Abstract
The detection of small molecule analytes (SMAs) is of great significance for food and drug testing, environmental monitoring, and disease diagnosis. However, the performance of commercially available SMA immunoassays is limited by their low sensitivity and specificity due to the competitive format, leaving significant room for improvement. In recent years, the application of noncompetitive immunoassays for the detection of SMAs has become a hot topic, especially with the rapid evolution of antibody development technology. The remarkable development and application of anti-immune complex (anti-IC) reagents targeting antigen-specific antibodies have garnered significant interest from researchers and diagnostic companies, particularly in the field of SMA detection. The discovery and development history of anti-IC antibodies, the advantages and limitations of different anti-IC reagent preparation methods, and the mechanisms of interaction between ICs and anti-IC antibodies are reviewed. A comprehensive overview of the application of anti-IC antibodies in SMAs assay, including pesticide residue detection, mycotoxin detection, and clinical testing, as well as current challenges and potential solutions in noncompetitive immunoassays, is also summarized to provide a reference for the rapid and accurate detection of SMAs.
1. Introduction
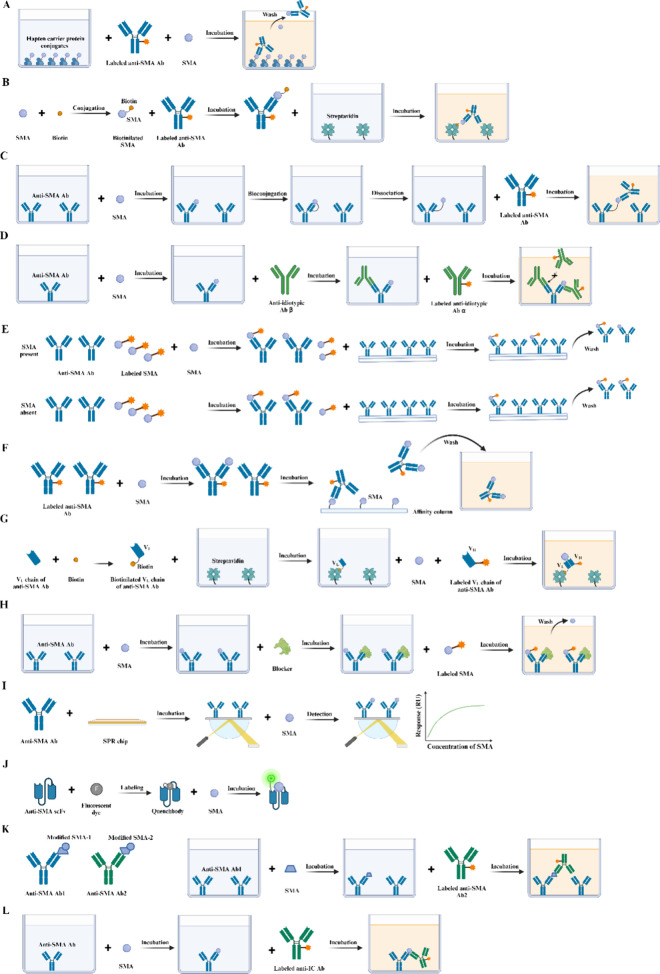
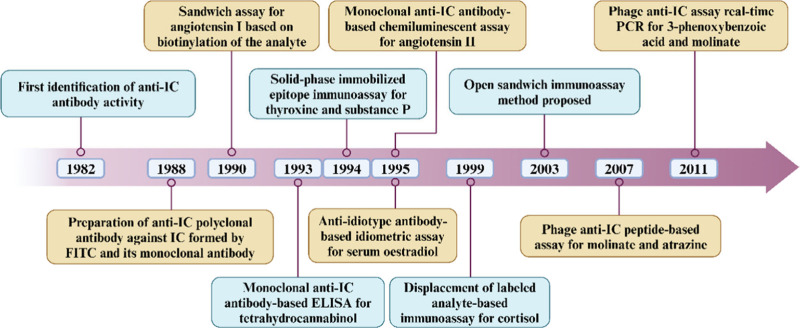
Haptens are low-molecular-weight molecules that, in the absence of other factors, are unable to elicit an immune response. This is primarily due to the absence of the requisite T cell epitopes, which are essential for T cell activation. This critical step is necessary for the stimulation of B cells and subsequent production of antibodies. When conjugated to a larger carrier, typically a protein, the carrier provides the essential T cell epitopes in the form of short peptide sequences that are presented to T cells via major histocompatibility complex (MHC) molecules. This T cell activation is a critical step in the B-cell-mediated immune response, enabling the hapten-carrier complex to trigger a full immune reaction. Strictly speaking, SMAs with a molecular weight of less than 1000 dt (Da) are classified as haptens. However, in a broader context, molecules with a molecular weight exceeding 1000 Da but remaining below 5000 Da can also be regarded as haptens. Quantitative determination of SMAs has widespread applications in agricultural product testing, food safety, environmental monitoring, clinical diagnostics, and biopharmaceuticals. The main involved SMAs include pesticides (e.g., organophosphate esters,1 carbamates,2 pyrethroids3), veterinary drugs (e.g., tetracyclines,4 sulfonamides,5 β-agonists6), mycotoxins (e.g., aflatoxins,7 ochratoxins,8 fumonisins9), and environmental pollutants (e.g., polychlorinated biphenyls, dioxins, polycyclic aromatic hydrocarbons). Additionally, food contaminants (e.g., melamine and bisphenol A), clinical biomarkers (e.g., hormones, drugs of abuse, therapeutic drugs), and pharmaceuticals (e.g., active pharmaceutical ingredients and their metabolites) are also critical targets. Accurate quantification of these SMAs plays a significant role in ensuring safety, regulatory compliance, and health monitoring through various analytical methods. The primary detection techniques include high-performance liquid chromatography (HPLC),10 liquid chromatography-tandem mass spectrometry (LC–MS/MS),11 gas chromatography–mass spectrometry (GC–MS/MS),12 and thin-layer chromatography (TLC).13 Despite their outstanding performance, these methods present several drawbacks such as the requirement for cumbersome sample preparation, low throughput, expensive instrumentation, and limited automation. These limitations collectively hinder their large-scale implementation in routine testing. The use of immunoassay in the detection of SMAs has increased significantly in recent years due to its simplicity of operation and rapid detection speed.14 SMA immunoassays can be classified into two main categories: competitive and noncompetitive. The competitive approach typically involves hapten-carrier protein conjugates and analytes in the sample competing for a limited number of antibody binding sites (Figure 1A). On the basis of this principle, the competition approach has the following disadvantages and limitations: (1) Limited sensitivity: Competitive assays often exhibit relatively low sensitivity due to the inherent nature of their design, where the analyte competes with a labeled counterpart for limited antibody binding sites. This results in detection limits that may not meet the requirements for detecting very low concentrations of analytes. The signal inversely correlates with the analyte concentration, which can lead to inaccuracies at low concentrations, making it difficult to distinguish between positive and negative samples. (2) Single epitope limitation: Competitive assays are often constrained to single-epitope binding, as the format usually requires the analyte to compete with a labeled analogue for a single antibody. This limitation significantly reduces their efficacy in detecting SMAs, which typically possess only one immunologically relevant site. The lack of multiple epitopes in SMAs restricts the application of more sensitive formats, such as sandwich assays, which would otherwise offer improved sensitivity and specificity. (3) Narrow dynamic range and poor precision: The competitive format is known to suffer from a narrower dynamic range and reduced precision at lower concentrations. This leads to lower repeatability of results and limited applicability for analytes present in trace amounts, which is often a critical requirement for environmental or food safety monitoring. (4) Cross-reactivity and specificity issues: Competitive assays often face challenges related to cross-reactivity, particularly when multiple structurally similar molecules are present in the intended samples. The lack of multiple recognition sites can lead to poor specificity, making it difficult to discriminate between structurally related analytes. (5) Longer incubation times: In competitive immunoassays, the time required to reach equilibrium can be prolonged, especially for reactions involving low analyte concentrations. This increases the total assay time, limiting the speed at which the results can be obtained. These limitations underscore the growing interest in noncompetitive formats that offer higher sensitivity, improved dynamic range, and better precision for detecting small molecules. Several methods for SMA noncompetitive immunoassays have been reported, including sandwich-type assay based on biotinylation of a target SMA15 (Figure 1B), solid-phase immobilized epitope immunoassay16 (Figure 1C), anti-idiotype antibody-based idiometric assay17 (Figure 1D), special separation-based immunoassay18 (Figure 1E), flow injection enzyme immunoassays19 (Figure 1F), open sandwich immunoassay20 (Figure 1G), displacement of labeled analyte-based immunoassay21 (Figure 1H), special sensor-based immunoassay22 (Figure 1I), quenchbody-based immunoassay23 (Figure 1J), and direct double antibodies sandwich assay24 (Figure 1K). Table 1 summarizes the ability of various noncompetitive immunoassay methods to overcome the specific limitations associated with competitive immunoassays, including sensitivity, dynamic range, cross-reactivity, and incubation time. The key developments of representative noncompetitive SMA immunoassays are listed in Figure 2.
Figure 1.
Principles of competitive and noncompetitive methods for the determination of SMAs. (A) Competitive approach. (B) Sandwich-type assay based on biotinylation of a target SMA. (C) Solid-phase immobilized epitope immunoassay. (D) Anti-idiotype antibody-based idiometric assay. (E) Special separation-based immunoassay. (F) Flow injection enzyme immunoassays. (G) Open sandwich immunoassay. (H) Displacement of labeled analyte-based immunoassay. (I) Special sensor-based immunoassay. (J) Quenchbody-based immunoassay. (K) Direct double antibody sandwich assay. (L) Anti-IC antibody-based immunoassay.
Table 1. Comparison of Noncompetitive Immunoassays in Overcoming the Limitations of Competitive Immunoassaysa.
| noncompetitive immunoassay | limitations
of competitive immunoassay |
reference | ||||
|---|---|---|---|---|---|---|
| limited sensitivity | single epitope limitation | narrow dynamic range and poor precision | cross-reactivity and specificity issues | longer incubation times | ||
| 1B | + | + | + | ± | – | (15) |
| 1C | + | – | + | + | ± | (16) |
| 1D | + | + | + | ± | ± | (17) |
| 1E | + | – | + | – | + | (18) |
| 1F | + | – | + | ± | + | (19) |
| 1G | + | + | + | ± | ± | (20) |
| 1H | + | – | + | ± | ± | (21) |
| 1I | + | – | + | ± | + | (22) |
| 1J | + | – | ± | ± | + | (23) |
| 1K | – | + | ± | + | ± | (24) |
Symbol: +: surmountable; −: insurmountable; ±: uncertain.
Figure 2.
Timeline of important events in the development of noncompetitive immunoassays for SMAs.
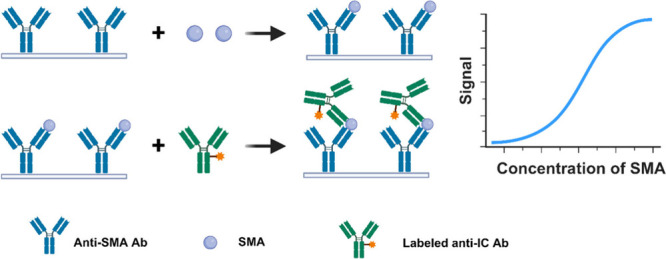
It is evident that the aforementioned noncompetitive methods come with inherent limitations. For instance, the sandwich-type assay based on biotinylation of a target SMA requires the analyte to have reactive groups, involves the use of harsh chemical reagents, and necessitates purification of the modified product, making the process complex and unsuitable for automated detection. Similarly, the solid-phase immobilized epitope immunoassay requires the presence of reactive groups in the analyte for conjugation to the anti-SMA antibody (Ab1) and involves harsh chemical treatments, resulting in complex and laborious procedures. The anti-idiotype antibody-based idiometric assay is hindered by the need to produce α- and β-type anti-idiotype antibodies (Ab2α and Ab2β), limiting its applicability. Moreover, this method lacks high specificity and is dependent on the properties of the anti-idiotype antibodies. Special separation-based immunoassays, flow injection enzyme immunoassays, and sensor-based immunoassays require specific separation devices or analytical equipment, which can be costly and limit their feasibility for routine use. Both the open sandwich immunoassay and quench-body-based immunoassay are homogeneous detection methods, which have limited sensitivity and are prone to matrix interference. The displacement of labeled analytes in immunoassays is essentially a variant of competitive assays, with sensitivity and specificity largely dependent on the characteristics of the displacement reagent. Lastly, the direct double-antibody sandwich assay for SMA faces significant technical challenges in generating high-affinity and high-specificity sandwich antibody pairs, which limits its applicability, with a few reported successful cases. In contrast, the anti-IC antibody- or anti-IC peptide-based assay effectively overcomes many of these limitations. This method offers high versatility across a wide range of analytes, regardless of whether they possess reactive groups, and is readily adaptable to automated detection without requiring harsh chemical treatments. Additionally, it is not prone to matrix interference and achieves both high sensitivity and specificity simultaneously, similar to large-molecule double-antibody sandwich assays. Recently, there has been growing interest in detection methods based on anti-IC antibodies (Figure 1L), with their use becoming increasingly prevalent in related fields.
Early in 1997, Voss et al.25 reviewed the principles and applications of anti-IC antibodies in immunoassay development. The review focused on the unique properties of anti-IC antibodies, including their ability to delay the dissociation rate of ligands from antibodies, thus enhancing the assay sensitivity. It also discussed the differential effects of polyclonal and monoclonal anti-IC antibodies on ligand dissociation rates and their implications for assay development. In recent years, numerous research groups have reviewed noncompetitive immunoassay methods for SMAs,26 consistently highlighting the application of anti-IC antibodies in detection. However, these reviews have generally overlooked the detailed preparation methods of anti-IC reagents and structural information related to their binding sites. Consequently, this review aims to fill these gaps by providing a comprehensive summary of recent advancements in anti-IC reagent development and their applications in SMA detection. The proposed review will encompass a comprehensive examination of the methodologies employed in the preparation of antimetatype antibodies and peptides, with a detailed comparative analysis presented of these techniques. The diverse applications of antimetatype antibodies and peptides in SMA immunoassays will be explored, with a particular emphasis placed on various detection platforms, and the advantages over traditional competitive assays elucidated. Additionally, structural studies concerning the binding sites of antimetatype antibodies will be delved into, providing insights into their molecular interactions. Finally, future trends and potential advancements in research related to antimetatype antibodies will be discussed, offering a forward-looking perspective on this rapidly evolving field.
2. Preparation Methods of Anti-IC Reagents
2.1. Traditional Immunization
The concept of anti-IC antibodies originated from some studies of rheumatoid factors and anti-Id antibodies. A new class of antigenic stimulants has been reported27 wherein novel epitopes are generated following the binding of antibodies to antigens. This type of stimulation led to the production of a new class of antibodies, which do not act via competitive inhibition but rather enhance the antigen–antibody and idiotype–antiidiotype antibody reactions. In their experiments, the researchers immunized CD rats with anti-p-azophenylarsonate (Ars) antibodies and obtained hybridoma 5 Ci and a series of anti-Id antibodies. They unexpectedly discovered that some cell supernatants could increase the binding strength between anti-Ars antibodies and 5 Ci, leading them to name these antibodies “enhancing antibodies”. Further studies have demonstrated that these enhancing hybridomas secrete IgM subtype antibodies, which can inhibit the dissociation of Id antibodies from anti-Id antibodies. However, this enhancement effect is only evident with the intact 5 Ci antibody and not with its two antigen-binding fragments Fab or F(ab′)2. They hypothesize that these IgM antibodies recognize epitopes on the Fc region of the antibody that are exposed due to antigen binding.
Following this work, Brown et al.28 also discovered that the combination with the antigen led to the emergence of new epitope sites on IgG antibody molecules. Following immunization of rabbits with human IgG-antigen ICs, antibodies against these new epitopes were detected in rabbit serum. However, when similar immunization was performed in mice, the proportion of such antibodies was very low, regardless of whether the mice were DBA/2 or C57BL/6 mice, which are generally tolerant of human IgG, or BALB/c mice, which are less tolerant. Despite this, Brown et al. succeeded in obtaining an IgM antibody, CE9, from BALB/c mice, which exhibited a reaction intensity to the human IgG-antigen that was 100 to 1,000 times stronger than that of human IgG. Similar to the study by Nemazee et al.,27 Brown also demonstrated that the binding of CE9 depends on the intact human IgG rather than F(ab′)2 fragments. The researchers compared this mAb (monoclonal antibody) to the rheumatoid factor, suggesting that exposure of the new epitope may result from conformational changes in the IgG molecule when both Fab fragments are involved in antigen binding. In another study on anti-Id antibodies, Sawutz et al.29 immunized rabbits with the β-adrenergic receptor antagonist alprenolol and obtained mAb 5B7. In addition to acquiring some 5B7 anti- idiotype serum, they discovered that serum from one rabbit (R9) could enhance the binding of [125I]iodo-cyanopindolol (ICYP) to the 5B7 antibody. ICYP and alprenolol are two small homologous molecules and can be considered as haptens in immunology. They found that the presence of R9 decreased the dissociation constant of ICYP to 0.3 from 20 nmol/L. The Fab fragments of 5B7 also exhibited similar effects, and R9 did not bind to ICYP in the absence of 5B7. These observations indicate that the enhancement effect of R9 serum is not due to binding with the Fc region of 5B7, but rather results from the formation of a new binding site through interaction with the Fab region.
Following these experiments, Voss et al.30 presented another example of anti-IC antibodies. The research team aimed to develop murine polyclonal antibodies against ICs formed by the covalent binding of high-affinity murine IgG2a mAb Fab with fluorescein. Unlike previous teams that accidentally discovered anti-IC antibodies, the Voss team was the first to develop antibodies specifically targeting ICs. Their strategy for producing anti-IC antibodies includes several key points: First, they preferred to use covalently conjugated ICs rather than reversible ICs for immunization. Second, they ensured that the antigen–antibody affinity was sufficiently high (2–3 × 1010 M–1) to maximize the intermolecular forces between the antigen and antibody. Third, nonspecific covalently bound hapten-antibody byproducts were specifically removed during the immunogen preparation process to focus the immune response on specific ICs. Fourth, they believed that allogeneic immunization would minimize the induction of antiallotypic and anti-isotypic antibodies. As a result, they successfully produced murine polyclonal ascitic fluid that did not recognize fluorescein or nonliganded antifluorescein antibodies but had high specificity for the fluorescein Fab fragments bound to the antigen. The newly exposed antigenic determinants, upon binding of antifluorescein antibodies to fluorescein, were termed “metatypes”. Antibodies recognizing these new determinants were named antimetatype antibodies. Further studies on the binding mechanism of antimetatype antibodies revealed that polyclonal antimetatype antibodies formed a 249 kDa complex around the antifluorescein antibody binding site, preventing the dissociation of fluorescein from the Ab1 binding site.31
On the basis of the aforementioned research, several research groups have successfully developed anti-IC antibodies against the SMA-Ab1 IC through immunization. These include THC,32 angiotensin II,33 digoxin,34 microcystin-LR,35 and ursodeoxycholic acid 7-N-acetylglucosaminide (UDCA 7-NAG).36
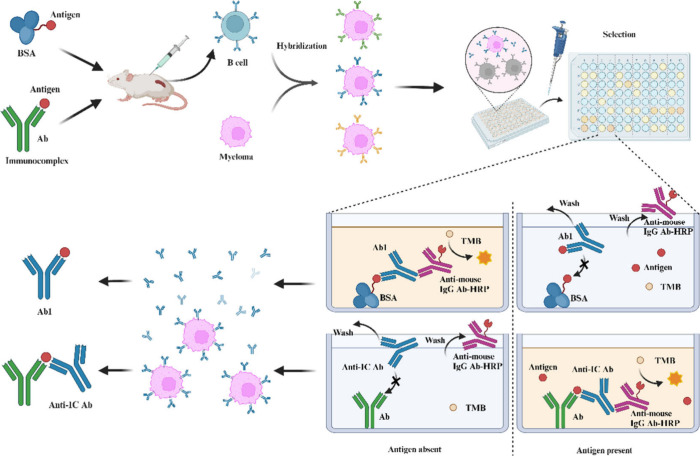
Conventional animal immunization can be conducted using species that are either homologous or heterologous to the source of Ab1. If Ab1 is a murine mAb, then it can be directly incubated with the corresponding SMA to form an IC, which then serves as the immunogen for immunization of mice (Figure 3). This immunization method helps to avoid the production of anti-isotype antibodies and antiallotype antibodies. Ab1 can be conjugated to a heterologous protein to enhance its immunogenicity, such as keyhole limpet hemocyanin (KLH) or bovine intestinal alkaline phosphatase. To prevent the dissociation of the IC after injection into the organism, the SMA can be chemically modified to introduce a reactive group. Once the SMA has bound to Ab1, it can form a stable covalent bond with amino acid residues near the binding site. As an example, Kobayashi et al.36 derivatized the free carboxyl group on the UDCA 7-NAG to form an N-succinimidyl ester. This ester was first incubated with Ab1 at pH 5.0 to form an IC. Subsequently, the pH was adjusted to 7–8 to restore the reactivity of the N-succinimidyl ester with ε-amino groups, allowing it to form an amide bond with lysine residues near the binding site. Despite the chemical cross-linking between the SMA and Ab1, a significant portion of the antibodies produced were antiidiotypic antibodies against Ab1. This indicates that a certain proportion of IC still dissociates within the body. If the SMA itself possessed reactive groups, then chemical modification can be omitted, and it can be directly conjugated to Ab1 using a cross-linking agent. For instance, Natalia et al.37 conjugated a SMA peptide and Ab1 directly using 1-ethyl-3-(3-(dimethylamino)propyl)carbodiimide (EDC), and the resulting conjugates were used as the immunogen. The precondition for coupling SMA and Ab1 with a cross-linking agent is the presence of at least one reactive group near the site of contact between the two parts. For some SMAs that do not contain a reactive group, a suitable linking arm can be introduced at a specific site, thus enabling the two to covalently couple into an IC. The site where the linking arm is introduced should not interfere with the binding of SMA to Ab1 and should have a certain degree of flexibility.
Figure 3.
Diagram of the preparation of Ab1 and anti-IC antibody by the hybridoma technique.
When an IC formed by conjugating a hapten with a murine mAb is used as an immunogen in rabbits or other animals, it includes anti-IC antibodies, Ab1s, and anti-idiotype antibodies against the murine mAb. To isolate anti-IC antibodies that specifically recognize the IC, the antiserum must be absorbed with solid-phase carriers conjugated with the hapten and murine mAb, respectively.
In our team’s development of noncompetitive assay reagents for 25-hydroxyvitamin D, estradiol (E2), and aldosterone, we attempted to prepare anti-IC antibodies targeting the SMA-Ab1 IC by immunizing mice. Our research revealed that when Ab1 is a murine antibody, the immunization effect is poor when it is incubated with the SMA. Better immunization results were obtained using nonmurine antibodies incubated with the SMA. When emulsified with Freund’s adjuvant, the IC formed by Ab1 and the SMA may dissociate under vigorous emulsification conditions, resulting in a low positive clonal rate of antibodies targeting new epitopes of the IC during subsequent screening. Another reason for the low positive rate could be that the conformational change of the IC upon antibody binding is minimal, with most of the SMA embedded within the antibody, making it difficult to form new effective epitopes.38 Thus, researchers are increasingly using phage display technology as an alternative to traditional immunization methods for the production of anti-IC antibodies.
2.2. Phage Displayed Library
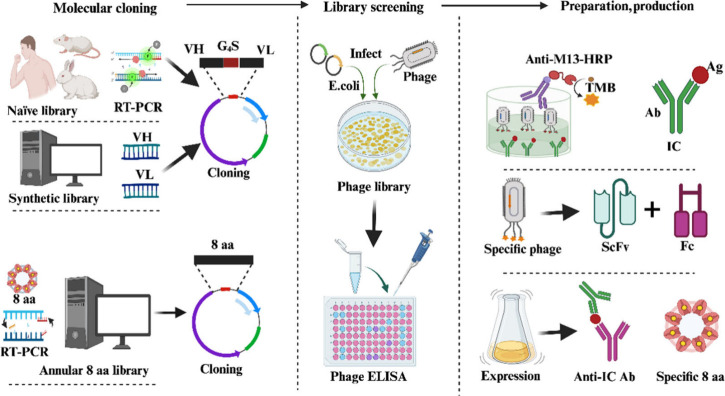
Phage display technology is an essential tool in antibody development, offering a robust, cost-effective, and straightforward approach for discovering antibodies against a variety of antigens.39 Antibody library methods include immunized libraries, naïve libraries, and synthetic libraries. The anti-IC antibodies produced can be in various forms, such as Fab fragments, single-chain variable fragments (scFv), VHH (variable domain of heavy chain),40 and peptides.41 The sources, diversity, and library capacity of antibody libraries are directly related to the affinity of the screened antibodies. Synthetic and naive libraries rely more on diversity, as their function is to serve as universal libraries, wherein a single library can be used for multiple projects. Immune libraries require smaller capacities as they have higher titers of antibodies specific to particular antigens.42 The naive antibody library integrates naïve immunoglobulin gene pools from multiple donors such as rabbits or humans. These libraries are constructed by separately cloning genes encoding the variable domains of the heavy chain (VH) and light chain (VL) from B cells. Random rearrangement of the VH and VL domains introduces additional diversity. Naïve antibody libraries are used to screen for antibodies that are difficult to obtain through immunization such as anti-IC antibodies, antitoxins, and autoantibodies. Human naive antibody libraries serve as crucial sources for selecting therapeutic human-derived antibodies.
Through immunization with specific antigens, immune libraries can be constructed using donor species, such as mice, rabbits, camels, chickens, and sheep. In theory, the choice of donor species is limited only by the availability of sufficient antibody sequence data to design specific primers for amplifying antibody libraries. However, practical considerations also include ethical restrictions. Immune libraries are typically constructed from genes of B cells from the mouse spleen and peripheral blood. Similar to naive libraries, the original pairing of VH and VL is lost during the process of separately amplifying the light and heavy chains before their recombination. This represents a disadvantage of libraries compared to hybridoma technology, which retains mature VH and VL pairs with preserved affinity (Figure 4).
Figure 4.
Workflow for preparation of anti-IC antibodies based on different phage libraries.
For recombinant antibodies, after specific variable domains are paired with complementary variable domain libraries, the optimal combination of VH and VL can be identified, thereby achieving a higher affinity. This approach is known as “chain-shuffling”.43 Synthetic libraries are generated through design and can be based on naive libraries to create partially engineered semisynthetic libraries, or they can be constructed de novo. Additionally, synthetic libraries can be developed by increasing the diversity of germline V gene segments and CDRs.44 During the design process, potential targets can be considered; for example, adjusting the length of CDR loops can help establish synthetic libraries tailored for proteins or small molecules.45
Additionally, chemically synthesized anti-IC peptides can serve as a substitute for phage-displayed anti-IC peptides. The primary distinction between synthetic peptides and phage-displayed peptides lies in their methods of preparation and the practical limitations they present in immunoassays. Phage-displayed peptides are presented on the surface of bacteriophages, which significantly increase their size and require additional steps for detection. In contrast, synthetic peptides are smaller and more easily labeled with detection elements such as fluorescein isothiocyanate (FITC), simplifying the assay process and accelerating the detection speed. Moreover, synthetic peptides eliminate the biological risks associated with phage particles.
The improvement in the sensitivity of the noncompetitive method compared to the competitive method is primarily determined by the affinity of the anti-IC peptide. In some cases, however, the competitive method is even more sensitive than the noncompetitive method. For example, in a study conducted by Chen et al.,46 the sensitivity of competitive and noncompetitive fluorescence immunoassays (FIAs) for the detection of benzothiostrobin was compared. The competitive FIA demonstrated an IC50 of 16.8 ng/mL with a detection range of 1.0–759.9 ng/mL, whereas the noncompetitive FIA exhibited an SC50 of 93.4 ng/mL and a detection range of 5.9–788.2 ng/mL. This can be attributed to the fact that in the competitive format, the lower affinity of the labeled mimetic peptide allows the analyte to compete more effectively with the immobilized antibody, thus enhancing sensitivity. In contrast, the noncompetitive method exhibited lower sensitivity due to insufficient affinity between the labeled peptide and the IC. To address this, Chen et al.47 used lysine as a scaffold to synthesize monomer, dimer, and tetramer dendrimer-like peptides, enhancing the valency of the tracers and improving their binding affinity for ICs. The affinity of the tracers increased significantly with higher valency, with tetramer tracers demonstrating a 10-fold greater affinity than monomer tracers. This multivalency effect leverages multiple binding sites to enhance overall binding strength and specificity, thereby improving sensitivity and the signal-to-noise ratio in fluorescence polarization immunoassays. Phage-displayed peptides tend to outperform synthetic peptides for two main reasons. First, the phage display technique facilitates the maintenance of the peptide’s specific conformation, which is crucial for binding. The structural integrity provided by the phage coat proteins ensures that the peptide adopts the necessary conformation to interact effectively with its target. Second, synthetic monovalent peptides often possess poorer binding affinity than their phage-displayed counterparts due to the absence of the multivalent binding sites naturally presented on the phage surface.
2.3. Autonomously Diversifying Library
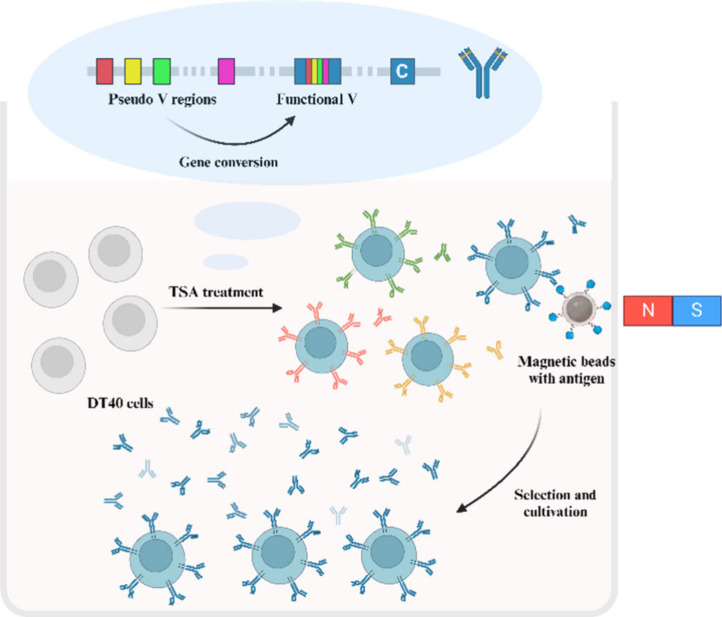
The autonomously diversifying library (ADLib) is another system for antibody production based on somatic hypermutation. This method employs chicken bursal lymphoma cells (DT40) and uses trichostatin A (TSA) to inhibit the histone deacetylase activity of DT40, promoting histone acetylation and causing high-frequency recombination of immunoglobulin genes. The DT40 cell line offers several advantages: (1) It is an immortalized chicken B-cell line that proliferates rapidly, with a population doubling time of approximately 7–8 h; (2) It is easy to manipulate; (3) It has a stable karyotype; (4) It can produce both secretory and membrane-bound IgM antibodies; (5) The functional immunoglobulin (Ig) locus undergoes low-level, continuous gene conversion; and (6) It allows gene modification via high-efficiency targeted homologous recombination. For these reasons, DT40 cells provide an excellent model for studying the role of histone acetylation in gene conversion in higher eukaryotes. DT40 cells can express membrane-bound IgM, making them a potential system for B-cell receptor (BCR) display and antibody production. However, the gene conversion frequency at the DT40 Ig locus is not high, with only a small proportion of cells undergoing gene conversion after several weeks of passage. Therefore, it is necessary to significantly enhance the Ig gene conversion. TSA-mediated histone acetylation leads to the display of a membrane IgM library on the cell surface. Antigen-specific cells are then sorted from the library using antigen-coated magnetic beads, and cultured to produce secretory IgM antibodies,48 as illustrated in Figure 5.
Figure 5.
Principle of ADLib for antibody production.
Kazuya et al.49 used the ICs formed by E2 with a mouse mAb and 25-hydroxyvitamin D with a sheep mAb as screening targets, selecting chicken monoclonal antibodies against these ICs using ADLib. Hidetaka et al.50 further developed ADLib by replacing the endogenous chicken immunoglobulin genes with human gene segments, constructing an ADLib expressing intact human IgG. From these libraries, human antibodies against vascular endothelial growth factor A (VEGF-A) and tumor necrosis factor α (TNF-α) were selected. Positive clones A033 and C018, specific for VEGF-A, were further subcloned without TSA induction for continued culture and affinity maturation. Surface plasmon resonance measurements showed that the KD values improved from 2.14 × 10–9 M and 1.61 × 10–9 M in the parent clones to 4.91 × 10–11 M and 1.49 × 10–11 M, respectively.
ADLib enables the rapid and convenient generation of specific IgM antibodies against various antigens without the need for animal immunization. This method is particularly useful for screening antigens that are conserved, self-antigens, or toxins, which can induce immune tolerance due to their weak or strong antigenicity, making it challenging to obtain high-affinity antibodies. Unlike phage display libraries, ADLib does not require DNA recombination, and high-density cultures can yield hundreds of micrograms of antibodies per milliliter. The DT40 cell line’s homologous gene targeting capability also facilitates gene insertion and knockout in positive clones. Constructing a humanized ADLib can further increase the library diversity. However, the affinity of antibodies selected by ADLib typically ranges from 10–7 to 10–8 M, necessitating further affinity maturation to achieve higher affinities. Additionally, IgM antibodies, being pentameric, are less stable and more difficult to purify compared to IgG.
Table 2 summarizes the comparison of different methods for preparing anti-IC antibodies.
Table 2. Comparison of Different Preparation Methods of Anti-IC Antibodies.
| hybridoma technology | nanoantibody (immune library) | nanoantibody (naive library) | single chain antibody fragment (synthetic library) | autonomously diversifying library | |
|---|---|---|---|---|---|
| antibody preparation cycle | 4–6 months | 3–5 months | 2–4 months | 2–4 months | 1–2 months |
| screening antibody form | intact mAb | VHH | VHH | ScFv | intact mAb |
| single antibody screening throughput | 10 000 to 30 000 cell clones | 108–109 clone sequences | 1010–1012 clone sequences | 1011–1013 clone sequences | 105–107 cell clones |
| antibody affinity | nM–pM | nM–pM | μM–nM | nM–pM | nM–pM |
| labor intensities | extreme | high | moderate | moderate | low |
| advantages | mature technology, low R&D cost | antibody affinity maturation, high affinity and high specific resistance | no immunizations required, large storage capacity, against all natural antigens | CDR3 sequence random, a variety of lengths, simulated antibody affinity maturation | quick screening, high affinity and high specific antibodies, high library diversity |
| limitations | cell lines are unstable, low efficiency and long cycle | storage capacity is relatively small, influenced by antigenic characteristics, different antigens need to be built separately | low specificity, low screening efficiency, lack of diversity, affinity immaturity | light and heavy chain unnatural pairing, high library costs | high R&D cost, technically complex |
3. Interaction between Anti-IC Antibodies and SMA-Ab1
Like other proteins, antibodies exist in a range of conformational states with different energies in solution, dynamically interchanging between these states.51 The idiotypic state (Id) refers to the dynamics of the antibody’s active site before antigen binding, while the metatypic state represents the conformational changes induced in the active site by antigen binding.
Many studies confirm that the binding of a SMA to an antibody’s active site induces conformational changes in the antibody. Németh et al.52 investigated the interaction between FITC (isomer I) as a hapten and specific monoclonal IgG1 using various spectroscopic methods. The results showed that upon FITC binding the hydration level of the specific binding region between the antibody hypervariable region and FITC significantly decreased, altering molecular movements. This change in the microenvironment affected the functions of both the antibody and the hapten. Furthermore, binding of the hapten induces conformational changes not only in the CDR region but also in the constant region of the antibody.53
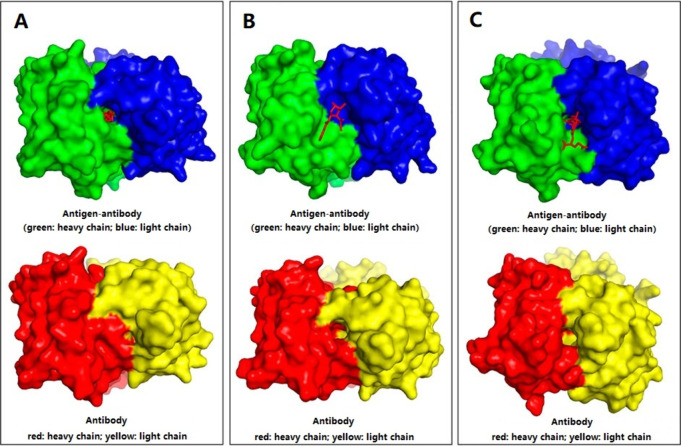
In the Fab fragment of the antibody, each peptide chain consists of approximately 230 amino acids, with the six CDRs comprising about 70 amino acid residues, forming a continuous surface of approximately 2800 Å2. Amino acid chains in the active centers of antibodies, particularly within the complementarity-determining regions (CDRs), play a crucial role in antigen binding. Antibodies consist of variable and constant domains with antigen-binding specificity localized within the variable domains (VH and VL) of the Fab fragments. Each variable domain comprises three hypervariable loops, known as complementarity-determining regions (CDRs) 1, 2, and 3. These loops collectively form the paratope and directly interact with the antigen. CDRs, especially CDR3, are responsible for modulating antibody–antigen interactions by adjusting their structure upon antigen binding, following mechanisms such as induced fit or isomerism. CDR3, being the most flexible, frequently undergoes significant conformational changes during antigen engagement, leading to the formation or closure of binding pockets depending on the antigen’s structural requirements. This structural adaptability allows the immune system to recognize a wide range of antigens. The classification of surface binding behaviors reflects the manner in which CDRs accommodate SMAs. Qaraghuli et al.54 compared the structures of SMA-Ab1 ICs with free antibodies, identifying three trends in the changes upon SMA binding to Ab1: (1) a pocket forms to accommodate the SMA; (2) the pocket disappears upon SMA binding; and (3) the pocket remains unchanged or nearly unchanged, as illustrated in Figure 6. The dynamics of these binding sites highlight the essential role of CDR loops, particularly CDR3, in adapting the antibody’s binding site to various antigenic structures. The structural flexibility and movement of CDRs, particularly CDR3, are key to recognizing how binding pockets form and adjust, facilitating efficient antigen recognition and binding.
Figure 6.
Binding sites surfaces. The binding surfaces can be grouped into three categories. (A)There is a pocket binding site on the antigen- antibody complex and not on the antibody-free counterparts. (B) There is a pocket binding site on the antibody-free structure and not on the antigen- antibody complex. (C)There is no (or only slight) change in the binding sites. The heavy and light chains were colored as denoted in the figure,and the SMA antigen is shown as a red line drawing. Reprinted with permission from Front. Mol. Biosci., 8, 633526. Copyright, 2021, Al Qaraghuli, Kubiak-Ossowska, Ferro and Mulheran.54
After the SMA binds to Ab1, the anti-IC antibody recognizes a conformational epitope composed of the SMA and the variable region of Ab1, rather than a linear sequence of amino acids. The binding of the anti-IC antibody to the IC can significantly reduce the first-order dissociation rate of the SMA, delaying it by several-fold or even up to hundreds or thousands of times. The mechanism by which the anti-IC antibody delays the SMA dissociation rate may be related to a significant reduction in the vibrational dynamics of the Ab1 variable region.
Niemi et al.55 explored the molecular recognition of (−)-Δ9-tetrahydrocannabinol (THC) by the anti-THC T3 Fab fragment, a mAb fragment. The crystal structures of T3 Fab in both its free form and complexed with THC were resolved, providing insights into the specific interactions that facilitate THC binding. The THC binding site on the T3 Fab is a narrow cavity between the variable domains of the heavy and light chains with the n-pentyl group of THC protruding deeply into this interface and the C10 monoterpene moiety partially exposed to the solvent. This binding involves significant hydrophobic interactions, predominantly with aliphatic residues, and is stabilized by several hydrogen bonds. Notably, Ser52H and Arg53H of the T3 Fab form hydrogen bonds with THC metabolites, contributing to higher affinity binding compared to THC itself. These structural details are crucial for understanding the enhanced specificity and sensitivity of the developed one-step, homogeneous fluorescence resonance energy transfer (FRET)-based immunoassay, which can detect THC at a limit of 20 ng/mL in saliva samples. The study’s findings highlight the importance of specific amino acid interactions and structural conformations in the recognition and binding of THC by antibody fragments.
Fu et al.56 presented the development of a sensitive noncompetitive immunoassay for the detection of ethyl carbamate (EC) in wine samples using IC binding peptides. The mechanism of binding of these peptides to the EC-antibody IC was explored through molecular modeling and computer simulations. It was found that the xanthydrol group of xanthyl ethyl carbamate and the Asn-32 and Asn-92 residues of the antibody light chain were primarily responsible for the interaction. The peptides exhibited high binding affinity due to strong hydrophobic interactions with the xanthydrol group and multiple hydrogen bonds with the antibody residues. This structural insight facilitated the development of a noncompetitive immunoassay with a limit of detection (LoD) of 0.54 ng/mL, which was significantly more sensitive than traditional competitive assays. The assay’s results were validated with ultraperformance liquid chromatography-quadrupole/orbitrap high-resolution mass spectrometry (UPLC-Q-Orbitrap HRMS), confirming its reliability and specificity for EC detection in wine samples.
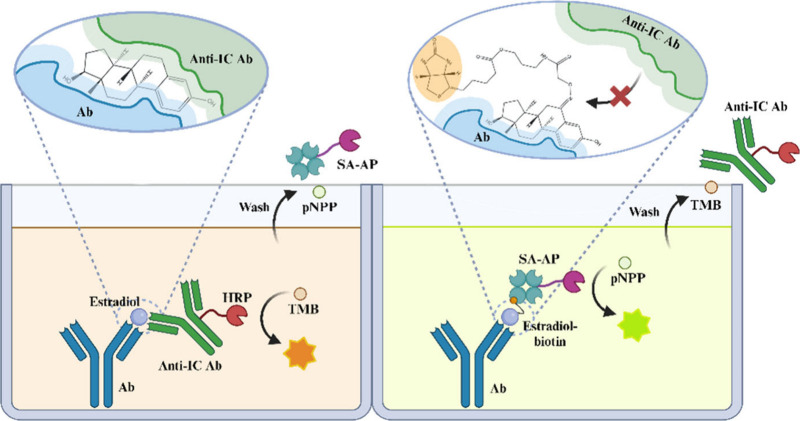
The increased specificity of the anti-IC reagent based noncompetitive assay lies in the specific interaction between the anti-IC antibody and the SMA-bound Ab1. The binding site of the anti-IC antibody is distinct in that it recognizes an epitope composed of both the hapten and the variable region of the Ab1 within the IC. This specificity is crucial for the sandwich immunoassay’s high sensitivity and specificity, which surpass those of traditional competitive immunoassays. For instance, Omi et al.49 developed a sensitive and specific noncompetitive assay for E2. The anti-IC antibody developed for the E2 sandwich immunoassay binds to the E2-mAb IC and shows minimal reaction when E2 is replaced with biotinylated E2 at the C6 position. This indicates that the anti-IC antibody recognizes part of E2 harboring the C6 position within the E2-mAb IC (Figure 7). Similarly, the antibody for the 25(OH)D sandwich assay showed weak reactivity with biotinylated 25(OH)D3, confirming that the anti-IC antibody specifically recognizes the region around the C3 position of 25(OH)D3 in the IC. These interactions are not merely due to the hapten alone but are significantly influenced by the conformational changes induced in Ab1 upon hapten binding. This dual recognition mechanism allows the sandwich immunoassay to achieve higher specificity and sensitivity, effectively improving the detection of small molecules like hormones, vitamins, drugs, and toxins in clinical samples. In summary, the structural recognition by anti-IC antibodies in hapten-antibody complexes is a sophisticated interplay between the hapten, the Ab1’s variable region, and the conformational changes induced upon binding. This intricate binding mechanism forms the basis of the superior performance of noncompetitive sandwich immunoassays over traditional competitive formats.
Figure 7.
Recognition of E2 and biotinylated E2 by anti-IC antibodies.
In another study, Solin et al. investigated the interactions between anti-IC antibodies and the THC-Ab1 IC. Specifically, the research focused on the immobilization of anti-IC antibodies onto a nanocellulose substrate via EDC/NHS (N-hydroxysuccinimide) coupling chemistry and how this affected the binding efficiency of the THC-Ab1 IC. They found that covalently linked biointerfaces, where anti-IC antibodies were chemically conjugated to nanocellulose, exhibited significantly stronger and more stable binding to the THC-Ab1 IC compared to physically adsorbed antibodies, which often showed loose attachment and diminished bioactivity. Additionally, the presence of both THC and anti-THC Fab antibody fragments was necessary for effective binding to the biointerface. The chemical conjugation not only improved antibody retention on the surface but also preserved bioactivity, leading to a more efficient THC detection mechanism. These findings highlight the advantages of using a nanocellulose-based anchor for the selective detection of THC, demonstrating superior detection sensitivity due to the robust immobilization of antibodies.
4. SMA Immunoassay Based on Anti-IC Reagents
To date, the noncompetitive methods developed using anti-IC reagents have been extensively employed in agricultural and food testing. The anti-IC-based noncompetitive immunoassay offers several key analytical advantages, as outlined below: (1) Higher sensitivity: The sensitivity of the anti-IC-based noncompetitive method can be several-fold to hundreds-fold higher than that of the competitive assay developed using the same Ab1, depending on the specific properties of the anti-IC antibody. (2) Improved specificity: In competitive assays, the binding interaction involves only a single antibody, rendering the assay more vulnerable to interference from analyte analogs and matrix effects (i.e., nonspecific interactions with sample components). In contrast, the anti-IC-based noncompetitive method uses two antibodies to enhance specificity and enables additional washing steps during incubation to reduce interference from analyte analogs. (3) Reduced sample volume requirement: Compared with the reagent-limited conditions in competitive assays, the anti-IC-based noncompetitive method is performed in a reagent-excess format, allowing for smaller sample volumes to be used. This is particularly advantageous in applications with limited sample availability as smaller volumes also reduce matrix effects. (4) Shorter incubation time: The reagent-excess format also allows for shorter incubation times, further enhancing assay efficiency. (5) Class-specific analyte detection: By rationally engineering Ab1 and anti-IC antibodies, it is possible to develop assays with satisfied affinity and specificity for a class of analytes, enabling the detection of structurally related compounds such as toxins. (6) Multiplex detection: By the combination of distinct Ab1 and anti-IC antibodies that specifically target different, structurally unrelated analytes, a multiplex assay can be developed to simultaneously detect multiple analytes in a single sample. This can be accomplished by labeling anti-IC antibodies with unique fluorophores, allowing for differentiation based on fluorescence emission. Table 3 summarizes the application of anti-IC reagents for the development of noncompetitive immunoassays for pesticides, veterinary drugs, mycotoxins, environmental pollutants, et al.
Table 3. Anti-IC Reagents for SMA Immunoassays.
| analyte | technology/source | assay principle | LoD | specificity | reference |
|---|---|---|---|---|---|
| microcystin and nodularin | ScFv, synthetic antibody library | TR-FRET (time-resolved Förster resonance energy transfer) immunoassay | ∼0.3 μg/L (microcystin) | broad specificity across different microcystin variants and nodularin-R | (57) |
| microcystin and nodularin | ScFv, synthetic antibody library | time-resolved immunofluorometry-based assay | ∼0.026 μg/L (1h assay time) | broad specificity across 11 commonly occurring hepatotoxins | (58) |
| ∼0.1 μg/L (10 min assay time) | |||||
| microcystin and nodularin | ScFv, synthetic antibody library | lateral-flow immunoassay | 0.7 μg/L | specificity for nine different microcystin variants and all toxin mixtures | (59) |
| microcystin and nodularin | ScFv, synthetic antibody library | ELISA | 0.2 μg/L (microcystin-LR) | cross-reactivities of tested analogues range from 30 to 127% relative to microcystin -LR | (60) |
| nodularin | ScFv, synthetic antibody phage library | time-resolved fluorometry-based assay | 0.03 μg/L (Nodularin-R) | minor cross-reactivity with nine different microcystin analogues | (61) |
| morphine | ScFv, naïve human phage display library | paper-based lateral-flow assay | 1 ng/mL, which is more sensitive compared to the competitive assay | (62) | |
| morphine | ScFv, naive scFv phage display library | FRET (fluorescence resonance energy transfer) assay | 5 ng/mL | with no cross-reactivity to codeine or heroin | (63) |
| HT-2 toxin | ScFv, naive human phage display library | ELISA | 13 μg/kg (wheat) | without cross-reactivity with structurally related fusarium mycotoxin T-2 | (64) |
| 4 μg/kg (barley) | |||||
| 16 μg/kg (oats), which is over 10 times more sensitive compared to the competitive assay | |||||
| HT-2 toxin | ScFv, naïve human phage display library | fluorescence immunoassay | 0.24 ng/mL | no cross-reactivity with other common toxins produced by Fusarium species | (65) |
| (−)-△9-tetrahydrocannabinol | ScFv, naïve human phage display library | homogeneous FRET immunoassay | 20 ng/mL | with no cross-reactivity with THC–COOH and 11-OH–THC | (55) |
| β-lactams | mouse mAbs and rabbit pAbs, immunization | ELISA | 1.65 ng/mL (ceftriaxone) | (66) | |
| gibberellins | peptide, phage display random peptide library | ELISA | ∼30 pg/reaction | without cross-reactivity with gibberellin1 | (67) |
| cannabis | Fab, naïve human phage display library | SPR-based assay | (68) | ||
| benzothiostrobin | synthetic peptide, isolated from a random peptide library | fluorescence polarization immunoassays | 9.27 ± 1.36 ng/mL | cross-reactivities with five analogs <0.1%, higher selectivity than the competitive assay | (47) |
| benzothiostrobin | synthetic peptide isolated from a cyclic 8-amino-acid random peptides library | fluorescence immunoassays | 5.9 ng/mL | cross-reactivities with tested analogues <0.1%, similar to the competitive assay | (46) |
| benzothiostrobin | phage-displayed peptide isolated from a cyclic 8-amino-acid random peptides library | ELISA | higher selectivity compared to the competitive assay | (69) | |
| benzothiostrobin | peptide, phage display random peptide libraries | Ratiometric signal immunoassay | 0.17 ng/mL, showed higher sensitivity | no cross-reactivities with four benzothiostrobin analogs | (70) |
| molinate | phage-displayed peptide isolated from a peptide library | CdS nanocrystals-phage-based electrochemical immunosensor | 0.034 ± 0.004 ng/mL | (71) | |
| molinate and clomazone | peptide, phage polypeptide library | ELISA | 1.2 ng/mL (molinate); 3.2 ng/mL (clomazone) up to 18-fold better than that of the competitive ELISA set up with the same antibody | only minor cross-reactivity with closely related thiocarbamate compounds | (72) |
| molinate and atrazine | peptides (10aa), phage display peptide library | PHAIA (Phage Anti-IC Assay) | 0.73 ng/mL (molinate), 0.016 ng/mL (atrazine) | similar to competitive assays | (73) |
| atrazine | peptide (10aa), phage display peptide library | PHAIEI | 0.2 pg/mL, 200-fold better than the LoD of the competitive ELISA using the same antibody | (74) | |
| atrazine | peptide (10aa), Phage polypeptide library | nanoAlphaLisa | 0.3 ng/mL, the sensitivity was similar to that obtained by competitive ELISA | cross-reactivities with other compounds of the triazine family were very similar to those obtained in conventional ELISA | (75) |
| organophosphorus pesticides (OPs) | peptides (8aa), isolated from a phage displayed library | magnetic-phage anti-immunocomplex assay (MPHAIA) | 4.07–14.19 ng/mL for 12 pesticides, approximately three times lower than that of the competitive method | broad specificities to the tested 12 pesticides | (76) |
| brominated diphenyl ether 47(BDE47) | peptide (11aa), isolated from a random peptide librar | PHAIA | the same as the heterologous ELISA | in agreement with the competitive assay | (77) |
| ethyl carbamate | peptide (9aa), isolated from a cyclic 7-amino-acid random peptides library | noncompetitive phage ELISA | 5.4 ng/mL, improved by 17-fold compared with the competitive assay | similar to that of mAb-based competitive immunoassay | (56) |
| imidacloprid (IMI) | Peptides (8–10aa), phage display cyclic peptide libraries | noncompetitive phage enzyme-linked immunosorbent assays (P-ELISAs) | 0.15 ng/mL, the sensitivity of noncompetitive P-ELISA was slightly higher than that of the competitive P-ELISA | noncompetitive P-ELISA significantly reduces the cross-reactivities of thianidam, thiacloprid, nittizpiram, and acetamidine | (78) |
| 3-phenoxybenzoic acid | peptide, phage 8 peptide librarys | sandwich electrochemical impedance spectroscopy assay (EIS) | 0.74 mg/mL, more sensitive than direct EIS immunoassay | (79) | |
| 3-phenoxybenzoic acid | peptide, phage 8 peptide librarys | phage anti-immunocomplex assay | 0.2 ng/mL, considerably improved assay sensitivity | (80) | |
| 3-phenoxybenzoic acid (3-PBA)and molinate | peptides (10aa), phage polypeptide library | phage Anti-Immunocomplex Real-Time PCR (PHAIA–PCR) | 20 pg/mL (3-PBA), which is 10-fold lower than that of the conventional PHAIA, and 0.2 ng/mL (molinate) | (81) | |
| estradiol (E2) and 25-hydroxyvitamin D [25(OH)D] | chicken antibodies, ADLib | ELISA for E2 and CLEIA for 25(OH)D | 3.13 pg/mL (E2), 2.1 ng/mL (25(OH)D) | the cross-reactivity with immunoreactive derivatives was effectively improved compared with the competitive assay | (49) |
| aldosterone | chicken antibodies, ADLib | CLEIA (two-site sandwich chemiluminescent enzyme immunoassays) | 0.1 ng/dL (aldosterone) | no significant cross-reactivity with corticosterone, cortisol, dexamethasone, spironolactone, progesterone, or 18-hydroxycorticosterone. | (82) |
| brain natriuretic peptide (BNP) | mAbs, hybridoma technology | single-epitope sandwich immunofluorescence assay | 0.4 ng/L | negligible cross-reactivity with atrial natriuretic peptide (ANP) and no cross-reactivity with C-type natriuretic peptide (CNP) | (37) |
| 25-Hydroxyvitamin D3 (25D3) and 9-cis-retinoic acid (9cRA) | scFv, phage display library | ELISA | 4 nmol (25D3), 0.50 nmol (9cRA) | the 9cRA assay was less specific, the stronger cross-reactivity with PP | (83) |
| clomazone | peptide(10aa), phage display peptide library | PHAIA (phage anti-immunocomplex assay) | 0.4 ng/mL, 10 times higher than that of the competitive format | cross-reactivity for aminoclomazone and phenoldiazo-clomazone were 110% and 20%, the competitive assay and 54% and <0.2% for PHAIA | (84) |
| phenoxybenzoic acid (PBA) | peptide(10aa), phage display peptide library | noncompetitive ELISA and PHAIA (phage anti-immunocomplex assay) | 0.05 ng/mL, 5-fold (heterologous) or 400-fold (homologous) higher than that of the competitive assay | negligible cross-reactivity, in agreement with the competitive assay | (85) |
| HT-2 Mycotoxin | Fab, phage display antibody library | TR-FRET (time-resolved Förster resonance energy transfer) immunoassay | 0.38 ng/mL | the competitive assay has 100% cross-reactivity for HT-2 and T-2 toxins, while the IC assay is highly specific for HT-2 alone | (86) |
| aflatoxin B1 (AFB1) | random linear 8-mer peptide, phage display library | nc-MCLEIA (noncompetitive magnetic-chemiluminescent enzyme-linked immunoassay) | 0.006 ng/mL, the sensitivity of Nc-MCLEIA was greatly improved. | no cross-reactivity with zearalenone (ZEN), deoxynivalenol (DON) and ochratoxin A (OTA) Cross-reactivity to AFB2, G1, G2 and M1 | (87) |
| clomazone | nanopeptamer, phage display Library | ELISA | 0.48 ng/mL, 8-fold better of the competitive assay | (88) | |
| angiotensin II | mAb, hybridoma technology | CLIA | 1 pg/mL | (33) | |
| clomazone | nanopeptamer(10aa), phage display Library | lateral-flow test | 2.5 ng/mL, with over a 10-fold increased in sensitivity | (89) | |
| 2,2′,4,4′-tetrabromodiphenyl ether | peptide (7aa), phage display Library | phage ELISA | 0.3 ng/L, the competitive and noncompetitive phage ELISAs demonstrated increases of 11-fold | the competitive phage ELISA format has superior specificity | (90) |
| aflatoxins | scFv, synthetic antibody repertoire | TRFIA | 70 pg/mL | cross-reactivities with aflatoxin variants ranged from 3% (AFM1) to 89% (AFG1) | (91) |
4.1. The Application of Anti-IC Reagents in Toxins and Drug of Abuse
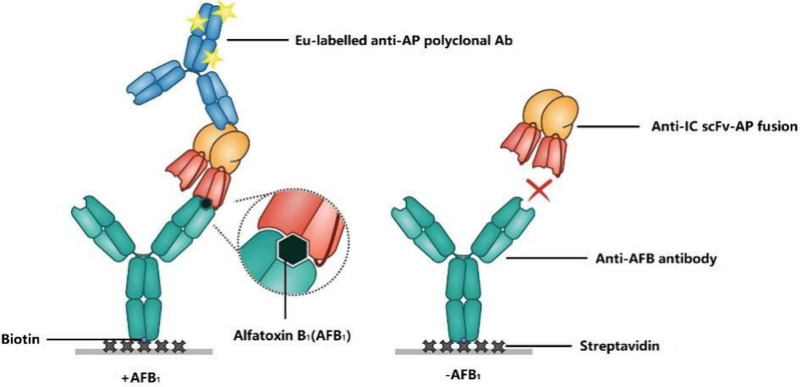
Peltomaa et al.91 utilized a monoclonal antiaflatoxin mouse antibody and an anti-IC scFv selected from a synthetic antibody library to establish a one-step sandwich fluorescent assay for rapid detection of aflatoxin. The LoD of this method was 70 pg/mL, with a detection time of within 15 min. In this assay, scFv was fused with alkaline phosphatase (AP), which avoided directly labeling scFv and thereby preserved its binding activity, as shown in Figure 8. The specificity of the assay was tested against various aflatoxins and other mycotoxins. It showed significant cross-reactivity with aflatoxins B2 and G1, but not with other mycotoxins like ochratoxin A (OTA) and deoxynivalenol (DON). This indicates that the assay is highly specific for aflatoxins while minimizing interference from other mycotoxins.
Figure 8.
Single-step noncompetitive immunoassay for aflatoxins B1 detection. Reprinted with permission from Food Chem.2022, 392, 133287, Copyright, 2022, Elsevier.91
In addition to scFv, phage display peptides can be utilized for small molecule sandwich detection. Zou et al.87 isolated a peptide with affinity for the aflatoxin B1-nanobody Nb28 complex from a random linear 8-mer peptide library using phage display, after four rounds of panning. Using Nb28-coated magnetic beads as the solid phase carrier and HRP-labeled phage peptides as the tracer, a magnetic particle chemiluminescence immunoassay for aflatoxin B1 was developed with an LoD of 6 pg/mL. The anti-IC antibody fragments or peptides prepared using phage display technology are presented on the surface of the phages, thus eliminating the need for purification and labeling as required for traditional polyclonal or monoclonal antibodies, facilitating subsequent applications.
Compared with competitive methods, noncompetitive methods employing dual-site binding significantly improve the sensitivity. González-Techera et al.73 compared the sensitivity of a noncompetitive phage peptide assay with a competitive ELISA based on the same Ab1. The results showed that the sensitivity of the noncompetitive method was approximately 30 times higher than that of the competitive method. Kim et al.81 combined a noncompetitive phage anti-IC assay (PHAIA) with immuno polymerase chain reaction (PCR), using antibody-coated magnetic beads as the separation carrier. They developed a detection method for 3-phenoxybenzoic acid with an LoD of approximately 60 pg/mL, which is 10 times more sensitive than traditional PHAIA, and also faster.
A method established by Solin et al.68 demonstrated significant improvements in practical THC detection by enhancing sensitivity, particularly for low-concentration samples, through more effective binding of the THC-anti-THC Fab IC. The nanocellulose-based biointerface proved versatile, functioning across both SPR systems and paper-based assays, supporting adaptability for point-of-care applications. Additionally, the hydrophilic properties of nanocellulose facilitate efficient detection in aqueous environments, preserving antibody bioactivity. The approach also showed promise for scalability with the nanocellulose substrate being compatible with mass-production techniques, making it suitable for large-scale diagnostic applications.
4.2. The Application of Anti-IC Antibodies in Clinical Diagonostics
In clinical testing, there have been few published reports on small molecule noncompetitive detection methods. These primarily include digoxin, angiotensin II,33 UDCA 7-NAG,36 brain natriuretic peptide,37 progesterone, E2, 25-hydroxyvitamin D,49,92 and aldosterone.82 In recent years, Fujirebio Inc. in Japan introduced sandwich methods for 25-hydroxyvitamin D and aldosterone on the LUMIPULSE platform, and Shenzhen New Industries Biomedical Engineering Co., Ltd. developed sandwich methods for E2, total triiodothyronine, and tacrolimus on the Maglumi system, which have garnered widespread attention in the clinical testing community.38 Our team has developed a chemiluminescent immunoassay sandwich detection method for E2 based on anti-IC antibodies, significantly improving the sensitivity compared to competitive methods. Additionally, for 286 clinical samples with concentration levels spanning the measurement range, the correlation coefficient (R2) between the test results and LC–MS/MS results) was greater than 0.9947.
Lanja et al.93 systematically evaluated the performance of the LUMIPULSE 25-hydroxyvitamin D sandwich method compared to three commercial competitive methods from Abbott, Beckman, and Roche. Using LC–MS/MS as the gold standard, they tested 100 clinical samples and found that the sandwich method had the best consistency with LC-MS/MS, with a linear correlation coefficient (R) of 0.986, and demonstrated superior precision, making it suitable as a routine clinical testing method. In clinical testing of 25-hydroxyvitamin D, it is required not only to detect 25-hydroxyvitamin D2 and D3 with equal capability but also to have a low cross-reactivity rate with numerous small molecule analogs present in serum, such as 3-epi-25-hydroxyvitamin D3, which have structures similar to 25-hydroxyvitamin D. Traditional competitive methods often struggle to meet these high standards. In contrast, the sandwich method leverages the precise recognition capability of anti-IC antibodies to the conformational changes in the SMA and Ab1 binding, not only enhancing detection sensitivity but also significantly improving specificity.
4.3. Application of Anti-IC Reagents in the Detection of Pesticide Residues
In pesticide residue detection, the application of anti-IC reagents has significantly enhanced sensitivity and enabled rapid on-site analysis for a variety of compounds, including for malachite green,94 leucomalachite green,94 clomazone,95 molinate,22,73 atrazine,73 3-phenoxybenzoic acid,80,81,85 and benzothiostrobin.69 The LoDs for these pesticides have been drastically reduced, facilitating better environmental monitoring and improving food safety assurance.
González-Techera et al.74 integrated phage anti-IC particles into an electrochemical sensor to establish a novel electrochemical detection method for atrazine. These particles specifically recognize the IC of atrazine and Ab1, with an LoD of 0.2 pg/mL, which is 200 times more sensitive than the competitive ELISA method established with the same Ab1. The measurement range was also extended 10 times. This detection method can use undiluted river water samples directly without pretreatment, making it suitable for rapid on-site detection.
Although phage particles have been proven to be robust and versatile reagent components, their biological characteristics as unconventional reagents might pose safety concerns. To overcome this limitation, Vanrell et al.72 replaced the phage particles with biotinylated synthetic anti-IC peptides, combined with streptavidin/avidin as core proteins, forming what they termed “nanopeptamers”. Using these, they established a lateral flow immunoassay for the herbicides fenoxapropethyl and propanil, with LoDs of 1.2 and 3.2 ng/mL, respectively. Carlomagno et al.88 further optimized the preparation of nanopeptamers by physically coupling anti-IC peptides, expressing them fused to either the N- or C-terminus of SA. They developed a noncompetitive ELISA method for propanil with an LoD of 0.48 ng/mL, which was similar in sensitivity to the phage anti-IC particle method. This approach did not require the synthesis of anti-IC peptides, significantly reducing production costs and freeing up biotin-binding sites. The recombinantly expressed nanopeptamers can be used in conjunction with any biotinylated reagents, including labeled enzymes, fluorophores, colloidal gold, and magnetic beads, enhancing the versatility of the system.
5. Summary and Outlook
In this review, the progress in the application of anti-IC reagents in SMA immunoassays have been discussed. The use of anti-IC reagents has shown great potential in overcoming the limitations of traditional competitive immunoassays, particularly in terms of sensitivity, precision, specificity, and dynamic range. First, regarding enhanced sensitivity, anti-IC-based noncompetitive methods demonstrated a several-fold to hundreds-fold increase in sensitivity over competitive assays, allowing for more accurate detection of SMAs. Second, in terms of improved specificity, the dual recognition mechanism, wherein Ab1 binds to the SMA and the anti-IC antibody recognizes the IC formed, provides higher specificity by reducing cross-reactivity and matrix interference. This enhanced specificity is particularly beneficial for detecting structurally similar analytes and ensuring accurate results. Third, the versatile applications of anti-IC antibodies have expanded the possibilities for SMA detection in fields such as clinical diagnostics, food safety, and environmental monitoring. This versatility is exemplified by their successful implementation in the detection of toxins, pesticides, and clinical biomarkers. Regarding future avenues of research, first, there is a need for a more comprehensive understanding of the conformational changes that occur upon IC formation. Further exploration of the relationship between SMA-Ab1 affinity and these structural changes will facilitate the production of anti-IC antibodies with optimal binding characteristics. Second, minimizing the cross-reactivity still remains a challenge. Research into the mechanisms by which anti-IC antibodies recognize ICs with improved selectivity, particularly in the presence of structurally related compounds, will be crucial in enhancing the assay specificity, especially in complex sample matrices. Finally, exploring new methods for anti-IC antibody preparation, such as the use of immune libraries and single B cell technology,96 could improve both the efficiency and the specificity of anti-IC antibody production. This could lead to more robust noncompetitive detection methods, offering promising solutions for SMA analysis. Overall, the use of anti-IC reagents represents a promising frontier in SMA detection, offering a pathway toward more sensitive, specific, and rapid immunoassays. Continued research in this area will further enhance our capabilities for the accurate and reliable analysis of small molecules in various fields.
Acknowledgments
The authors thank the financial support provided by the 2023 Second Batch of Special Fund Projects for Strategic Emerging Industries by the Development and Reform Commission of Shenzhen Municipality (Project Contract Number: XMHT20230215001).
Author Contributions
‡ These authors contributed equally to this work.
The authors declare no competing financial interest.
References
- Zhang W.; Giesy J. P.; Wang P. Organophosphate esters in agro-foods: Occurrence, sources and emerging challenges. Science of The Total Environment 2022, 827, 154271 10.1016/j.scitotenv.2022.154271. [DOI] [PubMed] [Google Scholar]
- Oliveira T. M. B. F.; Ribeiro F. W. P.; Sousa C. P.; Salazar-Banda G. R.; de Lima-Neto P.; Correia A. N.; Morais S. Current overview and perspectives on carbon-based (bio)sensors for carbamate pesticides electroanalysis. TrAC Trends in Analytical Chemistry 2020, 124, 115779 10.1016/j.trac.2019.115779. [DOI] [Google Scholar]
- Singh S.; Mukherjee A.; Jaiswal D. K.; de Araujo Pereira A. P.; Prasad R.; Sharma M.; Kuhad R. C.; Shukla A. C.; Verma J. P. Advances and future prospects of pyrethroids: Toxicity and microbial degradation. Science of The Total Environment 2022, 829, 154561 10.1016/j.scitotenv.2022.154561. [DOI] [PubMed] [Google Scholar]
- Chang D.; Mao Y.; Qiu W.; Wu Y.; Cai B. The Source and Distribution of Tetracycline Antibiotics in China: A Review. Toxics 2023, 11 (3), 214. 10.3390/toxics11030214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conde-Cid M.; Núñez-Delgado A.; Fernández-Sanjurjo M. J.; Álvarez-Rodríguez E.; Fernández-Calviño D.; Arias-Estévez M. Tetracycline and Sulfonamide Antibiotics in Soils: Presence, Fate and Environmental Risks. Processes 2020, 8 (11), 1479. 10.3390/pr8111479. [DOI] [Google Scholar]
- Zhang W.; Wang P.; Su X. Current advancement in analysis of β-agonists. TrAC Trends in Analytical Chemistry 2016, 85, 1–16. 10.1016/j.trac.2016.08.011. [DOI] [Google Scholar]
- Nazhand A.; Durazzo A.; Lucarini M.; Souto E. B.; Santini A. Characteristics, Occurrence, Detection and Detoxification of Aflatoxins in Foods and Feeds. Foods 2020, 9 (5), 644. 10.3390/foods9050644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingelhöfer D.; Braun M.; Schöffel N.; Oremek G. M.; Brüggmann D.; Groneberg D. A. Ochratoxin – Characteristics, influences and challenges of global research. Food Control 2020, 114, 107230 10.1016/j.foodcont.2020.107230. [DOI] [Google Scholar]
- Sohrabi H.; Arbabzadeh O.; Khaaki P.; Majidi M. R.; Khataee A.; Woo Joo S. Emerging electrochemical sensing and biosensing approaches for detection of Fumonisins in food samples. Critical Reviews in Food Science and Nutrition 2022, 62 (31), 8761–8776. 10.1080/10408398.2021.1932723. [DOI] [PubMed] [Google Scholar]
- Shephard G. S. Determination of mycotoxins in human foods. Chem. Soc. Rev. 2008, 37 (11), 2468–2477. 10.1039/b713084h. [DOI] [PubMed] [Google Scholar]
- Brandi J.; Siragusa G.; Robotti E.; Marengo E.; Cecconi D. Analysis of veterinary drugs and pesticides in food using liquid chromatography-mass spectrometry. TrAC Trends in Analytical Chemistry 2024, 179, 117888 10.1016/j.trac.2024.117888. [DOI] [Google Scholar]
- Jadhav M. R.; Pudale A.; Raut P.; Utture S.; Ahammed Shabeer T. P.; Banerjee K. A unified approach for high-throughput quantitative analysis of the residues of multi-class veterinary drugs and pesticides in bovine milk using LC-MS/MS and GC–MS/MS. Food Chem. 2019, 272, 292–305. 10.1016/j.foodchem.2018.08.033. [DOI] [PubMed] [Google Scholar]
- de Bairros A. V.; Dias D.; Bezerra A.; Wagner R.; Klein B.; Kommers G.; Stefanon E.; Miguel Pego A. An analytical strategy for the identification of carbamates, toxic alkaloids, phenobarbital and warfarin in stomach contents from suspected poisoned animals by thin-layer chromatography/ultraviolet detection. Toxicology Mechanisms and Methods 2019, 29 (7), 518–530. 10.1080/15376516.2019.1619213. [DOI] [PubMed] [Google Scholar]
- Yao J.; Wang Z.; Guo L.; Xu X.; Liu L.; Xu L.; Song S.; Xu C.; Kuang H. Advances in immunoassays for organophosphorus and pyrethroid pesticides. TrAC Trends in Analytical Chemistry 2020, 131, 116022 10.1016/j.trac.2020.116022. [DOI] [Google Scholar]
- Ishikawa E.; Tanaka K.; Hashida S. Novel and sensitive noncompetitive (two-site) immunoassay for haptens with emphasis on peptides. Clinical Biochemistry 1990, 23 (5), 445–453. 10.1016/0009-9120(90)90238-P. [DOI] [PubMed] [Google Scholar]
- Volland H.; Pradelles P.; Ronco P.; Azizi M.; Simon D.; Créminon C.; Grassi J. A solid-phase immobilized epitope immunoassay (SPIE-IA) permitting very sensitive and specific measurement of angiotensin II in plasma. Journal of Immunological Methods 1999, 228 (1), 37–47. 10.1016/S0022-1759(99)00097-6. [DOI] [PubMed] [Google Scholar]
- Shu M.; Xu Y.; Dong J.-x.; Zhong C.; Hammock B. D.; Wang W.-j.; Wu G.-p. Development of a noncompetitive idiometric nanobodies phage immumoassay for the determination of fumonisin B1. Food Agric. Immunol. 2019, 30 (1), 510–521. 10.1080/09540105.2019.1604637. [DOI] [Google Scholar]; Mares A.; De Boever J.; Osher J.; Quiroga S.; Barnard G.; Kohen F. A direct noncompetitive idiometric enzyme immunoassay for serum oestradiol. Journal of Immunological Methods 1995, 181 (1), 83–90. 10.1016/0022-1759(94)00332-Q. [DOI] [PubMed] [Google Scholar]
- Saha D.; Roy D.; Dhar T. K. Immunofiltration assay for aflatoxin B1 based on the separation of pre-immune complexes. Journal of Immunological Methods 2013, 392 (1), 24–28. 10.1016/j.jim.2013.03.003. [DOI] [PubMed] [Google Scholar]
- Lövgren U.; Kronkvist K.; Bäckström B.; Edholm L.-E.; Johansson G. Design of noncompetitive flow injection enzyme immunoassays for determination of haptens. Application to digoxigenin. Journal of Immunological Methods 1997, 208 (2), 159–168. 10.1016/S0022-1759(97)00144-0. [DOI] [PubMed] [Google Scholar]
- Chen L.; Tan R.; Zhou Y.; Zhang L.; Zhang S.; Li X.; Cong Y.; Li H.; Sun P.; Ueda H.; et al. Development of an Open sandwich ELISA for the detection of microcystin-LR. Microchemical Journal 2020, 158, 105325 10.1016/j.microc.2020.105325. [DOI] [Google Scholar]
- Bai Y.; Wang Y.; Li Q.; Dou L.; Liu M.; Shao S.; Zhu J.; Shen J.; Wang Z.; Wen K.; et al. Binding affinity-guided design of a highly sensitive noncompetitive immunoassay for small molecule detection. Food Chem. 2021, 351, 129270 10.1016/j.foodchem.2021.129270. [DOI] [PubMed] [Google Scholar]
- Di Tocco A.; Porcal G. V.; Lassabe G.; González-Techera A.; Zon M. A.; Fernández H.; González-Sapienza G.; Robledo S. N.; Arévalo F. J. Development of an electrochemical immunosensor for the determination of molinate by using phages labeled with CdS nanocrystals as a novel strategy to signal amplification. Sens. Actuators, B 2022, 367, 132126 10.1016/j.snb.2022.132126. [DOI] [Google Scholar]
- Jeong H.-J. Quenchbodies That Enable One-Pot Detection of Antigens: A Structural Perspective. Bioengineering 2023, 10 (11), 1262. 10.3390/bioengineering10111262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fei J.; Jiang J.; Bai Y.; Wu W.; Zhao X.; Yu W.; Wen K.; Yu X.; Shen J.; Wang Z. A Proof-of-Concept Sandwich Enzyme-Linked Immunoassay Development for Small Molecules. Anal. Chem. 2023, 95 (39), 14665–14674. 10.1021/acs.analchem.3c02557. [DOI] [PubMed] [Google Scholar]
- Voss E. W.; Mummert M. E. Anti-metatype antibodies in immunoassays. Microchimica Acta 1997, 126 (3), 193–202. 10.1007/BF01242320. [DOI] [Google Scholar]
- Liu A.; Anfossi L.; Shen L.; Li C.; Wang X. Noncompetitive immunoassay for low-molecular-weight contaminant detection in food, feed and agricultural products: A mini-review. Trends in Food Science & Technology 2018, 71, 181–187. 10.1016/j.tifs.2017.11.014. [DOI] [Google Scholar]; Li Y. S.; Zhang G. Z.; Mao X.; Yang S. P.; De Ruyck K.; Wu Y. N. High sensitivity immunoassays for small molecule compounds detection - Novel noncompetitive immunoassay designs. Trac-Trends Anal. Chem. 2018, 103, 198–208. 10.1016/j.trac.2018.04.008. [DOI] [Google Scholar]; Liang Y.-F.; Yang J.-Y.; Shen Y.-D.; Xu Z.-L.; Wang H.. A breakthrough of immunoassay format for hapten: recent insights into noncompetitive immunoassays to detect small molecules. Crit. Rev. Food Sci. Nutr., 2024, 1–11 10.1080/10408398.2024.2315473. [DOI] [PubMed] [Google Scholar]
- Nemazee D. A.; Sato V. L. Enhancing antibody: a novel component of the immune response. Proc. Natl. Acad. Sci. U. S. A. 1982, 79 (12), 3828–3832. 10.1073/pnas.79.12.3828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown E. J.; Bekisz J. Neoantigens appear in human IgG upon antigen binding: detection by antibodies that react specifically with antigen-bound IgG. J. Immunol. 1984, 132 (3), 1346–1352. 10.4049/jimmunol.132.3.1346. [DOI] [PubMed] [Google Scholar]
- Sawutz D. G.; Koury R.; Homcy C. J. Enhanced antigen-antibody binding affinity mediated by an anti-idiotypic antibody. Biochemistry 1987, 26 (17), 5275–5282. 10.1021/bi00391a010. [DOI] [PubMed] [Google Scholar]
- Voss E. W.; Miklasz S. D.; Petrossian A.; Dombrink-Kurtzman M. A. Polyclonal antibodies specific for liganded active site (metatype) of a high affinity anti-hapten monoclonal antibody. Molecular Immunology 1988, 25 (8), 751–759. 10.1016/0161-5890(88)90111-3. [DOI] [PubMed] [Google Scholar]
- Weidner K. M.; Denzin L. K.; Voss E. W. Molecular stabilization effects of interactions between anti-metatype antibodies and liganded antibody. J. Biol. Chem. 1992, 267 (15), 10281–10288. 10.1016/S0021-9258(19)50015-1. [DOI] [PubMed] [Google Scholar]
- Ullman E. F.; Milburn G.; Jelesko J.; Radika K.; Pirio M.; Kempe T.; Skold C. Anti-immune complex antibodies enhance affinity and specificity of primary antibodies. Proc. Natl. Acad. Sci. U. S. A. 1993, 90 (4), 1184–1189. 10.1073/pnas.90.4.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H.; Motz J.; Oroszlan P.; Zingel O. Sandwich immunoassay for the hapten angiotensin II a novel assay principle based on antibodies against immune complexes. Journal of Immunological Methods 1995, 181 (2), 167–176. 10.1016/0022-1759(94)00343-U. [DOI] [PubMed] [Google Scholar]
- Self C. H.; Dessi J. L.; Winger L. A. High-performance assays of small molecules: enhanced sensitivity, rapidity, and convenience demonstrated with a noncompetitive immunometric anti-immune complex assay system for digoxin. Clinical Chemistry 1994, 40 (11), 2035–2041. 10.1093/clinchem/40.11.2035. [DOI] [PubMed] [Google Scholar]
- Nagata S.; Tsutsumi T.; Yoshida F.; Ueno Y. A new type sandwich immunoassay for microcystin: production of monoclonal antibodies specific to the immune complex formed by microcystin and an anti-microcystin monoclonal antibody. Natural Toxins 1999, 7 (2), 49–55. . [DOI] [PubMed] [Google Scholar]
- Kobayashi N.; Oiwa H.; Kubota K.; Sakoda S.; Goto J. Monoclonal antibodies generated against an affinity-labeled immune complex of an anti-bile acid metabolite antibody: an approach to noncompetitive hapten immunoassays based on anti-idiotype or anti-metatype antibodies. Journal of Immunological Methods 2000, 245 (1), 95–108. 10.1016/S0022-1759(00)00291-X. [DOI] [PubMed] [Google Scholar]
- Tamm N. N.; Seferian K. R.; Semenov A. G.; Mukharyamova K. S.; Koshkina E. V.; Krasnoselsky M. I.; Postnikov A. B.; Serebryanaya D. V.; Apple F. S.; Murakami M. M.; et al. Novel Immunoassay for Quantification of Brain Natriuretic Peptide and Its Precursor in Human Blood. Clinical Chemistry 2008, 54 (9), 1511–1518. 10.1373/clinchem.2007.100545. [DOI] [PubMed] [Google Scholar]
- Du K.; Zhang Z.; Gao L.; He H.; Li T.; Rao W. Application of anti-idiotype antibodies in haptens immunoassay. Curr. Biotechnol. 2023, 13 (5), 690–697. 10.19586/j.2095-2341.2023.0066. [DOI] [Google Scholar]
- Ledsgaard L.; Kilstrup M.; Karatt-Vellatt A.; McCafferty J.; Laustsen A. H. Basics of Antibody Phage Display Technology. Toxins 2018, 10 (6), 236. 10.3390/toxins10060236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q.; Zhang F.; Lu Y.; Hu H.; Wang J.; Guo C.; Deng Q.; Liao C.; Wu Q.; Hu T.; et al. Highly potent multivalent VHH antibodies against Chikungunya isolated from an alpaca naïve phage display library. J. Nanobiotechnol. 2022, 20 (1), 231. 10.1186/s12951-022-01417-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledsgaard L.; Ljungars A.; Rimbault C.; Sørensen C. V.; Tulika T.; Wade J.; Wouters Y.; McCafferty J.; Laustsen A. H. Advances in antibody phage display technology. Drug Discovery Today 2022, 27 (8), 2151–2169. 10.1016/j.drudis.2022.05.002. [DOI] [PubMed] [Google Scholar]
- Qiu J.; Li J.; Zhang Z.; Dong S.; Ling X.; Fang Z.; Ling Q.; Huang Z.. Construction of an alpaca immune antibody library for the selection of nanobodies against Drosophila melanogaster proteins. Front. Bioeng. Biotechnol. 2023, 11 10.3389/fbioe.2023.1207048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim G. M.; Park D. R.; Nguyen T. T. H.; Kim J.; Kim J.; Sohn M.-H.; Lee W.-K.; Lee S. Y.; Shim H. Development of Anti-OSCAR Antibodies for the Treatment of Osteoarthritis. Biomedicines 2023, 11 (10), 2844. 10.3390/biomedicines11102844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y.; Li Q.; Luo L.; Duan C.; Shen J.; Wang Z. Application of germline antibody features to vaccine development, antibody discovery, antibody optimization and disease diagnosis. Biotechnology Advances 2023, 65, 108143 10.1016/j.biotechadv.2023.108143. [DOI] [PubMed] [Google Scholar]
- Contreras M. A.; Serrano-Rivero Y.; González-Pose A.; Salazar-Uribe J.; Rubio-Carrasquilla M.; Soares-Alves M.; Parra N. C.; Camacho-Casanova F.; Sánchez-Ramos O.; Moreno E. Design and Construction of a Synthetic Nanobody Library: Testing Its Potential with a Single Selection Round Strategy. Molecules 2023, 28 (9), 3708. 10.3390/molecules28093708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H.; Yang Q.; Ding Y.; Vasylieva N.; Bever C. S.; Hua X.; Wang M.; Hammock B. D. Competitive and noncompetitive immunoassays for the detection of benzothiostrobin using magnetic nanoparticles and fluorescein isothiocyanate-labeled peptides. Anal. Bioanal. Chem. 2019, 411 (2), 527–535. 10.1007/s00216-018-1478-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H.; You T.; Zong L.; Mukhametova L. I.; Zherdev D. O.; Eremin S. A.; Ding Y.; Wang M.; Hua X. Competitive and noncompetitive fluorescence polarization immunoassays for the detection of benzothiostrobin using FITC-labeled dendrimer-like peptides. Food Chem. 2021, 360, 130020 10.1016/j.foodchem.2021.130020. [DOI] [PubMed] [Google Scholar]
- Seo H.; Hashimoto S.-i.; Tsuchiya K.; Lin W.; Shibata T.; Ohta K. An ex vivo method for rapid generation of monoclonal antibodies (ADLib system). Nat. Protoc. 2006, 1 (3), 1502–1506. 10.1038/nprot.2006.248. [DOI] [PubMed] [Google Scholar]
- Omi K.; Ando T.; Sakyu T.; Shirakawa T.; Uchida Y.; Oka A.; Ise N.; Aoyagi K.; Goishi K. Noncompetitive Immunoassay Detection System for Haptens on the Basis of Antimetatype Antibodies. Clinical Chemistry 2015, 61 (4), 627–635. 10.1373/clinchem.2014.232728. [DOI] [PubMed] [Google Scholar]
- Seo H.; Masuda H.; Asagoshi K.; Uchiki T.; Kawata S.; Sasaki G.; Yabuki T.; Miyai S.; Takahashi N.; Hashimoto S.-i.; et al. Streamlined human antibody generation and optimization by exploiting designed immunoglobulin loci in a B cell line. Cellular & Molecular Immunology 2021, 18 (6), 1545–1561. 10.1038/s41423-020-0440-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mickert M. J.; Gorris H. H. Transition-State Ensembles Navigate the Pathways of Enzyme Catalysis. J. Phys. Chem. B 2018, 122 (22), 5809–5819. 10.1021/acs.jpcb.8b02297. [DOI] [PubMed] [Google Scholar]
- Nemeth P. Do Water Molecules Displaced by Hydrophobic Interactions Stabilize Antigen-Antibody Binding? Physico-chemical background of antigen-antibody reactions analyzed by fluorescent and Fourier-transform infrared spectroscopy on FITC–anti-FITC (IgG1) model. Southeastern European Medical Journal: SEEMEDJ. 2022, 6 (2), 1–19. 10.26332/seemedj.v6i2.257. [DOI] [Google Scholar]
- Crowley A. R.; Mehlenbacher M. R.; Sajadi M. M.; DeVico A. L.; Lewis G. K.; Ackerman M. E. Evidence of variable human Fcγ receptor-Fc affinities across differentially-complexed IgG. mAbs 2023, 15 (1), 2231128 10.1080/19420862.2023.2231128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Qaraghuli M. M.; Kubiak-Ossowska K.; Ferro V. A.; Mulheran P. A.. Structural Analysis of Anti-Hapten Antibodies to Identify Long-Range Structural Movements Induced by Hapten Binding. Front. Mol. Biosci. 2021, 8 10.3389/fmolb.2021.633526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemi M. H.; Turunen L.; Pulli T.; Nevanen T. K.; Höyhtyä M.; Söderlund H.; Rouvinen J.; Takkinen K. A Structural Insight into the Molecular Recognition of a (−)-Δ9-Tetrahydrocannabinol and the Development of a Sensitive, One-Step, Homogeneous Immunocomplex-Based Assay for Its Detection. J. Mol. Biol. 2010, 400 (4), 803–814. 10.1016/j.jmb.2010.05.048. [DOI] [PubMed] [Google Scholar]
- Fu H.-J.; Chen Z.-J.; Wang H.; Luo L.; Wang Y.; Huang R.-M.; Xu Z.-L.; Hammock B. Development of a sensitive noncompetitive immunoassay via immunocomplex binding peptide for the determination of ethyl carbamate in wine samples. J. Hazard. Mater. 2021, 406, 124288 10.1016/j.jhazmat.2020.124288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akter S.; Lamminmaki U. A 15-min noncompetitive homogeneous assay for microcystin and nodularin based on time-resolved Forster resonance energy transfer (TR-FRET). Anal. Bioanal. Chem. 2021, 413 (24), 6159–6170. 10.1007/s00216-021-03375-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akter S.; Vehniäinen M.; Spoof L.; Nybom S.; Meriluoto J.; Lamminmäki U. Broad-Spectrum Noncompetitive Immunocomplex Immunoassay for Cyanobacterial Peptide Hepatotoxins (Microcystins and Nodularins). Anal. Chem. 2016, 88 (20), 10080–10087. 10.1021/acs.analchem.6b02470. [DOI] [PubMed] [Google Scholar]
- Akter S.; Kustila T.; Leivo J.; Muralitharan G.; Vehniäinen M.; Lamminmäki U. Noncompetitive Chromogenic Lateral-Flow Immunoassay for Simultaneous Detection of Microcystins and Nodularin. Biosensors 2019, 9 (2), 79. 10.3390/bios9020079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akter S.; Vehniäinen M.; Meriluoto J.; Spoof L.; Lamminmäki U.. Noncompetitive ELISA with broad specificity for microcystins and nodularins. Adv. Oceanogr. Limnol. 2017, 8 ( (1), ) 10.4081/aiol.2017.6349. [DOI] [Google Scholar]
- Akter S.; Vehniainen M.; Kankaanpaa H. T.; Lamminmaki U. Rapid and Highly Sensitive Noncompetitive Immunoassay for Specific Detection of Nodularin. Microorganisms 2017, 5 (3), 58. 10.3390/microorganisms5030058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teerinen T.; Lappalainen T.; Erho T. A paper-based lateral flow assay for morphine. Anal. Bioanal. Chem. 2014, 406 (24), 5955–5965. 10.1007/s00216-014-8001-7. [DOI] [PubMed] [Google Scholar]
- Pulli T.; Höyhtyä M.; Söderlund H.; Takkinen K. One-Step Homogeneous Immunoassay for Small Analytes. Anal. Chem. 2005, 77 (8), 2637–2642. 10.1021/ac048379l. [DOI] [PubMed] [Google Scholar]
- Arola H. O.; Tullila A.; Nathanail A. V.; Nevanen T. K. A Simple and Specific Noncompetitive ELISA Method for HT-2 Toxin Detection. Toxins 2017, 9 (4), 145. 10.3390/toxins9040145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradanas-González F.; Glahn-Martínez B.; Benito-Peña E.; Arola H. O.; Nevanen T. K.; Moreno-Bondi M. C. Molecular super-gluing: a straightforward tool for antibody labelling and its application to mycotoxin biosensing. Anal. Bioanal. Chem. 2022, 414 (18), 5373–5384. 10.1007/s00216-021-03841-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai Y.; Dou L.; Wu W.; Lu Z.; Kou J.; Shen J.; Wen K.; Wang Z. Anti-Metatype Antibody Screening, Sandwich Immunoassay Development, and Structural Insights for β-Lactams Based on Penicillin Binding Protein. Molecules 2021, 26 (18), 5569. 10.3390/molecules26185569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaba J.; Nakamura S.; Shimizu K.; Asami T.; Suzuki Y. Anti-metatype peptides, a molecular tool with high sensitivity and specificity to monitor small ligands. Anal. Biochem. 2009, 388 (1), 63–70. 10.1016/j.ab.2009.02.004. [DOI] [PubMed] [Google Scholar]
- Solin K.; Vuoriluoto M.; Khakalo A.; Tammelin T. Cannabis detection with solid sensors and paper-based immunoassays by conjugating antibodies to nanocellulose. Carbohydr. Polym. 2023, 304, 120517 10.1016/j.carbpol.2022.120517. [DOI] [PubMed] [Google Scholar]
- Hua X. D.; Zhou L. L.; Feng L.; Ding Y.; Shi H. Y.; Wang L. M.; Gee S. J.; Hammock B. D.; Wang M. H. Competitive and noncompetitive phage immunoassays for the determination of benzothiostrobin. Anal. Chim. Acta 2015, 890, 150–156. 10.1016/j.aca.2015.07.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H.; Ding Y.; Li J.; Huang L. R.; Gonzalez-Sapienza G.; Hammock B. D.; Wang M. H.; Hua X. D. New Approach to Generate Ratiometric Signals on Immunochromatographic Strips for Small Molecules. Anal. Chem. 2022, 94 (20), 7358–7367. 10.1021/acs.analchem.2c00838. [DOI] [PubMed] [Google Scholar]
- Tocco A. D.; Porcal G. V.; Monti G. A.; Wendel A.; Palacios R.; Fernández H.; Pierini G.; Zon M. A.; Robledo S. N.; Arévalo F. J. Conjugation of phages with CdS nanocrystals. An alternative in the development of a new tracer for immunoassays. Microchemical Journal 2024, 196, 109634 10.1016/j.microc.2023.109634. [DOI] [Google Scholar]
- Vanrell L.; Gonzalez-Techera A.; Hammock B. D.; Gonzalez-Sapienza G. Nanopeptamers for the Development of Small-Analyte Lateral Flow Tests with a Positive Readout. Anal. Chem. 2013, 85 (2), 1177–1182. 10.1021/ac3031114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Techera A.; Vanrell L.; Last J. A.; Hammock B. D.; González-Sapienza G. Phage Anti-Immune Complex Assay: General Strategy for Noncompetitive Immunodetection of Small Molecules. Anal. Chem. 2007, 79 (20), 7799–7806. 10.1021/ac071323h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Techera A.; Zon M. A.; Molina P. G.; Fernandez H.; Gonzalez-Sapienza G.; Arevalo F. J. Development of a highly sensitive noncompetitive electrochemical immunosensor for the detection of atrazine by phage anti-immunocomplex assay. Biosens. Bioelectron. 2015, 64, 650–656. 10.1016/j.bios.2014.09.046. [DOI] [PubMed] [Google Scholar]
- Lassabe G.; Kramer K.; Hammock B. D.; Gonzalez-Sapienza G.; Gonzalez-Techera A. Noncompetitive Homogeneous Detection of Small Molecules Using Synthetic Nanopeptamer-Based Luminescent Oxygen Channeling. Anal. Chem. 2018, 90 (10), 6187–6192. 10.1021/acs.analchem.8b00657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi R. R.; Zhao Z. L.; Wang G. Q.; Zou W. T.; Zhao F. C.; Yang Z. Y. Development of a noncompetitive magnetic-phage anti-immunocomplex assay for detecting of organophosphorus pesticides with a thiophosphate group. Anal. Biochem. 2022, 646, 114632. 10.1016/j.ab.2022.114632. [DOI] [PubMed] [Google Scholar]
- Kim H. J.; Rossotti M. A.; Ahn K. C.; Gonzalez-Sapienza G. G.; Gee S. J.; Musker R.; Hammock B. D. Development of a noncompetitive phage anti-immunocomplex assay for brominated diphenyl ether 47. Anal. Biochem. 2010, 401 (1), 38–46. 10.1016/j.ab.2010.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You T.; Ding Y.; Huang Y.; Lu Y.; Wang M.; Hua X. Identification and Application of Two Promising Peptide Ligands for the Immunodetection of Imidacloprid Residue. Foods 2022, 11 (20), 3163. 10.3390/foods11203163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pali M.; Bever C. R. S.; Vasylieva N.; Hammock B. D.; Suni I. I. Impedance Detection of 3-Phenoxybenzoic Acid with a Noncompetitive Two-site Phage Anti-immunocomplex Assay. Electroanalysis 2018, 30 (11), 2653–2659. 10.1002/elan.201800457. [DOI] [Google Scholar]
- Kim H. J.; Ahn K. C.; Gonzalez-Techera A.; Gonzalez-Sapienza G. G.; Gee S. J.; Hammock B. D. Magnetic bead-based phage anti-immunocomplex assay (PHAIA) for the detection of the urinary biomarker 3-phenoxybenzoic acid to assess human exposure to pyrethroid insecticides. Anal. Biochem. 2009, 386 (1), 45–52. 10.1016/j.ab.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H. J.; McCoy M.; Gee S. J.; Gonzalez-Sapienza G. G.; Hammock B. D. Noncompetitive Phage Anti-Immunocomplex Real-Time Polymerase Chain Reaction for Sensitive Detection of Small Molecules. Anal. Chem. 2011, 83 (1), 246–253. 10.1021/ac102353z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teruyama K.; Naruse M.; Tsuiki M.; Kobayashi H. Novel chemiluminescent immunoassay to measure plasma aldosterone and plasma active renin concentrations for the diagnosis of primary aldosteronism. Journal of Human Hypertension 2022, 36 (1), 77–85. 10.1038/s41371-020-00465-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi N.; Oyama H.; Suzuki I.; Kato Y.; Umemura T.; Goto J. Oligosaccharide-Assisted Direct Immunosensing of Small Molecules. Anal. Chem. 2010, 82 (11), 4333–4336. 10.1021/ac100865p. [DOI] [PubMed] [Google Scholar]
- Rossotti M. A.; Carlomagno M.; González-Techera A.; Hammock B. D.; Last J.; González-Sapienza G. Phage Anti-Immunocomplex Assay for Clomazone: Two-Site Recognition Increasing Assay Specificity and Facilitating Adaptation into an On-Site Format. Anal. Chem. 2010, 82 (21), 8838–8843. 10.1021/ac101476f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Techera A.; Kim H. J.; Gee S. J.; Last J. A.; Hammock B. D.; Gonzalez-Sapienza G. Polyclonal antibody-based noncompetitive immunoassay for small analytes developed with short peptide loops isolated from phage libraries. Anal. Chem. 2007, 79 (23), 9191–9196. 10.1021/ac7016713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arola H. O.; Tullila A.; Kiljunen H.; Campbell K.; Siitari H.; Nevanen T. K. Specific Noncompetitive Immunoassay for HT-2 Mycotoxin Detection. Anal. Chem. 2016, 88 (4), 2446–2452. 10.1021/acs.analchem.5b04591. [DOI] [PubMed] [Google Scholar]
- Zou W.; Shi R.; Wang G.; Zhao Z.; Zhao F.; Yang Z. Rapid and sensitive noncompetitive immunoassay for detection of aflatoxin B1 based on anti-immune complex peptide. Food Chem. 2022, 393, 133317 10.1016/j.foodchem.2022.133317. [DOI] [PubMed] [Google Scholar]
- Carlomagno M.; Lassabe G.; Rossotti M.; Gonzalez-Techera A.; Vanrell L.; Gonzalez-Sapienza G. Recombinant Streptavidin Nanopeptamer Anti-Immunocomplex Assay for Noncompetitive Detection of Small Analytes. Anal. Chem. 2014, 86 (20), 10467–10473. 10.1021/ac503130v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassabe G.; Rossotti M.; Gonzalez-Techera A.; Gonzalez-Sapienza G. Shiga-Like Toxin B Subunit of Escherichia coli as Scaffold for High-Avidity Display of Anti-immunocomplex Peptides. Anal. Chem. 2014, 86 (11), 5541–5546. 10.1021/ac500926f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J.; Liu Z.; Li G.; Li J.; Kim H.-J.; Shelver W. L.; Li Q. X.; Xu T. Simultaneous development of both competitive and noncompetitive immunoassays for 2,2′,4,4′-tetrabromodiphenyl ether using phage-displayed peptides. Anal. Bioanal. Chem. 2013, 405 (29), 9579–9583. 10.1007/s00216-013-7364-5. [DOI] [PubMed] [Google Scholar]
- Peltomaa R.; Abbas A.; Yli-Mattila T.; Lamminmäki U. Single-step noncompetitive immunocomplex immunoassay for rapid aflatoxin detection. Food Chem. 2022, 392, 133287 10.1016/j.foodchem.2022.133287. [DOI] [PubMed] [Google Scholar]
- Cavalier E.; Lukas P.; Bekaert A.-C.; Peeters S.; Le Goff C.; Yayo E.; Delanaye P.; Souberbielle J.-C. Analytical and clinical evaluation of the new Fujirebio Lumipulse G noncompetitive assay for 25(OH)-vitamin D and three immunoassays for 25(OH)D in healthy subjects, osteoporotic patients, third trimester pregnant women, healthy African subjects, hemodialyzed and intensive care patients. Clinical Chemistry and Laboratory Medicine (CCLM) 2015, 54 (8), 1347–1355. 10.1515/cclm-2015-0923. [DOI] [PubMed] [Google Scholar]; Parra M.; Foj L.; Filella X. Automated measurement of 25-OH Vitamin D on the LUMIPULSE G1200: analytical verification and method comparison. British Journal of Biomedical Science 2016, 73 (3), 104–109. 10.1080/09674845.2016.1188478. [DOI] [PubMed] [Google Scholar]
- Saleh L.; Mueller D.; von Eckardstein A. Analytical and clinical performance of the new Fujirebio 25-OH vitamin D assay, a comparison with liquid chromatography-tandem mass spectrometry (LC-MS/MS) and three other automated assays. Clin. Chem. Lab. Med. 2016, 54 (4), 617–625. 10.1515/cclm-2015-0427. [DOI] [PubMed] [Google Scholar]
- Dong J. X.; Xu C.; Wang H.; Xiao Z. L.; Gee S. J.; Li Z. F.; Wang F.; Wu W. J.; Shen Y. D.; Yang J. Y.; et al. Enhanced Sensitive Immunoassay: Noncompetitive Phage Anti-Immune Complex Assay for the Determination of Malachite Green and Leucomalachite Green. J. Agric. Food Chem. 2014, 62 (34), 8752–8758. 10.1021/jf5019824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossotti M. A.; Carlomagno M.; Gonzalez-Techera A.; Hammock B. D.; Last J.; Gonzalez-Sapienza G. Phage Anti-Immunocomplex Assay for Clomazone: Two-Site Recognition Increasing Assay Specificity and Facilitating Adaptation into an On-Site Format. Anal. Chem. 2010, 82 (21), 8838–8843. 10.1021/ac101476f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedrioli A.; Oxenius A. Single B cell technologies for monoclonal antibody discovery. Trends in Immunology 2021, 42 (12), 1143–1158. 10.1016/j.it.2021.10.008. [DOI] [PubMed] [Google Scholar]