enhances the resistance of PC to 5-FU (Fig. 3C-E). Our results underscore the pivotal role of the mevalonate pathway in conferring 5-FU resistance in PC. Utilizing drug synergy software, we evaluated the synergistic effects of both agents. The optimally synergy concentrations of 5-FU and simvastatin were identified using the Loewe model. Among the three PC cell lines, the optimal synergy concentrations (1 μM 5-FU combined with 5 μM simvastatin in PANC-1, 1 μM 5-FU combined wih 0.5 μM simvastatin in Panc 02, and 1 μM 5-FU combined wih 1 μM simvastatin in MIA PaCa-2) and the optimal synergy scores (13.047, 11.213, 12.682) were found (Fig. 3F–N, Supplementary Fig. 1A–C, E–G). Furthermore, using the identified synergy concentrations, we conducted cell viability assays on three PC cell lines and found that the combined effect of 5-FU and simvastatin significantly enhanced the anti-tumor efficacy (Fig. 3O). The results revealed that simvastatin significantly enhances the cytotoxic effects of 5-FU against tumor cells, suggesting that targeting the mevalonate pathway may counteract tumor resistance to 5-FU.
Fig. 3.
Simvastatin combined with 5-FU is synergistic. A-B The sensitivity to 5-FU and simvastatin in three PC cell lines. C-E After exogenous GGPP (10 μM) supplementation, the sensitivity to 5-FU in three PC cell lines. F-N The synergistic effect of 5-FU and simvastatin was evaluated by collaborative analysis model, and the synergistic concentration was measured respectively. O Cell viability analysis of PANC-1, Panc 02, and MIA PaCa-2 cells after treatment with different conditions for 72 h. Results are presented as mean ± standard deviation. Student’s t test. *P < 0.05, **P < 0.01, ***P < 0.001
Targeting the mevalonate pathway combined with 5-FU significantly inhibited tumor progression
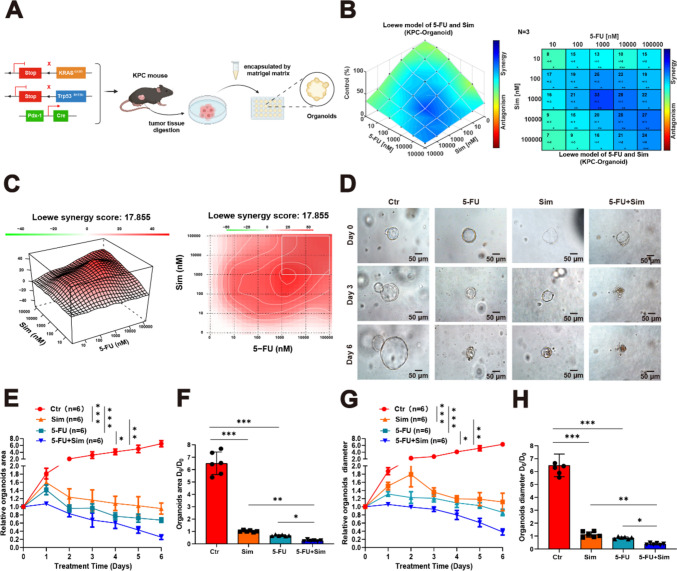
We demonstrated a strong synergistic effect of targeting the mevalonate pathway with 5-FU, and we continued to verify this effect with organoid models and genetically engineered mice with spontaneous PC. The KPC mice were obtained through breeding LSL-KrasG12D/+, LSL-Trp53R172H/+ and Pdx1-Cre and represent a successfully established murine model for PC and exhibit numerous characteristics resembling human PC [19, 20]. We harvested tumor tissues from KPC mice, isolated the stem cells through digestion, and subsequently cultured them in a 24-well plate using a matrix gel, thereby obtaining KPC organoids (Fig. 4A). Firstly, we investigated the synergistic effect of 5-FU and simvastatin on KPC organoids. The Loewe model indicated a strong synergistic interaction (Fig. 4B-C, Supplementary Fig. 1 D, H). Secondly, KPC organoids were treated with the identified optimal synergy concentration and categorized into three groups: control, single drug, and combination. Forty-eight hours later, the medium was replaced and the corresponding drugs were added (simvastatin; 5-FU), with this regimen continuing for 6 days. During this period, the medium was refreshed every 2 days and the appropriate drugs were administered. Morphological changes in the organoids were monitored and recorded using an electron microscope (Fig. 4D). Additionally, we tracked the growth diameters and areas of the organoids from Day 0 to Day 6 and conducted statistical analyses on the relative diameters and areas (Fig. 4E-H). We observed that simvastatin combined with 5-FU exerted a potent tumor-suppressive effect in KPC organoids.
Fig. 4.
5-FU combined with simvastatin significantly inhibited the organoid growth. A Graphical representation of the gene identification and organoid construction of KPC mouse model. B-C The synergy of 5-FU and simvastatin in the KPC organoid model was assessed, and the optimal concentration and synergy score were determined. D Representative images of the KPC organoids’ response to 5-FU and simvastatin (5-FU: 100 nM; simvastatin: 100 nM). E–H Relative growth rate, area, and diameter of KPC organoids’ response to 5-FU and simvastatin (n = 6). Results are presented as mean ± standard deviation. Student’s t test. *P < 0.05, **P < 0.01, ***P < 0.001
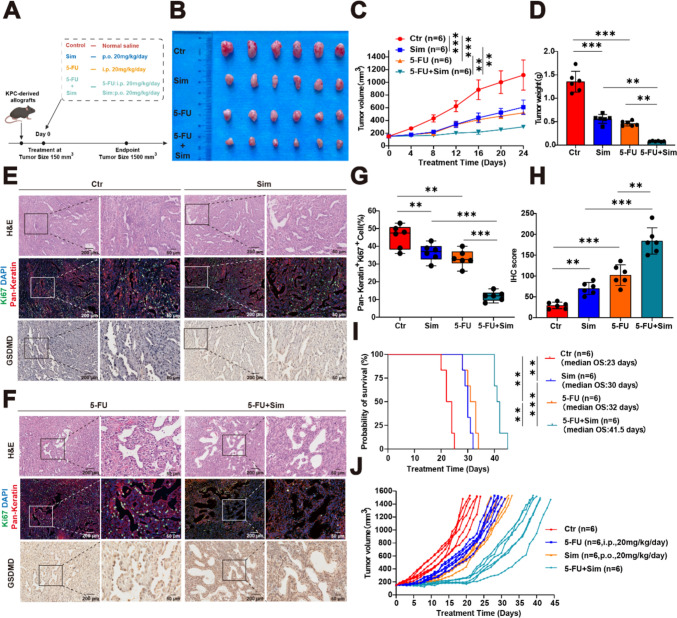
Next, we constructed the allograft models of KPC mice. Upon reaching a tumor volume of 150 mm3, these mice were arbitrarily divided into one of four groups: control, simvastatin group, 5-FU group, and the combination treatment group, with six mice in each group (Fig. 5A). In the first phase, we conducted the progression studies of the KPC-derived allografts, and when the tumor volume of a mouse reached 1500 mm3, we surgically excised all tumor tissues and recorded the tumor volume to plot curves and the tumor weights (Fig. 5B-D). The results illustrated that the combination of 5-FU and simvastatin effectively suppressed tumor growth in KPC-derived allografts. We also performed H&E, IF, and IHC staining on the excised tumor tissues. Statistical analysis confirmed that the combined treatment with 5-FU and simvastatin had a significant therapeutic effect (Fig. 5E-H). In the subsequent phase, we conducted survival studies on the KPC-derived allografts. During the treatments, tumor tissues were surgically removed when the tumor volume of each mouse reached 1500 mm3. Survival curves were plotted based on the observed survival days to evaluate the efficacy of the treatments (Fig. 5I-J). Based on these experimental results, we assessed the combined treatment effect of simvastatin and 5-FU in both KPC organoids and KPC-derived allografts. As hypothesized, the combination therapy demonstrated superior efficacy compared to monotherapy.
Fig. 5.
Targeting the mevalonate pathway combined with 5-FU significantly inhibited tumor progression. A Graphical representation of the treatment for KPC-derived allografts. B Tumor tissue photographs. (C-D) Tumor volume and weight statistics. E–F Representative H&E staining, IF staining (pan-keratin, Ki67), and IHC staining images of GSDMD in tumor tissue. G-H Quantification of pan-keratin, Ki67, and GSDMD staining in tumor tissue from KPC-derived allografts. I-J Kaplan–Meier analysis and tumor growth of KPC-derived allografts treated with 5-FU, simvastatin, or both. Results are presented as mean ± standard deviation (C, D, G, H, I). Student’s t test. **P < 0.01, ***P < 0.001
Overall, our results offer compelling evidence for the efficacy of targeting the mevalonate pathway in combination with 5-FU in treating PC. These insights open new avenues for targeted therapy in PC patients, suggesting a promising direction for future treatment strategies.
Discussion
As a frontline treatment for PC, the FOLFIRINOX regimen demonstrates a longer median survival time compared to the treatment regimen combining gemcitabine with albumin-bound paclitaxel [21].Central to this regimen is 5-FU, which serves as a cornerstone by irreversibly inhibiting thymidylate synthase, thereby exerting its anti-tumor effects. Here, we investigate the mechanisms of chemotherapy resistance using PC clinical samples and PC cell lines such as PANC-1, Panc 02, and MIA PaCa-2, with the goal of improving treatment outcomes for PC.
The mevalonate pathway is the central pathway for cholesterol synthesis, and its aberrant activation is a hallmark of tumorigenesis [22]. HMGCR is an essential enzyme in the mevalonate pathway that is primarily responsible for limiting the rate of catalytic sterol and isoprene biosynthesis, and is a target of statin therapy for hypercholesterolemia. HMGCR can promote tumor proliferation to some extent [23].GGPP, as a subsequent product of the mevalonate pathway, also promotes tumor progression through its abnormal accumulation in PC [24, 25].Simvastatin, in addition to inhibiting cholesterol synthesis, can also target HMGCR and inhibit tumor progression by reducing GGPP and blocking protein prenylation [18, 26, 27]. Our results demonstrate that the abnormal accumulation of HMGCR and GGPP influences chemoresistance to 5-FU to varying degrees. It is worth noting that our study was dedicated solely to the effects of HMGCR and GGPP in the mevalonate pathway on 5-FU. The abnormal activation of the mevalonate pathway in other types of tumors and the effects of other metabolic products on 5-FU warrant further investigation.
Pyroptosis, a proinflammatory form of regulated cell death, is distinct from autophagy and ferroptosis [28]. Pyroptosis is characterized by inducing GSDM (A-E), a member of the aerogel protein superfamily, to promote cell lysis and release pro-inflammatory cytokines IL-18 and IL-1β. GSDMD is cleaved by caspase 1 to activate the inflammasome to induce pyroptosis [29]. Recent studies have found that the inhibition of HMGCR and HMGS1 by simvastatin can induce pyroptosis and then promote the anti-tumor effect [17], and our study also confirms this result. We also observed a connection between the mevalonate pathway and GSDMD through clinical phenomena, and this relationship has not yet been reported in the context of pancreatic cancer. Recent research indicates that GSDMC and GSDME play a facilitating role in the progression of pancreatic cancer [30–33]. Additionally, there are no reports on the roles of GSDMA and GSDMB in pancreatic cancer, although they are known to promote tumor growth in other cancers [34–36].Targeting the mevalonate pathway to induce pyroptosis is also worth further investigation in other types of tumors.
Several limitations are evident in our study. Firstly, recent research has shown that in ovarian cancer, the accumulation of GGPP and mevalonate can inhibit the anti-tumor effects of pyroptosis in tumor cells. However, in our study, we did not demonstrate this effect in PC. Secondly, we validated the combined therapeutic efficacy of 5-FU and simvastatin using PC cell lines, organoid models, and genetically engineered mice. However, we did not further verify this in patient-derived xenograft models. Thirdly, through literature review, we found that there are additional mechanisms by which simvastatin enhances 5-FU [37–39]. These results suggest that targeting the mevalonate pathway to enhance 5-FU by regulating pyroptosis is not the only mechanism.
Conclusion
To sum up, our findings demonstrate a distinct synergy between simvastatin and 5-FU. This combination effectively suppresses the intrinsic activities of the mevalonate pathway in PC, while concurrently promoting pyroptosis in tumor cells to enhance their anticancer effects. Therefore, we expect our research to significantly influence the development of treatment strategies to overcome chemotherapy resistance in PC.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Not applicable.
Author contributions
Conceptualization: Zeyuan Yu and Xiangyan Jiang. Methodology: Zongrui Xing, Yong Ma, Huiguo Qing, Xiangyan Jiang, Yuxia Wu, Shengfu Che, Zhongti Gao, Keshen Wang, Tao Wang, Qichen He, Zhigang Li, Bin Zhao, Wenbo Liu, and Haonan Sun. Visualization: Zongrui Xing, Yong Ma, Huiguo Qing, Xiangyan Jiang. Funding acquisition and supervision: Zeyuan Yu. Writing—original draft preparation: Zongrui Xing. Writing—review and editing: Zeyuan Yu.
Funding
This work was supported by the National Natural Sciences Foundation of China (82360601 and 82160487 to Z.Y.), Cuiying Scientific and Technological Innovation Program of the Lanzhou University Second Hospital (CY2022-MS-A05 to Z.Y.).
Data availability
All data and material are available within the article.
Declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
This research was conducted in accordance with the ethical standards and approved by the Human Research Ethics Committee of Lanzhou University Second Hospital (Permit No. 2023A-173). The animal research was reviewed and approved by the Animal Experiment Ethics Committee of Lanzhou University Second Hospital (Permit No. D2023–M2).
Consent for Publication
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Zongrui Xing, Yong Ma, Xiangyan Jiang, and Huiguo Qing have equally contributed to this work.
References
- 1.Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73(1):17–48. [DOI] [PubMed] [Google Scholar]
- 2.Amrutkar M, Gladhaug IP. Pancreatic cancer chemoresistance to gemcitabine. Cancers (Basel). 2017. 10.3390/cancers9110157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Conroy T, Castan F, Lopez A, Turpin A, Ben Abdelghani M, Wei AC, Mitry E, Biagi JJ, Evesque L, Artru P, et al. Five-Year outcomes of FOLFIRINOX vs gemcitabine as adjuvant therapy for pancreatic cancer: a randomized clinical trial. JAMA Oncol. 2022;8(11):1571–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Longley DB, Harkin DP, Johnston PG. 5-fluorouracil: mechanisms of action and clinical strategies. Nat Rev Cancer. 2003;3(5):330–8. [DOI] [PubMed] [Google Scholar]
- 5.Öman M, Wettergren Y, Odin E, Westermark S, Naredi P, Hemmingsson O, Taflin H. Pharmacokinetics of preoperative intraperitoneal 5-FU in patients with pancreatic ductal adenocarcinoma. Cancer Chemother Pharmacol. 2021;88(4):619–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klein B, Sadikov E, Mishaeli M, Levin I, Figer A. Comparison of 5-FU and leucovorin to gemcitabine in the treatment of pancreatic cancer. Oncol Rep. 2000;7(4):875–7. [DOI] [PubMed] [Google Scholar]
- 7.Sethy C, Kundu CN. 5-Fluorouracil (5-FU) resistance and the new strategy to enhance the sensitivity against cancer: implication of DNA repair inhibition. Biomed Pharmacother. 2021;137:111285. [DOI] [PubMed] [Google Scholar]
- 8.Xiang F, Luo F. Stem cell factor modulates HIF-1α levels and diminishes 5-FU sensitivity in 5-FU resistant pancreatic cells by altering the anabolic glucose metabolism. J Biochem Mol Toxicol. 2023;37(12):e23487. [DOI] [PubMed] [Google Scholar]
- 9.Wang W-B, Yang Y, Zhao Y-P, Zhang T-P, Liao Q, Shu H. Recent studies of 5-fluorouracil resistance in pancreatic cancer. World J Gastroenterol. 2014;20(42):15682–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang H, Xie S, Liang B, Tang Q, Liu H, Wang D, Huang G. Exosomal IDH1 increases the resistance of colorectal cancer cells to 5-Fluorouracil. J Cancer. 2021;12(16):4862–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cao J, Sun X, Zhang X, Chen D. 6PGD upregulation is associated with chemo- and immuno-resistance of renal cell carcinoma via AMPK signaling-dependent NADPH-mediated metabolic reprograming. Am J Med Sci. 2020;360(3):279–86. [DOI] [PubMed] [Google Scholar]
- 12.Cotte AK, Aires V, Fredon M, Limagne E, Derangère V, Thibaudin M, Humblin E, Scagliarini A, de Barros J-PP, Hillon P, et al. Lysophosphatidylcholine acyltransferase 2-mediated lipid droplet production supports colorectal cancer chemoresistance. Nat Commun. 2018;9(1):322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gao Y, Zhao Q, Mu X, Zhu H, Liu B, Yao B, Liu X, Xue W, Wang B, Liu S. SREBP1 promotes 5-FU resistance in colorectal cancer cells by inhibiting the expression of caspase7. Int J Clin Exp Pathol. 2019;12(3):1095–100. [PMC free article] [PubMed] [Google Scholar]
- 14.Huang B, Song B-L, Xu C. Cholesterol metabolism in cancer: mechanisms and therapeutic opportunities. Nat Metab. 2020;2(2):132–41. [DOI] [PubMed] [Google Scholar]
- 15.Waller DD, Park J, Tsantrizos YS. Inhibition of farnesyl pyrophosphate (FPP) and/or geranylgeranyl pyrophosphate (GGPP) biosynthesis and its implication in the treatment of cancers. Crit Rev Biochem Mol Biol. 2019;54(1):41–60. [DOI] [PubMed] [Google Scholar]
- 16.Gbelcová H, Rimpelová S, Knejzlík Z, Šáchová J, Kolář M, Strnad H, Repiská V, D’Acunto WC, Ruml T, Vítek L. Isoprenoids responsible for protein prenylation modulate the biological effects of statins on pancreatic cancer cells. Lipids Health Dis. 2017;16(1):250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou W, Liu H, Yuan Z, Zundell J, Towers M, Lin J, Lombardi S, Nie H, Murphy B, Yang T, et al. Targeting the mevalonate pathway suppresses ARID1A-inactivated cancers by promoting pyroptosis. Cancer Cell. 2023. 10.1016/j.ccell.2023.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiang W, Hu J-W, He X-R, Jin W-L, He X-Y. Statins: a repurposed drug to fight cancer. J Exp Clin Cancer Res. 2021;40(1):241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ariston Gabriel AN, Jiao Q, Yvette U, Yang X, Al-Ameri SA, Du L, Wang Y-S, Wang C. Differences between KC and KPC pancreatic ductal adenocarcinoma mice models, in terms of their modeling biology and their clinical relevance. Pancreatology. 2020;20(1):79–88. [DOI] [PubMed] [Google Scholar]
- 20.Jiang X, Ma Y, Wang T, Zhou H, Wang K, Shi W, Qin L, Guan J, Li L, Long B, et al. Targeting UBE2T potentiates gemcitabine efficacy in pancreatic cancer by regulating pyrimidine metabolism and replication stress. Gastroenterology. 2023;164(7):1232–47. [DOI] [PubMed] [Google Scholar]
- 21.Klein-Brill A, Amar-Farkash S, Lawrence G, Collisson EA, Aran D. Comparison of FOLFIRINOX vs gemcitabine plus nab-paclitaxel as first-line chemotherapy for metastatic pancreatic ductal adenocarcinoma. JAMA Netw Open. 2022;5(6):e2216199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Göbel A, Rauner M, Hofbauer LC, Rachner TD. Cholesterol and beyond - the role of the mevalonate pathway in cancer biology. Biochim Biophys Acta Rev Cancer. 2020;1873(2):188351. [DOI] [PubMed] [Google Scholar]
- 23.Kim Y, Jee W, An E-J, Ko HM, Jung JH, Na Y-C, Jang H-J. Timosaponin A3 inhibits palmitate and stearate through suppression of SREBP-1 in pancreatic cancer. Pharmaceutics. 2022;14(5):945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haney SL, Varney ML, Chhonker YS, Shin S, Mehla K, Crawford AJ, Smith HJ, Smith LM, Murry DJ, Hollingsworth MA, Holstein SA. Inhibition of geranylgeranyl diphosphate synthase is a novel therapeutic strategy for pancreatic ductal adenocarcinoma. Oncogene. 2019;38(26):5308–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haney SL, Varney ML, Chhonker Y, Talmon G, Smith LM, Murry DJ, Holstein SA. In vivo evaluation of combination therapy targeting the isoprenoid biosynthetic pathway. Pharmacol Res. 2021;167:105528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stine JE, Guo H, Sheng X, Han X, Schointuch MN, Gilliam TP, Gehrig PA, Zhou C, Bae-Jump VL. The HMG-CoA reductase inhibitor, simvastatin, exhibits anti-metastatic and anti-tumorigenic effects in ovarian cancer. Oncotarget. 2016;7(1):946–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thurnher M, Nussbaumer O, Gruenbacher G. Novel aspects of mevalonate pathway inhibitors as antitumor agents. Clin Cancer Res. 2012;18(13):3524–31. [DOI] [PubMed] [Google Scholar]
- 28.Lin L, Zhang M-X, Zhang L, Zhang D, Li C, Li Y-L. Autophagy, Pyroptosis, and Ferroptosis: new regulatory mechanisms for atherosclerosis. Front Cell Dev Biol. 2021;9:809955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rao Z, Zhu Y, Yang P, Chen Z, Xia Y, Qiao C, Liu W, Deng H, Li J, Ning P, Wang Z. Pyroptosis in inflammatory diseases and cancer. Theranostics. 2022;12(9):4310–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yan C, Niu Y, Li F, Zhao W, Ma L. System analysis based on the pyroptosis-related genes identifies GSDMC as a novel therapy target for pancreatic adenocarcinoma. J Transl Med. 2022;20(1):455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu R, Li J, Aicher A, Jiang K, Tondi S, Dong S, Zheng Q, Tang S, Chen M, Guo Z, et al. Gasdermin C promotes Stemness and immune evasion in pancreatic cancer via pyroptosis-independent mechanism. Adv Sci (Weinh). 2024. 10.1002/advs.202308990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li S, Yue M, Xu H, Zhang X, Mao T, Quan M, Ma J, Wang Y, Ge W, Wang Y, et al. Chemotherapeutic drugs-induced pyroptosis mediated by gasdermin E promotes the progression and chemoresistance of pancreatic cancer. Cancer Lett. 2023;564:216206. [DOI] [PubMed] [Google Scholar]
- 33.Lv J, Liu Y, Mo S, Zhou Y, Chen F, Cheng F, Li C, Saimi D, Liu M, Zhang H, et al. Gasdermin E mediates resistance of pancreatic adenocarcinoma to enzymatic digestion through a YBX1-mucin pathway. Nat Cell Biol. 2022;24(3):364–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hergueta-Redondo M, Sarrió D, Molina-Crespo Á, Megias D, Mota A, Rojo-Sebastian A, García-Sanz P, Morales S, Abril S, Cano A, et al. Gasdermin-B promotes invasion and metastasis in breast cancer cells. PLoS ONE. 2014;9(3):e90099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.He H, Yi L, Zhang B, Yan B, Xiao M, Ren J, Zi D, Zhu L, Zhong Z, Zhao X, et al. USP24-GSDMB complex promotes bladder cancer proliferation via activation of the STAT3 pathway. Int J Biol Sci. 2021;17(10):2417–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang R, Song Q, Lin X, Du B, Geng D, Gao D. GSDMA at the crossroads between pyroptosis and tumor immune evasion in glioma. Biochem Biophys Res Commun. 2023;686:149181. [DOI] [PubMed] [Google Scholar]
- 37.Lee J, Jung KH, Park YS, Ahn JB, Shin SJ, Im S-A, Oh DY, Shin DB, Kim TW, Lee N, et al. Simvastatin plus irinotecan, 5-fluorouracil, and leucovorin (FOLFIRI) as first-line chemotherapy in metastatic colorectal patients: a multicenter phase II study. Cancer Chemother Pharmacol. 2009;64(4):657–63. [DOI] [PubMed] [Google Scholar]
- 38.Luput L, Sesarman A, Porfire A, Achim M, Muntean D, Casian T, Patras L, Rauca VF, Drotar DM, Stejerean I, et al. Liposomal simvastatin sensitizes C26 murine colon carcinoma to the antitumor effects of liposomal 5-fluorouracil in vivo. Cancer Sci. 2020;111(4):1344–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ahn KS, Sethi G, Aggarwal BB. Reversal of chemoresistance and enhancement of apoptosis by statins through down-regulation of the NF-kappaB pathway. Biochem Pharmacol. 2008;75(4):907–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data and material are available within the article.