Abstract
Age-related hearing loss is a risk factor for mobility problems and falls, possibly due to poor access to spatial sounds or the higher allocation of attention required to listen, thereby reducing cognitive resources to support mobility. Introducing stabilizing spatial sounds or reducing cognitive load through hearing aids could possibly improve balance performance; however, evidence is mixed. Few studies have evaluated the effects of hearing aids and spatial sounds on balance during realistic, multisensory, dual-tasking conditions. This study used virtual reality to simulate a listening-while-balancing task in 22 older adults with normal hearing and 22 hearing aid users, tested with their aids on versus off. Participants performed a competing digits listening task (two, four digits) and a standing postural task, alone and in combination (dual task) under different visual, postural, and acoustical loads. Listening accuracy and postural outcomes (centre of pressure mean velocity, anterior–posterior standard deviation, medial–lateral standard deviation) were collected. With respect to listening accuracy, as expected, normal hearing adults had higher listening accuracy than those with hearing loss (aided better than unaided) and both groups performed better with eyes closed (vs. open) and under lower postural loads (firm vs. compliant). With respect to postural performance, hearing aids did not remarkably improve balance overall, with no effects on dual-task costs to posture. Other factors related to the complexity of the conditions (i.e., listening, visual, postural demands) differently influenced dual-task costs to posture in individuals with and without hearing loss. Overall, these results contribute to our understanding of how age-related hearing loss and hearing aids affect balance-related outcomes under realistic, complex, multisensory, multitasking conditions.
Keywords: Age-related hearing loss, Older adults, Dual task, Listening effort, Standing Balance, Hearing aids
Subject terms: Risk factors, Health care
Introduction
Age-related hearing loss is associated with balance and mobility-related problems such as unstable posture1–6, slower walking speed7, greater risk of falls8–10, and higher vestibular perceptual thresholds11 and functioning12. Individuals with hearing loss are 3 times more likely to fall compared to those with normal hearing and with every 10 dB increase in hearing loss, there is a 1.4 times greater risk of falling9. However, the causal mechanisms underlying the associations between age-related hearing loss and mobility problems are poorly understood.
One hypothesis to explain this association is the cognitive load hypothesis. This hypothesis suggests that poorer hearing (such as declines in suprathreshold auditory processing) can lead to greater listening effort (requiring more cognitive resources to listen) at the expense of concurrently performed and competing mobility-related tasks13. Indeed, it is well-established that cognition is involved in the regulation of posture and gait, particularly in older adults14. In order to test the cognitive load hypothesis, our group and others have implemented multi-tasking paradigms, requiring participants to listen and balance/walk at the same time, as a way of evaluating dual-task costs (the cost of performing two tasks simultaneously compared to one) to posture and gait stability14–18. While many studies have demonstrated a postural prioritization in older adults14,16–18, far fewer studies have investigated dual-task costs to posture in older adults with hearing loss specifically, for whom task prioritization may be more difficult to manage16,18.
Another hypothesis is the spatial sounds hypothesis, which suggests that hearing loss restricts the use of acoustical cues from the environment that can be used to perceive self-orientation in space, thereby compromising balance. Specifically, sound level differences between each ear (interaural level differences) and sound arrival time differences between each ear (interaural time differences;19,20) provide important information about direction and localization, which may be compromised by age-related hearing loss. However, the literature is mixed on whether spatial sound cues can indeed support or impede balance and very little is understood about how these effects change with older age. Most previous studies have focused on younger adults or have sampled across a broad range of ages, with some demonstrating that stationary sound cues in the environment may serve as auditory anchors to support balance and orientation by providing a “spatial map”21–27. Other studies have not observed similar posture-stabilizing effects of sound cues11,28,29. Some of these inconsistencies across studies may be related to the nature of the tasks used and/or the complexity of the experimental environment. In particular, very little is known about whether spatial sound cues are used to support balance in the context of more realistic environmental conditions (visual and acoustically complex) or under greater task demands (e.g., multi-tasking), particularly in older adults for whom these spatial acoustical signals may be less reliable due to for example, high-frequency hearing loss30.
Ecologically valid testing environments: virtual reality
While most everyday conditions occur in complex, multisensory environments, the majority of previous experimental research investigating the effects of hearing on balance have been conducted in highly controlled, isolated laboratory settings or sound booths, which rely on the use of less complex, low-level stimuli (e.g., white-noise, pure tones). It is possible, that the most pronounced effects of dual-task costs to balance associated with hearing loss may be observed within complex, realistic, environments and/or when performing more ecologically valid tasks31–33. One method of increasing the ecological validity of testing paradigms, without compromising internal validity, is to use immersive, multisensory virtual reality environments. Such testing environments may allow for more reliable and accurate estimates of sensory-cognitive-motor interactions supporting listening-while-balancing in older adults with hearing loss during more natural, everyday situations. Further, one factor that realistic virtual reality systems can also address is the role that complex visual information might play in older adults’ ability to manage competing demands during realistic listening-while-balancing tasks. Specifically, while it is known that vision provides strong cues to support balance (e.g., orientation cues, obstacle avoidance, safe navigation34), listening performance may actually be compromised by the introduction of irrelevant visual content, as it may compete for perceptual or attentional resources35.
The dual-task effects of cognitive tasks on postural control to balance36 and walking37 have previously been characterized as an inverted U-shaped function26,36–40. Specifically, dual-task effects on posture depends on whether the cognitive task introduces very low cognitive demands (postural task improvements) or high cognitive demands (lack of postural task improvements37). However, the relative extent to which cognitive demands are considered low vs. high differs across individuals based on their unique abilities. For example, how these patterns of dual-task effects are observed when performing concurrent listening tasks (e.g., tasks of central auditory processing) and postural tasks is not well known, particularly for older adults with typical age-related changes to hearing, as well as older adults with age-related hearing loss. Age-related changes can include suprathreshold changes to central auditory processing within clinically normal ranges, as well as sensorineural hearing loss meeting the threshold for clinical impairment. Comparing older adults with and without age-related hearing loss would allow for the evaluation of how typical age-related changes to hearing (e.g., suprathreshold changes to central auditory processing) influence postural-related outcomes when having to manage listening while standing concurrently compared to clinically significant hearing loss (i.e., sensorineural hearing loss). Further, comparing performance of older adult hearing aid users with and without the use of their hearing aids could provide added insights into whether amplification reduces dual-task costs to balancing while listening.
The effects of hearing aids on balance and falls-risk
Hearing aids are largely designed to improve speech communication and have also been shown to improve performance across several different domains of cognitive abilities41. However, there is far less consensus on whether hearing aids support balance and/or reduce falls-risk21,33,42–56. It could be argued that binaural amplification of spatial sound cues could support localization and orientation behaviours. However, sound cues used for localization are not well-preserved by hearing aids, thereby potentially limiting their benefits for spatial orientation supporting balance57. In order to evaluate this, it would be important to compare the effects of hearing aids on balance when auditory landmarks are present versus absent.
It is also possible that hearing aids may support balance through reductions in listening effort (i.e., reduced cognitive load). Specifically, reductions in listening effort may minimize the competition for cognitive resources between listening-related tasks and concurrently performed mobility-related tasks. In order to evaluate this, it would be important to assess the effects of hearing aids on postural performance when performing listening tasks of varying difficulties (i.e., lower demands vs. higher demands) and compare these dual-task postural effects to postural conditions involving no competing listening task (e.g., a single-task with standing only).
Ultimately, the existing literature investigating the effects of hearing aids on balance and falls-risk is highly inconsistent33,42–45. While some studies have demonstrated improvements to balance and/or a link to falls-risk with hearing aid use21,46–50, other studies have not observed beneficial effects or associations51–56. These inconsistencies may be related to the lack of experimental control over, or consideration of, factors such as the presence of spatial sound cues in the testing environment, competing visual distractions, postural loads, and/or the introduction of concurrent listening tasks varying in difficulty.
Current research
The current study used an immersive, multisensory, virtual reality environment to address our primary objectives of examining the effects of hearing aids (aided vs. unaided) and hearing status (normal hearing older adults vs. older adults with hearing loss) on listening accuracy and dual-task costs to balance during realistic multisensory conditions. It was predicted that older adults with hearing loss would demonstrate greater dual-task costs to balance than older adults with normal hearing, particularly when unaided compared to when aided. Secondary objectives included the effects of listening, visual, postural, and acoustical loads on dual-task costs to balance and/or interactions of these factors with hearing loss/aid use. It was predicted that greater overall, cumulative demands would result in greater dual-task costs to balance, up to a threshold where the two tasks would no longer be manageable (resulting in low/no dual-task costs to posture, which will be prioritized over listening). This demand threshold was predicted to be lower in older adults with hearing loss compared to older adults with normal hearing.
Methods
Participants
Twenty-two older adults with normal hearing (Mage = 73.55 years, SD = 3.92; 14F, 8 M) and 22 older adults with age-related hearing loss who use bilateral hearing aids (Mage = 75.14 years, SD = 7.39; 12F, 10 M) participated in this study. Age was not significantly different between the two groups determined by a two-tailed t-test (p = 0.38). Participants were recruited through posters and flyers distributed throughout Toronto and online social media platforms. Participants were required to have corrected-to-normal or near-normal vision, speak English fluently and started learning English before age 5 (except for n = 1 in the hearing loss group who learned English between 25–30 years of age). The hearing loss group had worn their current hearing aids (n = 19 behind-the-ear; n = 1 receiver-in-canal, n = 1 in-the-canal; n = 1 in-the-ear) for a minimum of 1 month (M = 2.33 years, range = 2 months-5 years). The average number of years of having used any hearing aids was 8.45 (SD = 12.22, range = 6 months–47 years). On average, participants used their hearing aids for 10.86 h per day (SD = 4.99, range = 0–18) and their hearing had been assessed by an audiologist within the previous 5.95 months on average.
Participants were excluded if they had a prior history of neurological diseases, psychiatric illnesses (unless controlled), mild cognitive impairment, dementia, a recent stroke, claustrophobia, motion sickness, seizure disorder or epilepsy, arthritis or severe injury of the leg or feet, or any mobility-related impairments (e.g., required use of mobility aids, diagnosed vestibular disorder, or major musculoskeletal problems) that would prevent them from participating in their everyday activities. During the baseline session, participants were required to have normal cognition, which was screened using the Montreal Cognitive Assessment (MoCA cutoff ≥ 2358), as well as corrected-to-normal or near-normal visual acuity as assessed using the Early Treatment Diabetic Retinopathy Study59 with − 0.2–0.5 logMAR units in the better-eye60. In order to stratify participants in the correct groups (hearing loss versus normal hearing), we assessed their hearing using SHOEBOX™ Audiometry61 and grouped participants with a pure tone average (PTA) cutoff of ≤ 25 dB HL in the normal hearing group62 and ≥ 26 dB HL in the hearing loss group with no asymmetry (no more than 15 dB right/left ear difference at more than two adjacent frequencies). Note that while the new World Health Organization standards recommend a cutoff of < 20 dB hearing loss63; we used the previously recommended cutoff of ≤ 25 dB hearing loss to be consistent and comparable with previous research in this area. Participants provided written consent at the beginning of each session and were compensated $20/hour for their time. This study was approved by the University Health Network’s (CAPCR ID: 11–033) and University of Toronto’s (RAISE ID: 39,734) Research Ethics Boards. All methods were performed in accordance with the relevant guidelines and regulations.
Baseline session
Additional assessments beyond those used to determine eligibility were administered to better characterize the participant sample with respect to sensory, cognitive, motor abilities that could influence the interpretation of the results (see Table 1 for a summary) and to conduct correlations between these baseline and experimental outcome measures (see Results section below for summary of results, Supplementary Baseline Measures section and Supplementary Figs. S1–3 for heatmaps online). A general Health History Questionnaire (HHQ) was also administered online using Qualtrics survey software (Qualtrics, Provo, Utah).
Table 1.
Demographics and baseline measures of participants.
| Sample Size (n) | Hearing Loss Group (n = 22) | Sample Size (n) | Normal hearing group (n = 22) | ||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | p value | |||
| Demographics | |||||||
| Age (years) | n = 22 | 75.14 | 7.39 | n = 22 | 73.55 | 3.92 | 0.35 |
| Education (years) | n = 22 | 17.32 | 3.48 | n = 22 | 16.91 | 2.37 | 0.61 |
| Hearing | |||||||
| CDTT SRT1 | n = 22 | − 3.82 | 4.22 | n = 19 | − 8.18 | 6.74 | 0.02* |
| Better Ear PTA (dB HL)2 | n = 22 | 48.45 | 11.45 | n = 22 | 16.91 | 4.59 | < 0.001* |
| HHIE3 | n = 21 | 21.52 | 8.67 | n = 22 | 3.18 | 4.52 | < 0.001* |
| Vision | |||||||
| Better Eye ETDRS4 | n = 22 | 0.08 | 0.14 | n = 22 | 0.04 | 0.12 | 0.32 |
| Pelli Robson5 | n = 22 | 1.58 | 0.14 | n = 21 | 1.67 | 0.16 | 0.05 |
| Horizontal DVA6 | n = 18 | 0.09 | 0.15 | n = 19 | 0.17 | 0.19 | 0.18 |
| Cognition | |||||||
| MoCA7 | n = 22 | 27.05 | 2.06 | n = 22 | 26.5 | 1.68 | 0.49 |
| RAVLT Total8 | n = 21 | 43.57 | 9.82 | n = 22 | 42.72 | 8.79 | 0.76 |
| RAVLT Delayed Recall9 | n = 20 | 9.10 | 3.63 | n = 22 | 8.55 | 2.77 | 0.73 |
| WAIS-III Digit Span10 | n = 21 | 17.19 | 3.96 | n = 22 | 18.63 | 2.92 | 0.34 |
| Digit Symbol Substitution11 | n = 21 | 55.95 | 20.92 | n = 22 | 60.95 | 10.97 | 0.74 |
| Trail Making B-A (sec) | n = 21 | 53.40 | 42.33 | n = 21 | 54.65 | 40.85 | 0.52 |
| Stroop Inhibition12 | n = 22 | 12.18 | 2.74 | n = 21 | 11.90 | 2.05 | 0.42 |
| Stroop Inhibition/Switching12 | n = 21 | 11.48 | 1.94 | n = 21 | 11.57 | 2.94 | 0.68 |
| Mobility | |||||||
| TUG (seconds)13 | n = 21 | 10.90 | 2.04 | n = 21 | 10.41 | 1.56 | 0.70 |
Summary statistics of sample size, mean and standard deviation were calculated for each group and baseline measure. In order to determine whether differences were observed between the normal hearing and hearing loss groups, t-tests were conducted with significance set at *p < 0.05.
1 Canadian Digit Triplet Test (CDTT) speech reception threshold (SRT).
2 Better ear pure-tone average (BPTA) of thresholds at 0.5, 1, 2, and 4 kHz.
3 The Hearing Handicap Inventory for the Elderly (HHIE) score out of 40.
4 Better eye Early Treatment Diabetic Retinopathy Study (ETDRS) score for corrected vision in logMAR units.
5 Pelli Robson Contrast Sensitivity Test score using both eyes.
6 Dynamic Visual Acuity Test (DVA) score for corrected vision in logMAR units.
7 Montreal Cognitive Assessment (MoCA) total score out of 30 adjusted for years of education.
8 Rey Auditory Verbal Learning Test (RAVLT) total score out of 75.
9 Rey Auditory Verbal Learning Test (RAVLT) delayed recall score out of 15.
10 Wechsler Adult Intelligence Scale, Third Edition (WAIS-III) Digit Span Test score out of 30.
11 Digit Symbol Substitution Test score out of 133.
12 Stroop Test scaled score equivalent out of 19.
13 Averaged time of two Timed Up and Go (TUG) trials.
Experimental session
Virtual reality testing environment
The Experimental session was conducted in StreetLab, a fully immersive, projection-based, virtual reality simulator housed in the Challenging Environment Assessment Laboratory at the KITE-Toronto Rehabilitation Institute – University Health Network (see Fig. 1). This simulator has a high resolution, 240° horizontal by + 15° to − 90° vertical field-of-view projection system displayed on a curved screen. The virtual environment consisted of a 6-lane intersection in Toronto created using OpenScene Graph (www.openscenegraph.org). StreetLab was outfitted with an AMTI BP12001200 strain gauge force platform to collect posturography outcome measures (i.e., average center of pressure mean velocity and variability measures in the anterior–posterior and medial–lateral directions). A loose-fitting harness was used to ensure participants could not fall to the ground surface but did not provide body-weight support during the study while standing. A surround sound system with seven speakers (Meyersound MP-4XP; Meyersound Laboratories, Inc., Berkeley, CA) was used to present auditory stimuli. The speakers are at a distance of 2.14 m from the participant and are approximately aligned with the participant’s head. A subwoofer (Meyersound MP-10XP) is positioned in the floor under the centre speaker. Speakers are positioned at 0° azimuth across a horizontal plane at ± 28° (right front and left front), ± 90° (right side and left side), and ± 127° (right rear and left rear). A sound level metre was used to determine the lab’s ambient noise at 51 decibels sound pressure level during quiet standing conditions (for full acoustical information of StreetLab refer to64).
Fig. 1.
(a) Exterior view of StreetLab. (b) Interior view of StreetLab showing a participant standing on the force plate, facing the projection screen displaying the simulated city intersection. Informed consent was obtained from the participant in panel B to be published in an online open access publication. Photos by Tim Fraser.
Procedure
Potential participants were pre-screened over the phone to ensure they met all self-reported inclusion and exclusion criteria. All pre-screened eligible participants first completed the in-person baseline session. Participants who obtained scores that met all eligibility criteria for audiometry, MoCA, and ETDRS were invited to complete the experimental session. When participants first arrived at the lab to complete the experimental session, an initial pre-screening “audibility task” was administered in StreetLab to ensure participants could hear the experimental stimuli (i.e., the digits presented, see Listening Task section below). This audibility task consisted of six trials at 100% volume, where two numbers were presented one after the other, one from the left speaker and one from the right speaker and participants were required to report back the digits as accurately as they could. Subsequently, all participants completed the full experimental session, which was comprised of a single-task condition (i.e., standing only) and a dual-task condition (e.g., listening-while-standing).
Standing task
For the standing task, participants were instructed to stand on the force plate, facing the screen with feet approximately shoulder-width apart with their hands down by their sides. Participants stood on either a firm surface (directly on the force plate; i.e., low postural load) for half of the trials, or on a compliant surface (a Temper® foam, i.e., high postural load) for the other half of the trials. Ground reaction forces at the feet were captured using an AMTI MSA-6 MiniAmp strain gauge amplifier. The outcome measures for the posturography data included average center of pressure mean velocity (mm/s) and variability (standard deviation) measures in the anterior–posterior (mm) and medial–lateral (mm) directions. Data for each balance trial was, on average, 45.97 s (SD = 5.12 s).
Listening task
For the listening task, participants completed the dichotic digits task65, which assesses central auditory processing and involves listening to and repeating back a series of digits. Specifically, during the low listening load condition, two digits were presented simultaneously, while during the high listening load condition, four digits were presented in two sequential pairs (two simultaneous digits followed by two more simultaneous digits). Participants were asked to repeat back all digits in any order or to take their best guess if unsure. Participants were told that a closed set of digits from 1–9, excluding 7 (the only two-syllable digit in this range) would be randomly presented. In total, there were three listening task conditions: 1) low listening load (2-digits); 2) high listening load (4-digits) and 3) no listening (standing only, no-digits). Participants had a chance to practice the low and high listening loads (2 numbers simultaneously × 6 trials and 4 numbers in two sequential pairs × 6 trials, respectively) prior to the main experimental trials. All listening conditions were counterbalanced for order across participants.
Since the physical act of talking can affect postural measurement, we ensured that there were an equal number of utterances during the response phase of each condition66. Specifically, in the no listening condition, participants were still asked to produce the word “beep” four times; during the low listening load condition (2-digits) participants were asked to repeat the two digits followed by the word “beep” two times. This ensured that for all three listening conditions, the amount of talking was equal (4 utterances). Participants completed six listening trials per condition.
The outcome measure for listening performance is digit recognition accuracy. Accuracy scores were calculated based on the percentage of the 6 trials in which participants reported both of the two digits correctly for low load trials and all four digits correctly for high load trials. Partially correct digit sets such as 1 out of 2 digits correct in the low load trials or 1, 2, or 3 digits correct in the high load trials were marked as “incorrect”. Percentages across the 6 trials were calculated for each condition to determine a digit recognition accuracy score, with higher scores reflecting better listening performance.
Dual-task condition: listening while standing task
During dual-task conditions, participants completed the standing and listening tasks simultaneously across all postural, visual, acoustical and listening load conditions (see below for details). Data for each balance trial was comprised of the duration it took the participant to complete all 6 listening trials per condition (M = 59.46 s, SD = 7.29 s).
Postural, Visual, Acoustical Manipulations and the Effects of Hearing Aids.
Postural load
Participants stood on a firm surface or on a compliant surface (50% each). It was expected that the compliant surface would be more challenging compared to the firm surface.
Visual load
Participants were asked to open their eyes or close their eyes (blindfolded) on different trials (50% each). It was expected that visual cues would support postural stability but could be detrimental to listening task performance.
Acoustical load
Trials included those with no background noise, white noise, and spatial noise. In the spatial noise condition, there were three noises that commonly occur at busy city intersections played simultaneously: a truck backing up (54 dB-A), pedestrian alert bells (59–64 dB-A) and running fountain water (52 dB-A). Participants were asked to identify each spatial noise (briefly describe what they heard) and localize where the spatial sound was coming from (left, right, or centre) to measure their ability to identify and localize each spatial noise. If localizable, it was expected that spatial sounds could support postural stability by providing auditory anchors. While there is some evidence that even white noise may support balance (e.g., through stochastic resonance67), it is also possible that in the context of the current study, given that speech-based listening tasks were performed at the same time, background noise may interfere with listening68, thereby increasing cognitive load to the detriment of posture. While all normal hearing participants were able to identify all sounds, 36% (n = 16/44) of the participants in the hearing loss group were not able to identify all sounds. In terms of localization, 30% (n = 13/44) of participants in the normal hearing group and 66% (n = 29/44) of participants in the hearing loss group could not accurately localize all sounds.
Hearing aids
The effects of hearing aids on postural stability and listening performance were evaluated by having the hearing loss group complete all conditions with their hearing aids on (aided) and off (unaided); counterbalanced across two days. The normal hearing group performed the same trials twice, once on each day. Day 1 and day 2 were separated by 8.77 days on average. Breaks were offered throughout the experiment.
Anxiety, fatigue, and motivation ratings
Previous research has shown that emotions (e.g., anxiety) can influence postural stability [see69 for a review]. Hearing loss can contribute to anxiety and fatigue, and the degree of motivation can also influence performance13. In order to capture information about their general emotional status, each participant answered the following three questions at the end of the experimental session: (1) Please rate your level of anxiety on a scale from 1–10, with “1” being “not anxious” and 10 being “very anxious”, (2) Please rate your level of fatigue on a scale from 1–10, with “1” being “no fatigue” and 10 being “very fatigued”, (3) Please rate your level of motivation on a scale from 1–10, with “1” being “no motivation” and 10 being “very motivated.”
Experimental design and statistical analyses
As mentioned above, the primary outcomes included the effects of hearing aids (aided vs. unaided) and hearing status (normal hearing vs. hearing loss) on dual-task costs to balance while listening. Secondary outcomes included the effects of listening, visual, postural, and acoustical loads on dual-task costs to balance and/or interactions of these factors with hearing loss/aid use. Specifically, the overall experimental design included the following primary factors: 3 participant groupings (normal hearing, hearing loss aided, hearing loss unaided) and 3 listening loads (no listening load, low listening load, high listening load). Secondary factors included 2 visual loads (eyes open, eyes closed) × 2 postural loads (firm surface, compliant surface) × 3 acoustical loads (none, white, spatial background noise).
All analyses were completed using R-studio (Version 1.4.1717). ANOVAs were used for all analyses and normality was assessed using a Shapiro-Wilk test. Homogeneity of variance and normality of residuals were visually checked and a Levene’s test was used to compare variances across groups objectively. All assumptions were largely met but an ANOVA was still deemed appropriate due to its robustness from minor deviations from normality and the equal sample sizes across groups (22 normal hearing; 22 hearing loss70). Post-hoc tests were conducted with a Tukey’s HSD correction. For the ANOVA analyses, outliers were not removed, and a few extreme values were included in analyses. This is because older adults are a heterogenous sample, and these extreme values were still deemed reflective of the population. However, for all graphs all data points below or above 3.5 standard deviations from the mean were winsorized to ensure that the scale of the y-axis was not so large as to compress the visualization of significant differences between conditions. For the ANOVAs, if participants could not complete the most challenging postural task (compliant surface) because it was too difficult, their postural value was replaced by the maximum value from the sample. If they could not complete a trial for any other reason (e.g., spatial sounds were too loud to tolerate for their hearing aids, n = 1 hearing loss) their values were replaced with the mean for that group and condition. For the postural data, MATLAB was used to remove the first five seconds of each trial and the remaining data points were processed using a Butterworth filter with a sampling frequency of 1000 Hz. Anterior–posterior standard deviation (mm), medial–lateral standard deviation (mm) and centre of pressure mean velocity (mm/s) were extracted for the duration it took to complete all 6 listening trials. Since there were no significant effects of background noise (acoustical load) for any outcome measures, the trials were pooled across each acoustical load condition.
To address our primary objective of examining the effects of hearing aids (aided vs. unaided) on dual-task costs to balance, we first examined the hearing loss group alone (aided vs. unaided). To address our other primary objective of examining differences between older adults with normal hearing (typical age-related changes) to older adults with age-related hearing loss in dual-task costs to balance, we ran another set of analyses, first comparing normal hearing participants to hearing loss participants when aided, and second, comparing normal hearing participants to hearing loss participants when unaided. For each set of comparisons, we first report the results of the listening task performance (% correct accuracy) as a manipulation check to confirm that manipulations to listening difficulty/load were observed as poorer listening task performance as was intended by the design.
With respect to the postural data, we compared dual-task postural outcomes (listening-while-standing) to single-task postural outcomes (no listening, just standing) to determine if there was a cost to balance when completing two tasks at the same time (dual-task costs). Dual-task costs were calculated by subtracting the single-task condition from the dual-task condition for each postural outcome measure (dual-task outcomes – single-task outcomes for each participant, averaged across participants in each group). Note that dual-task costs were not conducted for listening data, as we did not include a single-task listening outcome (a condition where participants were just listening and not standing). Since the postural stance used in this study was a shoulder-width stance, it was expected that postural outcomes would be more evident in the anterior–posterior direction than the medial–lateral direction, given the relatively wide base of support along the medial–lateral axis71–74. Therefore, we have only reported centre of pressure anterior–posterior standard deviation (spatial measure) and mean velocity (temporal measure) in the main text, while medial–lateral standard deviation results are reported in Supplementary Figs. 4–5.
For both the listening task performance and dual-task postural performance measures, in order to address our primary objectives related to hearing aids and listening load we conducted a repeated measures ANOVA (in the case of aided vs. unaided) and for hearing status and listening load we conducted a mixed factorial ANOVA (in the case of normal hearing vs. aided and normal hearing vs. unaided) with the factors of group and listening load. In order to address our secondary objectives of exploring the effects of visual and postural manipulations we then conducted secondary analyses by integrating the additional factors of visual load (eyes open vs. closed), postural load (firm vs. compliant surface) to the ANOVAs described above. Correlations were conducted to see if there were any associations between baseline measures and experimental outcome measures (see Results section below for summary of results, Supplementary Baseline section and Figs. S1–3 for assessment descriptions and heatmaps online).
Results
Aided vs. unaided
Listening task performance
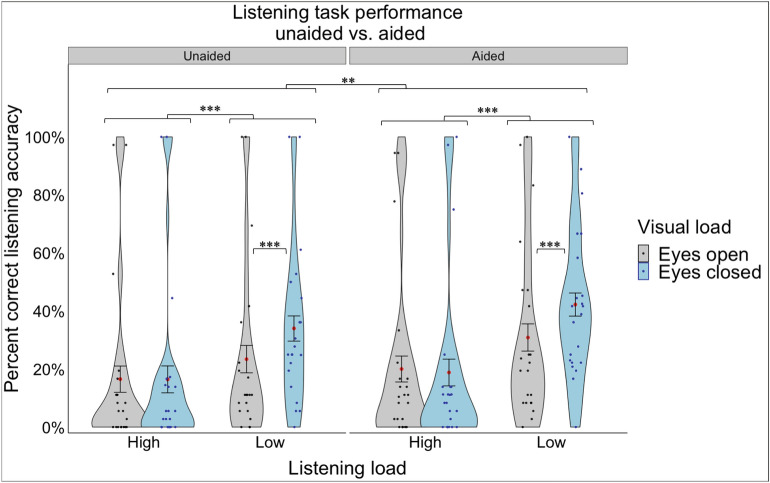
A 2 aid use (unaided vs. aided) × 2 listening load (high vs. low) repeated-measures ANOVA was conducted on listening accuracy (percent correct) in participants with hearing loss. A significant main effect of aid use was observed (F(1,20) = 6.04, p = 0.023, ηp2 = 0.232, Fig. 2), indicating that listening accuracy was higher when aided than when unaided. There was also a significant main effect of listening load (F(1,20) = 52.61, p < 0.001, ηp2 = 0.725, Fig. 2) indicating that listening accuracy was higher in the low load compared to the high load listening condition.
Fig. 2.
Percent correct listening accuracy for the hearing loss group (unaided vs. aided) under different listening loads (high, low) and visual loads (eyes closed, eyes open). The violin plot represents the frequency of the data at a particular value along the y-axis with the standard error. The red dot represents the mean. The individual points represent each participant’s average for each listening and visual load combination. Statistical significance was determined using an ANOVA with a significance level of p < 0.05*.
We repeated the same repeated-measures ANOVA, with the addition of visual (eyes open/closed) and postural (compliant/firm) loads as within-subject factors. There was a significant main effect of vision (F(1,20) = 8.83, p = 0.008, ηp2 = 0.306) demonstrating that eyes closed resulted in higher listening performance than eyes open. These effects were qualified by two significant interaction effects. First, a significant listening load x vision interaction (F(1,20) = 18.55, p < 0.001, ηp2 = 0.481) demonstrated that the beneficial effects of closing one’s eyes were only observed during low listening load conditions and not high listening load conditions (where listening accuracy was worse overall; (t(20) = 4.220, p = 0.0004, Fig. 2). Second, there was a significant listening load x postural load interaction (F(1,20) = 5.34, p = 0.032, ηp2 = 0.211) suggesting that listening accuracy was higher when standing on a compliant surface compared to a firm surface, but only during high listening loads (not low listening loads; (t(20) = 2.774, p = 0.0117)). There were no other significant main or interaction effects.
Having now established that the listening manipulations were consistent with expectations we next report the primary outcome of interest related to dual-task costs to balance.
Balance task performance
Centre of pressure anterior–posterior standard deviation
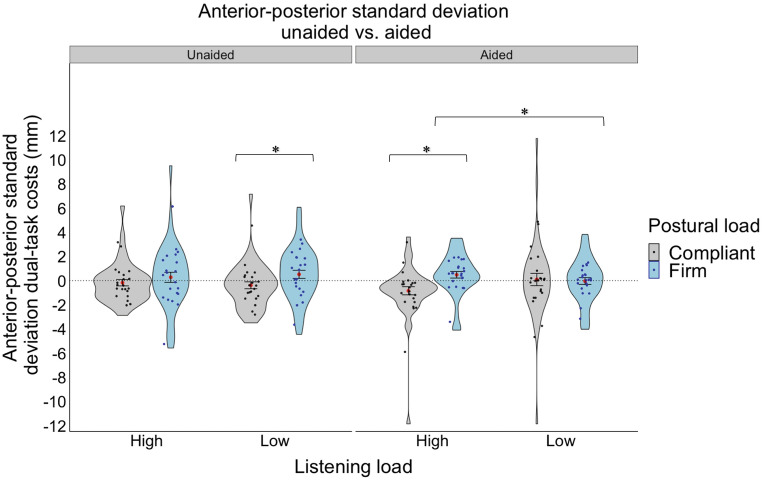
A 2 aid use (unaided vs. aided) × 2 listening load (high, low) repeated-measures ANOVA was conducted on dual-task costs to anterior–posterior standard deviation in participants with hearing loss. There were no significant main or interaction effects. We repeated the same repeated-measures ANOVA with the addition of visual (eyes open/closed) and postural (compliant/firm) loads as within-subject factors. There were no significant main effects; however, there was a significant three-way interaction effect between aid use x listening load x postural load (F(1,20) = 4.81, p = 0.040, ηp2 = 0.194). Specifically, it was observed that the effects of listening load and the effects of postural load were mostly observed for the aided condition. For example, high listening loads resulted in higher dual-task costs to posture (higher variability) than low listening loads when standing on a firm surface (t(20) = -2.724, p = 0.0131, see Fig. 3), but only when aided. Interestingly, in the high listening load condition only (not low load), compliant standing resulted in lower dual-task costs to posture (less variability) than firm standing (t(20) = − 2.295, p = 0.0327, see Fig. 3), only when aided. It is possible that this most difficult condition of compliant standing while performing high load listening, resulted in a “rigidity” or “stiffening” strategy as a way of protecting posture under high degrees of listening and postural load. While we cannot assess rigidity directly in this study given that no measures of relative joint/body position or muscle activation were included, reduced variability of the centre of pressure at the feet may be associated with a use of this strategy75. Further, in the low listening load, compliant standing resulted in lower dual-task costs to posture (less variability) than firm standing (t(20) = -2.140, p = 0.0448, see Fig. 3) when unaided. There were no other significant main or interaction effects.
Fig. 3.
Dual-task costs for anterior–posterior standard deviation for the hearing loss group (unaided vs. aided) under different listening loads (high, low) and postural loads (compliant, firm). The violin plot represents the frequency of the data at a particular value along the y-axis with the standard error. The red dot represents the mean. The individual points represent each participant’s average for each listening and postural load combination. Statistical significance was determined using an ANOVA with a significance level of p < 0.05*.
Centre of pressure mean velocity
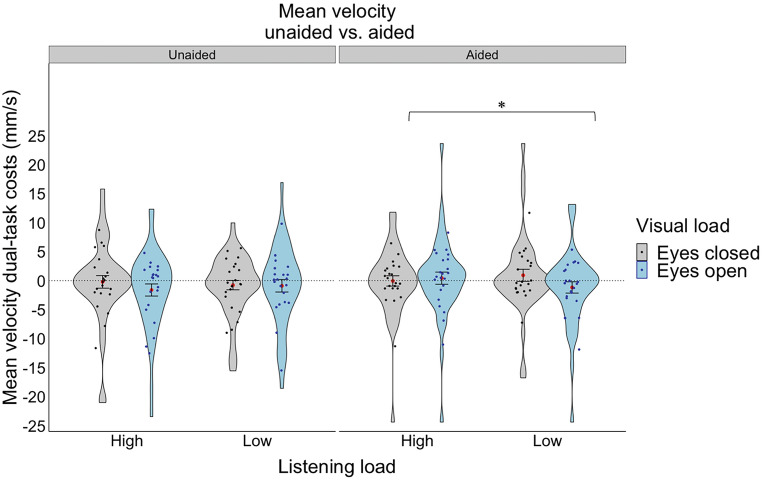
A 2 aid use (aided vs. unaided) × 2 listening load (low vs. high) within-subject ANOVA was conducted on dual-task costs to mean velocity, demonstrating no significant main or interactions effects. We repeated the same repeated-measures ANOVA with the addition of visual (eyes open/closed) and postural (compliant/firm) loads as within-subject factors. Again, no significant main effects were observed; however, there was a significant two-way interaction between vision x posture (F(1,20) = 7.69, p = 0.012, ηp2 = 0.278) and a significant three-way interaction between aid use x listening load x vision (F(1,20) = 5.03, p = 0.036, ηp2 = 0.201). Specifically, the vision x posture interaction demonstrated that having one’s eyes open resulted in lower dual-task costs to mean velocity (higher stability) than having one’s eyes closed, but only for the compliant standing condition and not the firm standing condition (t(20) = 2.211, p = 0.0388). The significant aid use x listening load x vision interaction demonstrated that only in the aided condition and only when their eyes were open was there an effect of listening load, whereby higher listening loads resulted in higher dual-task costs to mean velocity (less stability) than lower listening loads (t(20) = -2.673, p = 0.0146, see Fig. 4). There were no other significant main or interaction effects.
Fig. 4.
Dual-task costs for mean velocity for the hearing loss group (unaided vs. aided) under different listening loads (high, low) and visual loads (eyes closed, eyes open). The violin plot represents the frequency of the data at a particular value along the y-axis with the standard error. The red dot represents the mean. The individual points represent each participant’s average for each listening and visual load combination. Statistical significance was determined using an ANOVA with a significance level of p < 0.05*.
Normal hearing vs. hearing loss aided
Listening task performance
A 2 group (hearing loss aided, normal hearing) × 2 listening load (high, low) mixed factorial ANOVA was conducted on percent correct scores, where group was a between-subject factor and listening load was a within-subject factor. There was a significant main effect of group (F(1,42) = 38.88, p < 0.001, ηp2 = 0.481) with the normal hearing group demonstrating higher listening accuracy than the hearing loss group. There was also a significant main effect of listening load (F(1,42) = 78.19, p < 0.001, ηp2 = 0.651) demonstrating that listening accuracy was higher in the low load compared to the high load listening condition.
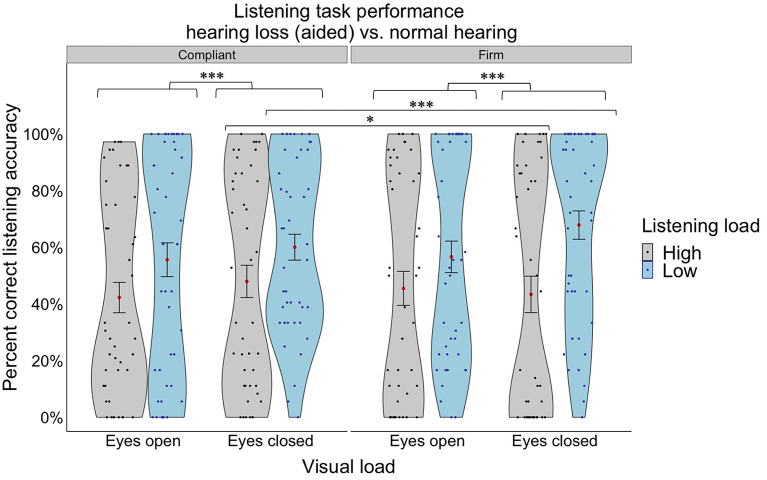
We then repeated the same mixed factorial ANOVA with the addition of visual (eyes open/closed) and postural (compliant/firm) loads as additional within-subject factors. We found a significant main effect of vision (F(1,42) = 12.67, p < 0.001, ηp2 = 0.232, Fig. 5), with eyes closed resulting in higher listening performance than eyes open. However, these significant main effects were qualified by a significant two-way interaction effect (group x posture), and two, significant three-way interaction effects (group x listening load x vision and listening load x vision x posture). Specifically, the significant group x posture interaction (F(1,42) = 8.90, p = 0.05, ηp2 = 0.175) demonstrated that the effect of posture was only observed in the normal hearing group (who had much higher accuracy overall), whereby higher accuracy was observed when standing on a firm surface compared to a compliant surface (t(42) = -3.349, p = 0.0017). The hearing loss group had much worse accuracy overall, demonstrating no worsening of accuracy when standing on a compliant surface compared to a firm surface (a possible “floor” effect in performance). The significant group x listening load x vision interaction (F(1,42) = 9.25, p = 0.004, ηp2 = 0.181) demonstrated that the beneficial effects of closing one’s eyes on listening accuracy was only observed during high listening load conditions for the normal hearing group (t(42) = 2.261, p = 0.0290) and only during the low listening load conditions in the hearing loss group (t(42) = 4.166, p = 0.0002). This may be because for the normal hearing group during low load listening, they were performing at, or near, ceiling, so there were no benefits to closing their eyes; whereas for the hearing loss group during high listening loads, they were at floor, so there were also no added benefits from closing their eyes. It may be that during these moderately difficult conditions (which may be scaled uniquely for each group) closing one’s eyes benefits listening accuracy. Finally, the significant listening load x posture x vision interaction (F(1,42) = 12.70, p < 0.001, ηp2 = 0.232) demonstrated that compliant standing resulted in higher listening accuracy than firm standing under high listening loads when eyes were closed (t(42) = 2.626, p = 0.0120, Fig. 5). In contrast, firm standing resulted in higher listening accuracy than compliant standing under low listening loads when eyes were closed (t(42) = -3.157, p = 0.0029, Fig. 5). There were no other significant main or interaction effects.
Fig. 5.
Percent correct listening accuracy collapsed across the hearing loss group (aided) and normal hearing group under different listening loads (high, low) and visual loads (eyes open, eyes closed). The violin plot represents the frequency of the data at a particular value along the y-axis with the standard error. The red dot represents the mean. The individual points represent each participant’s average for each listening and visual load combination. Statistical significance was determined using an ANOVA with a significance level of p < 0.05*.
Balance task performance
Centre of pressure anterior–posterior standard deviation
A 2 group (hearing loss aided, normal hearing) × 2 listening load (high, low) mixed factorial ANOVA was conducted on dual-task costs to anterior–posterior standard deviation, where group was a between-subject variable and listening load was a within-subject variable. There were no significant main or interaction effects. We then repeated the same mixed factorial ANOVA with the addition of visual (eyes open/closed) and postural (compliant/firm) loads as additional within-subject factors. There were no significant main effects. A significant listening load x postural load interaction was observed (F(1,42) = 5.52, p = 0.024, ηp2 = 0.116). Specifically, there were an effect of listening load during firm (but not compliant) standing, with high listening loads having more dual-task costs than low listening loads (t(1,42) = -4.083, p = 0.0002). There was also an effect of postural load during high listening loads (not low listening loads) with firm having more dual-task costs than compliant (t(42) = -2.962, p = 0.0050). There were no other significant main or interaction effects.
Centre of pressure mean velocity
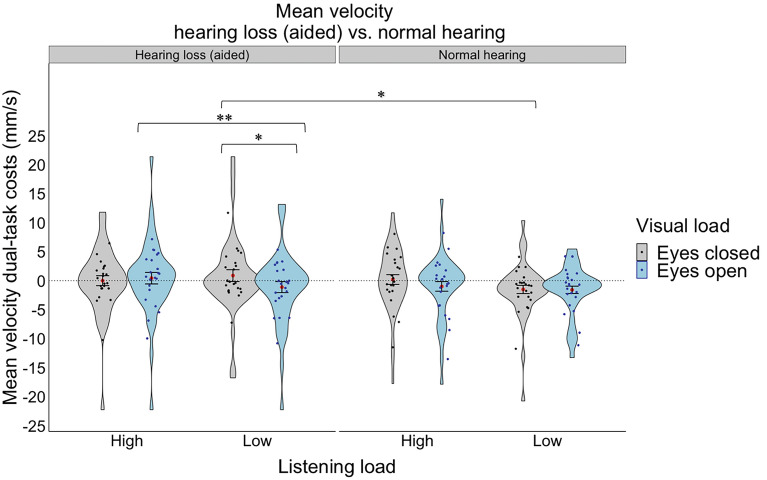
A 2 group (hearing loss aided, normal hearing) × 2 listening load (high, low) mixed factorial ANOVA was conducted on dual-task costs to mean velocity, where group was a between-subject variable and listening load was a within-subject variable. There were no significant main or interaction effects. We then repeated the same mixed factorial ANOVA with the addition of visual (eyes open/closed) and postural (compliant/firm) loads as additional within-subject factors. There were no significant main effects. However, there was a significant three-way interaction effect between group x listening load x vision (F(1,42) = 6.07, p = 0.018, ηp2 = 0.126). Specifically, in the hearing loss aided group only, and only during low listening loads, there was an effect of vision, whereby closing the eyes resulted in higher dual-task costs to mean velocity than having the eyes open (t(42) = 2.379, p = 0.0220, see Fig. 6). Further, for the hearing loss aided group only and only with eyes open, high listening load resulted in a higher dual-task cost to velocity mean than lower listening loads (t(42) = − 3.155, p = 0.0030, see Fig. 6). Moreover, the normal hearing group had less variability compared to the hearing loss (aided) group, but only with eyes closed during low listening load (t(42) = 2.356, p = 0.0232, see Fig. 6). There were no other significant main or interaction effects.
Fig. 6.
Dual-task costs for mean velocity for the hearing loss group (aided) compared to the normal hearing group under different listening (high, low) and visual loads (eyes closed, eyes open). The violin plot represents the frequency of the data at a particular value along the y-axis with the standard error. The red dot represents the mean. The individual points represent each participant’s average for each listening and visual load combination. Statistical significance was determined using an ANOVA with a significance level of p < 0.05*.
Normal hearing vs. hearing loss unaided
Listening task performance
A 2 group (hearing loss unaided, normal hearing) × 2 listening load (high, low) mixed factorial ANOVA was conducted on percent correct scores, where group was a between-subject factor and listening load was a within-subject factor. The same results were observed here as were reported in the normal hearing vs. hearing loss aided analyses reported above. Specifically, there was a significant main effect of group (F(1,41) = 45.47, p < 0.001, ηp2 = 0.526) with the normal hearing group demonstrating higher listening accuracy than the hearing loss group. There was also a significant main effect of listening load (F(1,41) = 80.14, p < 0.001, ηp2 = 0.662) demonstrating that listening accuracy was higher in the low load compared to the high load listening condition.
We repeated the same mixed factorial ANOVA with group and listening load, with the addition of visual (eyes open/closed) and postural (compliant/firm) loads as additional within-subject factors. Again, we found a significant effect of vision (F(1,41) = 16.36, p < 0.001, ηp2 = 0.285) with eyes closed resulting in higher listening performance than eyes open (t(41) = 4.044, p = 0.0002). Also, we found a significant main effect of posture (F(1,41) = 4.89, p = 0.033, ηp2 = 0.106) with firm resulting in higher listening performance than compliant (t(41) = -2.210, p = 0.0327). Also, we found a significant four-way interaction with group x listening load x vision x posture (F(1,41) = 5.24, p = 0.027, ηp2 = 0.113) demonstrating similar results to those reported for the normal hearing vs. hearing loss aided comparison above. There were no other significant main or interaction effects.
Balance task performance
Centre of pressure anterior–posterior standard deviation
A 2 group (hearing loss unaided, normal hearing) × 2 listening load (high, low) mixed factorial ANOVA was conducted on dual-task costs to anterior–posterior standard deviation, where group was a between-subject variable and listening load was a within-subject variable. There were no significant main or interaction effects. We then repeated the same mixed factorial ANOVA with the addition of visual (eyes open/closed) and postural (compliant/firm) loads as additional within-subject factors. There was a significant main effect of posture (F(1,41) = 5.78, p = 0.021, ηp2 = 0.123), where compliant standing resulted in lower dual-task costs to variability (a potentially stiffer posture) compared to firm standing. There was also a significant group x listening load x posture interaction (F(1,42) = 4.96, p = 0.031, ηp2 = 0.108). Specifically, it was observed that the effect of posture was only observed for the hearing loss group, such that standing on a compliant surface resulted in lower dual-task costs to variability (potentially stiffer posture) than standing on a firm surface (t(41) = − 2.273, p = 0.0284) in low load listening only. Further, the effect of listening load was only observed for the normal hearing group during firm standing, whereby higher listening loads resulted in higher dual-task costs (higher variability) than lower listening loads (t(41) = − 2.023, p = 0.0497). There were no other significant main or interaction effects.
Centre of pressure mean velocity
A 2 group (hearing loss unaided, normal hearing) × 2 listening load (high, low) mixed factorial ANOVA was conducted on dual-task costs to mean velocity, where group was a between-subjects variable and listening load was a within-subject variable. There were no significant main or interaction effects. We repeated the same mixed factorial ANOVA with the addition of visual (eyes open/closed) and postural (compliant/firm) loads as additional within-subject factors and there were no significant main effects, but there was a significant two-way interaction between listening load x vision (F(1,41) = 7.44, p = 0.009, ηp2 = 0.154), where low listening load had lower dual-task costs to velocity (higher stability) compared to high listening load, but only when eyes were closed (t(41) = − 2.605, p = 0.0127) and not when eyes were open. There were no other significant main or interaction effects.
Correlations
Correlations were conducted to explore whether there were any associations among baseline measures (demographic, sensory, cognitive, motor) and experimental outcome measures (visual, postural, and listening loads). While no pronounced and highly consistent patterns were observed, the most notable associations were between baseline assessments of cognitive functions and certain experimental postural outcomes. For example, there were negative correlations showing that better cognitive performance on RAVLT was associated with less postural variability (less dual-task costs in anterior–posterior sway) particularly when unaided (see Supplementary Baseline section and Figs. S1–3 for heatmaps online for more details).
Anxiety, fatigue and motivation
Three ANOVAs were conducted with anxiety, fatigue, and motivation ratings (averaged across day 1 & 2 and aided/unaided) as the dependent factors and group (normal hearing vs. hearing loss) as the independent factor. Although not significant, the normal hearing group had less anxiety (M = 1.97/10) and slightly more motivation (M = 8.84/10) compared to the hearing loss group (M = 2.53/10, M = 8.31/10, respectively). However, the hearing loss group reported significantly more fatigue (M = 4.26) compared to the normal hearing group (M = 2.29), (F(1,42) = 12.902, p < 0.001, ηp2 = 0.235). When we examined whether there was an effect of aid use, there were no significant effects of aid use on anxiety, motivation, or fatigue.
Discussion
This study incorporated a realistic simulation to examine the effects of hearing aids (aided vs. unaided) and hearing status (normal hearing vs. hearing loss) on listening accuracy and dual-task costs to balance. To address secondary objectives, we explored whether the effects of visual, postural, and acoustical loads affected listening accuracy and dual-task costs to balance and/or interacted with the effects of hearing loss/aid use. When examining listening performance, as expected, the hearing loss group demonstrated better accuracy when aided compared to unaided and higher accuracy was observed in the normal hearing group compared to the hearing loss group. Both groups had higher accuracy in the easier low listening load condition (compared to high), when their eyes were closed (compared to open) and when the standing surface was firm (compared to compliant). When looking at postural performance, a key finding was that hearing aids did not remarkably improve balance overall. Other factors related to the complexity of the conditions (i.e., visual load, postural load, listening load) differently influenced individuals with and without hearing loss with respect to balance-related outcomes. There was some evidence that an inverted U-shaped function was observed for some postural parameters, with the difficulty threshold of these functions being lower for individuals with hearing loss compared to those with normal hearing. Interestingly, we did not find any effects of spatial sounds on postural outcomes indicating that, under these complex, conditions, spatial sound cues did not appear to stabilize posture.
Effects of hearing aids and hearing loss on listening under different cognitive, motor, and perceptual loads
As mentioned above when examining listening performance, as expected, individuals with hearing loss performed better when aided than when unaided. Further, older adults with normal hearing demonstrate higher listening performance overall compared to the those with hearing loss. Lower listening loads also resulted in better listening performance than higher listening loads. These findings verified that relative listening demands were effectively modulated as intended, thereby supporting that these manipulations served as a valid strategy for evaluating the effects of varying listening loads on postural outcomes. Perhaps the most interesting and novel result, was that the presence of a complex, realistic visual environment negatively influenced listening accuracy. Specifically, both the normal hearing and hearing loss groups (aided and unaided) demonstrated better listening accuracy when their eyes were closed compared to open. This suggests that the visual inputs, which were not relevant to listening performance in this situation, may have introduced distractions or perceptual loads that negatively affected listening accuracy. This negative effect of visual inputs on listening accuracy is very different from situations when visual inputs can be helpful for augmenting auditory information to support behaviors – such as when navigating traffic or during speech communication. In fact, there are typically multisensory benefits observed when sensory inputs are redundant or complementary (e.g., reading lips while listening to voices76–78). However, in the case of the current study, visual inputs were irrelevant to the listening task, and as such may have interfered with listening accuracy. This finding is consistent with previous work demonstrating that auditory-visual speech perception is negatively affected by non-relevant visual distractions and that older adults may be more susceptible to visual distractions compared to younger adults79.
Effects of hearing aids and hearing loss on dual-task costs to posture under different cognitive, motor, and perceptual loads
Previous literature examining the effects of hearing aids on postural outcomes have been mixed, with some studies demonstrating improvements21,46–50 and others showing no effects51–56. The current study was unique in that it evaluated the effects of hearing aids on postural outcomes during complex, multisensory conditions that more closely mimic the types of conditions and challenges that older adults experience during their everyday lives. It was initially predicted that hearing aids could support postural outcomes by either providing access to spatial sound cues and/or by reducing listening load; however, results demonstrated that spatial sound cues had no effects on posture for either the normal hearing group or hearing loss group, irrespective of whether they were aided or unaided. We also observed that while listening accuracy was indeed higher when aided compared to unaided, suggesting that perhaps listening load was reduced through hearing aid use, there were no clear associated benefits on posture. By examining the effects of amplification in the context of other balance-relevant factors (e.g., visual load and postural load), this provides a more holistic understanding of how these factors interact. For example, in the current study it was observed that the presence of irrelevant, complex visual information resulted in poorer listening performance, perhaps due to increased distractions, and yet complex visual information is known to provide very important orientation cues to support balance; two effects that may ultimately cancel each other out with respect to evidenced changes in postural-related outcomes.
In this study, it was initially predicted that higher cognitive, sensory, and postural demands would result in progressively larger dual-task costs to postural outcomes, particularly for older adults with hearing loss. For example, it was expected that higher (compared to lower) listening loads, standing on a compliant (compared to firm) surface, and closing (compared to opening) one’s eyes would lead to overall less stable posture (e.g., higher center of pressure variability and velocity), and that these additional loads would lead to compounded effects on postural stability when combined. However, the observed results were more complex and nuanced, demonstrating that, in several cases, the lowest dual-task costs were actually observed during both the easiest and hardest conditions. These results may reflect an inverted U-shaped function for the effects of sensory/cognitive/postural demands on dual-task costs rather than a linear relationship26,36–40. More specifically, when dual-task demands were low (e.g., low listening load, eyes open), there may have been minimal adverse effects on posture; in fact, low load tasks may actually result in dual-task benefits by shifting away from attentionally-driven postural control, which can decrease postural efficiency, towards automated control processes75,80–84. Conversely, when dual-task demands were too high (e.g., high listening load, eyes closed), participants may have disengaged from the highly effortful cognitive-listening task and abandon this task all together in favour of protecting the prioritized task, which may be more safety critical (i.e., maintaining balance13,15–18). This redistribution in the allocation of cognitive resources would result in dual-task cost values near zero; specifically evidenced by no differences between single-task and dual-task performance given that the listening task is abandoned and is no longer consuming cognitive resources. As a result, the most pronounced dual-task costs would be observed during the moderately difficult tasks (e.g., low listening load, eyes closed; or high listening load, eyes open). Indeed, this is the exact pattern that we observed in the current study for certain postural parameters (see Fig. 3 aided, Fig. 4 aided, Fig. 6 aided). Importantly, however, what is considered “moderately” difficult is entirely relative and depends on many factors, including individual capabilities across different domains of functioning. For example, what is moderately difficult for some individuals (e.g., older adults with hearing loss) may be more easily managed by other individuals (e.g., older adults with normal hearing). As such, older adults with hearing loss may have an inverted U-shape function that is shifted along the dual-task demand scale (lower demand thresholds), compared to the normal hearing older adult function, which is partially represented in the current data as an incomplete function (see Fig. 6).
This interpretation of an inverted U-shaped function is clearly supported by cross-referencing the patterns observed for listening performance to those observed for the dual-task costs. Specifically, we would expect the poorest listening accuracy to be observed under the most demanding conditions (e.g., high listening load, eyes open, unaided), which would support a task-abandonment interpretation. We indeed observed this pattern in the current study (see Fig. 2), where performance was near floor (M = 17% correct for the hearing loss group when unaided). Similarly, we would expect to see the highest listening performance during the least demanding conditions (e.g., low listening load, eyes closed, aided), whereby the dual-task costs would be minimized (or dual-task benefits observed). This pattern was again observed in the current study (e.g., M = 42% correct for hearing loss group when aided).
Limitations and future directions
Effects of spatial sounds
In order to evaluate the effects of spatial sounds on balance we presented sound cues from three stationary sound sources within a semi-naturalistic, reverberant environment. Due to the nature of the experimental environment, the perceptual locations of the sound cues could not be precisely controlled compared to, for example, perfectly sound-attenuated and acoustically controlled spaces. Indeed, when asked to localize the sound cues, many participants in both groups were not able to accurately localize each sound when individually and sequentially presented in the virtual environment (30% of the normal hearing group and 66% of the hearing loss group). It is, therefore, possible that manipulating the factor of spatial sound cues (no noise vs. white noise vs. spatial noise) had no influence on any postural outcomes due to uncontrolled acoustical factors. That said, by using a highly immersive, multisensory, non-acoustically controlled virtual reality simulation, we were perhaps better able to recreate common real-world conditions that are indeed reverberant and noisy in nature, making these results potentially more generalizable to the effects observed during everyday experiences.
Hearing aids
In the current study, we did not control for the type of hearing aids participants used and instead allowed them to wear their current, familiar, hearing aids, whatever the type. While this ensured that participants were comfortable with and well-adapted to their hearing aids, there was a great deal of heterogeneity in terms of, for example, the length of time they used their current hearing aids (M = 2.33 years, range = 2 months-5 years), frequency of use (M = 10.86 h/day, SD = 4.99, range = 0–18), fit (hearing had been assessed by an audiologist within the previous 5.95 months on average), and model type (n = 19 behind-the-ear; n = 1 receiver-in-canal, n = 1 in-the-canal; n = 1 in-the-ear). Factors related to hearing aid use/types may be relevant for mobility-related outcomes. For example, recent evidence suggests that consistency of hearing aid use may be a predictor of falls-risk47, such that more consistent use is associated with lower falls-risk. Therefore, novice hearing aid users may not benefit from their aids in terms of supporting balance to the same extent as more experienced users. Also, hearing aids have various features, such as directional microphones, which are designed to amplify sounds from the front/facing direction of the user (typically directed towards a conversational partner) in order to improve speech intelligibility in noise. However, this also has the effect of reducing peripheral and spatial sound cues that could be important for mobility/navigation that occur from all different directions85. Across the general population of hearing aid users, there remains heterogeneity in hearing aid types and use habits, which could be related to a number of factors, such as cost and access to hearing care services86. Therefore, the results of the current study may reflect the general influence of hearing aids on balance across the population, rather than what might be observed under “ideal”, highly controlled conditions (i.e., experimentally controlled hearing aid types/models, fit quality, use frequency). Recent research has also shown that hearing-related interventions (hearing aids with audiology counselling) may reduce cognitive decline, but primarily in older adults who are at higher risk of cognitive decline (e.g., due to poor cardiovascular health), compared to those who are at lower risk87. Therefore, similar effects may be observed with respect to falls risk mitigation whereby perhaps it is the individuals who are at greater risk of falling due to other comorbidities or health conditions who benefit most from hearing health interventions, such as hearing aids.
Emotional factors
Research has shown that emotional factors (e.g., anxiety, fatigue, motivation) can influence both postural outcomes69 and listening performance and effort13. In the current study, when exploring the emotional status of participants with respect to anxiety, fatigue, and motivation, we collected this information at the end of each of the two testing days. However, participants’ levels of anxiety, fatigue and motivation may have fluctuated across the experimental conditions, particularly as the conditions varied by visual, listening, acoustical or postural loads and across dual- vs. single-task conditions. Therefore, collecting emotional ratings specifically after each block (i.e., immediately after each condition type), rather than at the very end of the study, would provide a better indication of participants’ specific emotional status with respect to specific task conditions.
Study sample
Due to the strict participant eligibility criteria of this study, we recruited community-dwelling older adults who had generally very high levels of sensory, cognitive, and motor functioning and very few serious health conditions. However, “older adults” as a population are highly heterogeneous and often experience a spectrum of abilities (e.g., different hearing, cognitive, motor abilities, and other common age-related health conditions). Therefore, while we aimed to control the variability within each of our two groups, the overall sample used in this study provides a biased representation of how sensory, cognitive, and motor abilities interact and/or are compromised during complex, everyday behaviours. It is likely that these results offer a conservative underestimation of how these various sensory, cognitive, postural factors influence dual-task costs to balance in the broader population.
Listening-alone task
As we were primarily interested in the effects of hearing and listening on balance-related outcomes, we prioritized measuring dual-task costs to balance and did not measure dual-task costs to listening. In order to measure dual-task costs to listening, a “listening only” task would need to be included. In previous studies using a very similar paradigm15,88, a seated listening task was implemented to represent single-task listening (in comparison to dual-task listening-while-standing). In these studies, there were minimal differences observed between listening task performance during single (seated) and dual (standing) trials. As such, given that dual-task costs to posture were our primary outcome measure, that there was a lack of previously observed differences in similar studies between single- and dual-task listening performance, and to keep our relatively lengthy protocol within reasonable limits (e.g., to limit fatigue etc.), we did not include a listening alone condition in the current study. Nevertheless, we recognize that this limits the ability to consider how proportional dual-task costs compare for listening relative to postural performance.
Conclusions
This study implemented a listening-while-balancing task varying in listening, visual, acoustical, and postural loads in older adults with normal hearing and hearing loss (aided vs. unaided). Overall, normal hearing adults had higher listening accuracy than individuals with hearing loss (aided better than unaided) with both groups generally demonstrating higher listening accuracy in the less demanding conditions (low listening load, firm surface, eyes closed) compared to the more demanding conditions (high listening load, compliant surface, eyes open). When looking at postural performance, hearing aids did not remarkably improve balance overall. Other factors related to the complexity of the conditions (i.e., visual load, postural load, listening load) differently influenced dual-task costs to posture in individuals with and without hearing loss with respect to balance-related outcomes. Overall, these results contribute to our understanding of how age-related hearing loss and hearing aids affect balance-related outcomes under realistic, complex, multisensory, multitasking conditions.
Supplementary Information
Acknowledgements
We would like to thank Colin Stoddart, Roger Montgomery, Bruce Haycock and Susan Gorski for their technical assistance, and Kristen Arnold and Kelly Qi with assistance in piloting and collecting the baseline data.
Author contributions
All authors conceptualized and designed the study; NM, LM and GAG collected and transcribed the data; NM, LM, GAG, JC analyzed the data; JC and NM wrote the manuscript, NM and LM made the tables and figures, all authors contributed to reviewing and editing the manuscript.
Data availability
The datasets generated during and/or analysed during the current study are not publicly available due to the Research Ethics Board at KITE-Toronto Rehabilitation Institute and University of Toronto but are available from the corresponding author on reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-79933-8.
References
- 1.Chen, D. S. et al. Association of hearing impairment with declines in physical functioning and the risk of disability in older adults. J. Gerontol. Series A70(5), 654–661 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Viljanen, A. et al. Hearing as a predictor of falls and postural balance in older female twins. J. Gerontol. Series A64(2), 312–317 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berge, J. E., Nordahl, S. H. G., Aarstad, H. J. & Goplen, F. K. Hearing as an independent predictor of postural balance in 1075 patients evaluated for dizziness. Otolaryngol.-Head Neck Surgery161(3), 478–484 (2019). [DOI] [PubMed] [Google Scholar]
- 4.Thomas, E. et al. Decreased postural control in people with moderate hearing loss. Medicine10.1097/MD.0000000000010244 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Agmon, M., Lavie, L. & Doumas, M. The association between hearing loss, postural control, and mobility in older adults: a systematic review. J. Am. Acad. Audiol.28(06), 575–588 (2017). [DOI] [PubMed] [Google Scholar]
- 6.Da, H. K., Lee, J. D. & Lee, H. J. Relationships among hearing loss, cognition and balance ability in community-dwelling older adults. J. Phys. Ther. Sci.27(5), 1539–1542 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li, L., Simonsick, E. M., Ferrucci, L. & Lin, F. R. Hearing loss and gait speed among older adults in the United States. Gait Posture38(1), 25–29 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gopinath, B., McMahon, C. M., Burlutsky, G. & Mitchell, P. Hearing and vision impairment and the 5-year incidence of falls in older adults. Age Ageing45(3), 409–414 (2016). [DOI] [PubMed] [Google Scholar]
- 9.Lin, F. R. & Ferrucci, L. Hearing loss and falls among older adults in the United States. Arch. Int. Med.172(4), 369–371 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jiam, N. T. L., Li, C. & Agrawal, Y. Hearing loss and falls: A systematic review and meta-analysis. Laryngoscope126(11), 2587–2596 (2016). [DOI] [PubMed] [Google Scholar]
- 11.Gabriel, G. A. et al. Vestibular perceptual thresholds in older adults with and without age-related hearing loss. Ear Hearing43(2), 420–435 (2022). [DOI] [PubMed] [Google Scholar]
- 12.Zuniga, M. G. et al. Association between hearing loss and saccular dysfunction in older individuals. Otol. Neurotol.33(9), 1586–1592 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pichora-Fuller, M. K. et al. Hearing impairment and cognitive energy: The framework for understanding effortful listening (FUEL). Ear Hearing37, 5S-27S (2016). [DOI] [PubMed] [Google Scholar]
- 14.Li, K. Z., Bherer, L., Mirelman, A., Maidan, I. & Hausdorff, J. M. Cognitive involvement in balance, gait and dual-tasking in aging: a focused review from a neuroscience of aging perspective. Front. Neurol.9, 413669 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carr, S., Pichora-Fuller, M. K., Li, K. Z. & Campos, J. L. Effects of age on listening and postural control during realistic multi-tasking conditions. Hum. Movement Sci.73, 102664 (2020). [DOI] [PubMed] [Google Scholar]
- 16.Bruce, H. et al. The effects of age and hearing loss on dual-task balance and listening. J. Gerontol.74(2), 275–283 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nieborowska, V., Lau, S. T., Campos, J., Pichora-Fuller, M. K., Novak, A., & Li, K. Z. Effects of age on dual-task walking while listening. Journal of Motor Behavior. (2018). [DOI] [PubMed]
- 18.Lau, S. T., Pichora-Fuller, M. K., Li, K. Z., Singh, G. & Campos, J. L. Effects of hearing loss on dual-task performance in an audiovisual virtual reality simulation of listening while walking. J. Am. Acad. Audiol.27(7), 567–587 (2016). [DOI] [PubMed] [Google Scholar]
- 19.Hartmann, W. M. How we localize sound. Phys. Today52(11), 24–29 (1999). [Google Scholar]
- 20.Campos, J., Ramkhalawansingh, R. & Pichora-Fuller, M. K. Hearing, self-motion perception, mobility, and aging. Hearing Res.369, 42–55 (2018). [DOI] [PubMed] [Google Scholar]
- 21.Vitkovic, J., Le, C., Lee, S. L. & Clark, R. A. The contribution of hearing and hearing loss to balance control. Audiol. Neurotol.21(4), 195–202 (2016). [DOI] [PubMed] [Google Scholar]
- 22.Easton, R. D., Greene, A. J., DiZio, P. & Lackner, J. R. Auditory cues for orientation and postural control in sighted and congenitally blind people. Exp. Brain Res.118, 541–550 (1998). [DOI] [PubMed] [Google Scholar]
- 23.Zhong, X. & Yost, W. A. Relationship between postural stability and spatial hearing. J. Am. Acad. Audiol.24(09), 782–788 (2013). [DOI] [PubMed] [Google Scholar]
- 24.Gandemer, L., Parseihian, G., Kronland-Martinet, R. & Bourdin, C. Spatial cues provided by sound improve postural stabilization: evidence of a spatial auditory map?. Front. Neurosci11, 357 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stevens, M. N., Barbour, D. L., Gronski, M. P. & Hullar, T. E. Auditory contributions to maintaining balance. J. Vestibular Res.26(5–6), 433–438 (2016). [DOI] [PubMed] [Google Scholar]
- 26.Deviterne, D., Gauchard, G. C., Jamet, M., Vançon, G. & Perrin, P. P. Added cognitive load through rotary auditory stimulation can improve the quality of postural control in the elderly. Brain Res. Bull.64(6), 487–492 (2005). [DOI] [PubMed] [Google Scholar]
- 27.Mangiore, R. J. The effect of an external auditory stimulus on postural stability of participants with cochlear implants. Independent Studies and Capstones. Program in Audiology and Communication Sciences, Washington University School of Medicine, 639 (2012).
- 28.Soames, R. W. & Raper, S. A. The influence of moving auditory fields on postural sway behaviour in man. Eur. J. Appl. Physiol. Occup. Physiol.65, 241–245 (1992). [DOI] [PubMed] [Google Scholar]
- 29.Raper, S. A. & Soames, R. W. The influence of stationary auditory fields on postural sway behaviour in man. Eur. J. Appl. Physiol. Occup. Physiol.63, 363–367 (1991). [DOI] [PubMed] [Google Scholar]
- 30.Allen, P. D. & Eddins, D. A. Presbycusis phenotypes form a heterogeneous continuum when ordered by degree and configuration of hearing loss. Hearing Res.264(1–2), 10–20 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Keidser, G. et al. The quest for ecological validity in hearing science: What it is, why it matters, and how to advance it. Ear Hearing41(Suppl 1), 5S (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Campos, J. L. & Launer, S. From healthy hearing to healthy living: A holistic approach. Ear Hearing41, 99S-106S (2020). [DOI] [PubMed] [Google Scholar]
- 33.Carpenter, M. G. & Campos, J. L. The effects of hearing loss on balance: a critical review. Ear Hearing41, 107S-119S (2020). [DOI] [PubMed] [Google Scholar]
- 34.Lord, S. R., Smith, S. T. & Menant, J. C. Vision and falls in older people: risk factors and intervention strategies. Clin. Geriatr. Med.26(4), 569–581 (2010). [DOI] [PubMed] [Google Scholar]
- 35.Cohen, J. I. & Gordon-Salant, S. The effect of visual distraction on auditory-visual speech perception by younger and older listeners. J. Acoustical Soc. Am.10.1121/14983399 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huxhold, O., Li, S. C., Schmiedek, F. & Lindenberger, U. Dual-tasking postural control: aging and the effects of cognitive demand in conjunction with focus of attention. Brain Res. Bull.69(3), 294–305 (2006). [DOI] [PubMed] [Google Scholar]
- 37.Lövdén, M., Schaefer, S., Pohlmeyer, A. E. & Lindenberger, U. Walking variability and working-memory load in aging: a dual-process account relating cognitive control to motor control performance. J. Gerontol. Series B63(3), P121–P128 (2008). [DOI] [PubMed] [Google Scholar]
- 38.Riley, M. A., Baker, A. A. & Schmit, J. M. Inverse relation between postural variability and difficulty of a concurrent short-term memory task. Brain Res. Bull.62(3), 191–195 (2003). [DOI] [PubMed] [Google Scholar]
- 39.Vuillerme, N. & Nougier, V. Attentional demand for regulating postural sway: the effect of expertise in gymnastics. Brain Res. Bull.63(2), 161–165 (2004). [DOI] [PubMed] [Google Scholar]
- 40.Vuillerme, N., Nougier, V. & Camicioli, R. Veering in human locomotion: modulatory effect of attention. Neurosci. Lett.331(3), 175–178 (2002). [DOI] [PubMed] [Google Scholar]
- 41.Sarant, J. et al. The effect of hearing aid use on cognition in older adults: can we delay decline or even improve cognitive function?. J. Clin. Med.9(1), 254 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mahafza, M. T., Wilson, W. J., Brauer, S., Timmer, B. H. & Hickson, L. A systematic review of the effect of hearing aids on static and dynamic balance in adults with hearing impairment. Trends Hearing26, 23312165221121016 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lavie, L., Tobia, N., Slav-Zarfati, N., Castel, S. & Banai, K. Are current data sufficient to infer that hearing aids contribute to postural control and balance in older adults? A systematic review. Folia phoniatrica et logopaedica76(3), 232–244 (2024). [DOI] [PMC free article] [PubMed]
- 44.Borsetto, D. et al. The influence of hearing aids on balance control: A systematic review. Audiol. Neurotol.26(4), 209–217 (2021). [DOI] [PubMed] [Google Scholar]
- 45.Lubetzky, A. V. et al. Auditory input and postural control in adults: a narrative review. JAMA Otolaryngol.-Head Neck Surgery146(5), 480–487 (2020). [DOI] [PubMed] [Google Scholar]
- 46.Behtani, L. et al. Hearing Aid Amplification Improves Postural Control for Older Adults With Hearing Loss When Other Sensory Cues Are Impoverished. Trends Hearing28, 23312165241232220 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Campos, L. et al. Consistent hearing aid use is associated with lower fall prevalence and risk in older adults with hearing loss. J. Am. Geriatrics Soc.10.1111/jgs.18461 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Negahban, H. & Nassadj, G. Effect of hearing aids on static balance function in elderly with hearing loss. Gait Posture58, 126–129 (2017). [DOI] [PubMed] [Google Scholar]
- 49.Rumalla, K., Karim, A. M. & Hullar, T. E. The effect of hearing aids on postural stability. Laryngoscope125(3), 720–723 (2015). [DOI] [PubMed] [Google Scholar]
- 50.Ibrahim, I., da Silva, S. D., Segal, B. & Zeitouni, A. Postural stability: assessment of auditory input in normal hearing individuals and hearing aid users. Hearing Balance Commun.17(4), 280–287 (2019). [Google Scholar]
- 51.McDaniel, D. M., Motts, S. D. & Neeley, R. A. Effects of bilateral hearing aid use on balance in experienced adult hearing aid users. Am. J Audiol.27(1), 121–125 (2018). [DOI] [PubMed] [Google Scholar]
- 52.Lacerda, C. F. & e Silva, L. O., de Tavares Canto, R. S., & Cheik, N. C.,. Effects of hearing aids in the balance, quality of life and fear to fall in elderly people with sensorineural hearing loss. Int. Arch. Otorhinolaryngol.16(02), 156–162 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Weaver, T. S., Shayman, C. S. & Hullar, T. E. The effect of hearing aids and cochlear implants on balance during gait. Otol. Neurotol.38(9), 1327–1332 (2017). [DOI] [PubMed] [Google Scholar]
- 54.Kowalewski, V., Patterson, R., Hartos, J. & Bugnariu, N. Hearing loss contributes to balance difficulties in both younger and older adults. J. Prevent. Med.10.21767/2572-5483.100033 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chia, E. M. et al. Hearing impairment and health-related quality of life: the Blue Mountains Hearing Study. Ear Hearing28(2), 187–195 (2007). [DOI] [PubMed] [Google Scholar]
- 56.Riska, K. M., Peskoe, S. B., Gordee, A., Kuchibhatla, M. & Smith, S. L. Preliminary evidence on the impact of hearing aid use on falls risk in individuals with self-reported hearing loss. Am. J. Audiol.30(2), 376–384 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Akeroyd, M. A. & Whitmer, W. M. Spatial hearing and hearing aids. ENT Audiol. News20(5), 76 (2011). [PMC free article] [PubMed] [Google Scholar]
- 58.Carson, N., Leach, L. & Murphy, K. J. A re-examination of Montreal Cognitive Assessment (MoCA) cutoff scores. Int. J. Geriatr. Psychiatr.33(2), 379–388 (2018). [DOI] [PubMed] [Google Scholar]
- 59.Ferris, F. L. III., Kassoff, A., Bresnick, G. H. & Bailey, I. New visual acuity charts for clinical research. Am. J. Ophthalmol.94(1), 91–96 (1982). [PubMed] [Google Scholar]
- 60.Colenbrander, A. Visual standards: aspects and ranges of vision loss with emphasis on population surveys. In Report prepared for the International Council of Ophthalmology at the 29th International Congress of Ophthalmology Sydney, Australia. (2022).
- 61.Bastianelli, M. et al. Adult validation of a self-administered tablet audiometer. J. Otolaryngol Head Neck Surgery48, 1–9 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.World Health Organization. Report of the informal working group on prevention of deafness and hearing impairment Programme planning, Geneva, 18–21 June 1991 (No. WHO/PDH/91.1. Unpublished). World Health Organization. (1991).
- 63.World Health Organization. World report on hearing. World Health Organization. (2021). [DOI] [PMC free article] [PubMed]
- 64.Campos, J. L. et al. The importance of acoustics in multimodal virtual reality research and application. Can. Acoust.46, 29–40 (2018). [Google Scholar]
- 65.Musiek, F. E. Assessment of central auditory dysfunction: the dichotic digit test revisited. Ear Hearing4(2), 79–83 (1983). [DOI] [PubMed] [Google Scholar]
- 66.Dault, M. C., Yardley, L. & Frank, J. S. Does articulation contribute to modifications of postural control during dual-task paradigms?. Cogn. Brain Res.16(3), 434–440 (2003). [DOI] [PubMed] [Google Scholar]
- 67.Ross, J. M. & Balasubramaniam, R. Auditory white noise reduces postural fluctuations even in the absence of vision. Exp. Brain Res.233, 2357–2363 (2015). [DOI] [PubMed] [Google Scholar]
- 68.Helfer, K. S., Merchant, G. R. & Wasiuk, P. A. Age-related changes in objective and subjective speech perception in complex listening environments. J. Speech Lang. Hearing Res.60(10), 3009–3018 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Adkin, A. L. & Carpenter, M. G. New insights on emotional contributions to human postural control. Front. Neurol.9, 411342 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sawyer, S. F. Analysis of variance: the fundamental concepts. J. Manual Manipulative Ther.17(2), 27E-38E (2009). [Google Scholar]
- 71.Day, B. L., Steiger, M. J., Thompson, P. D. & Marsden, C. D. Effect of vision and stance width on human body motion when standing: implications for afferent control of lateral sway. J. Physiol.469(1), 479–499 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wing, A. M., Clapp, S. & Burgess-Limerick, R. Standing stability in the frontal plane determined by lateral forces applied to the hip. Gait Posture3(1), 38–42 (1995). [Google Scholar]
- 73.Winter, D. A., Patla, A. E., Prince, F., Ishac, M. & Gielo-Perczak, K. Stiffness control of balance in quiet standing. J. Neurophysiol.80(3), 1211–1221 (1998). [DOI] [PubMed] [Google Scholar]
- 74.Bonnet, C. T. Broad stance conditions change postural control and postural sway. J. Motor Behav.44(2), 125–131 (2012). [DOI] [PubMed] [Google Scholar]
- 75.Lajoie, Y., Jehu, D. A., Richer, N. & Chan, A. Continuous and difficult discrete cognitive tasks promote improved stability in older adults. Gait Posture55, 43–48 (2017). [DOI] [PubMed] [Google Scholar]
- 76.Peiffer, A. M., Mozolic, J. L., Hugenschmidt, C. E. & Laurienti, P. J. Age-related multisensory enhancement in a simple audiovisual detection task. Neuroreport18(10), 1077–1081 (2007). [DOI] [PubMed] [Google Scholar]
- 77.Freiherr, J., Lundström, J. N., Habel, U. & Reetz, K. Multisensory integration mechanisms during aging. Front. Hum. Neurosci.7, 863 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jones, S. A. & Noppeney, U. Ageing and multisensory integration: A review of the evidence, and a computational perspective. Cortex138, 1–23 (2021). [DOI] [PubMed] [Google Scholar]
- 79.Cohen, J. I. & Gordon-Salant, S. The effect of visual distraction on auditory-visual speech perception by younger and older listeners. J. Acoustical Soc. America10.1121/14983399 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lajoie, Y., Richer, N., Jehu, D. A. & Tran, Y. Continuous cognitive tasks improve postural control compared to discrete cognitive tasks. J. Motor Behav.48(3), 264–269 (2016). [DOI] [PubMed] [Google Scholar]
- 81.Richer, N., Saunders, D., Polskaia, N. & Lajoie, Y. The effects of attentional focus and cognitive tasks on postural sway may be the result of automaticity. Gait Posture54, 45–49 (2017). [DOI] [PubMed] [Google Scholar]
- 82.Polskaia, N., Richer, N., Dionne, E. & Lajoie, Y. Continuous cognitive task promotes greater postural stability than an internal or external focus of attention. Gait Posture41(2), 454–458 (2015). [DOI] [PubMed] [Google Scholar]
- 83.Lajoie, Y., Teasdale, N., Bard, C. & Fleury, M. Attentional demands for static and dynamic equilibrium. Exp. Brain Res.97, 139–144 (1993). [DOI] [PubMed] [Google Scholar]
- 84.Richer, N., Polskaia, N. & Lajoie, Y. Continuous cognitive task promotes greater postural stability than an internal or external focus of attention in older adults. Exp. Aging Res.43(1), 21–33 (2017). [DOI] [PubMed] [Google Scholar]
- 85.Jespersen, C. T., Kirkwood, B. C. & Groth, J. Increasing the effectiveness of hearing aid directional microphones. In Seminars Hearing42(03), 224–236 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lin, F. R., Hazzard, W. R. & Blazer, D. G. Priorities for improving hearing health care for adults: a report from the National Academies of Sciences, Engineering, and Medicine. JAMA316(8), 819–820 (2016). [DOI] [PubMed] [Google Scholar]
- 87.Lin, F. R. et al. Hearing intervention versus health education control to reduce cognitive decline in older adults with hearing loss in the USA (ACHIEVE): a multicentre, randomised controlled trial. Lancet402(10404), 786–797 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Carr, S., Pichora-Fuller, M. K., Li, K. Z., Phillips, N. & Campos, J. L. Multisensory, multi-tasking performance of older adults with and without subjective cognitive decline. Multisens. Res.32(8), 797–829 (2019). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analysed during the current study are not publicly available due to the Research Ethics Board at KITE-Toronto Rehabilitation Institute and University of Toronto but are available from the corresponding author on reasonable request.