Abstract
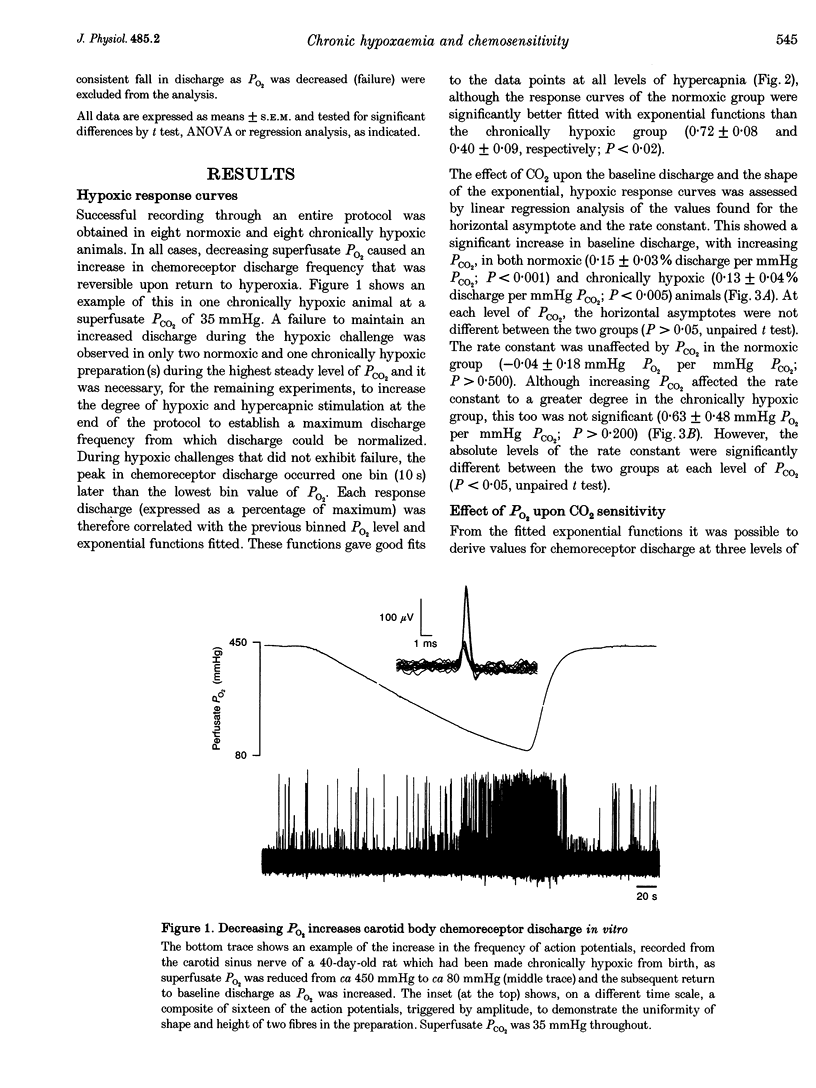
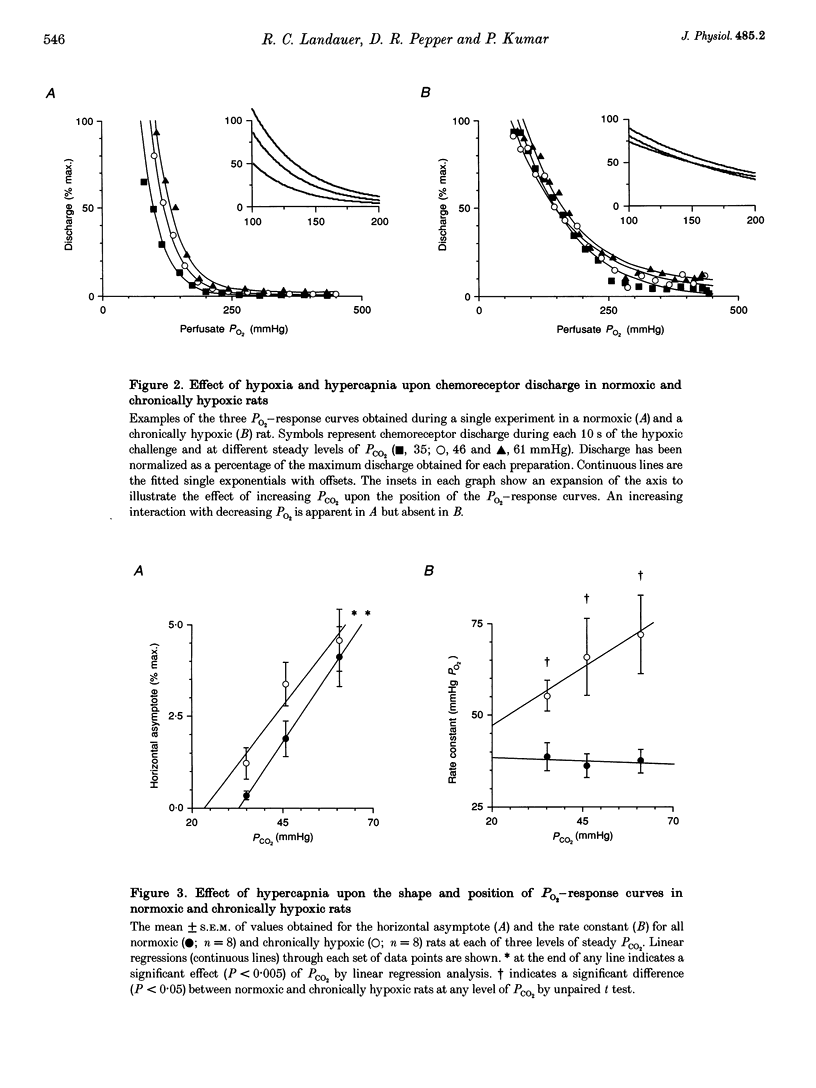
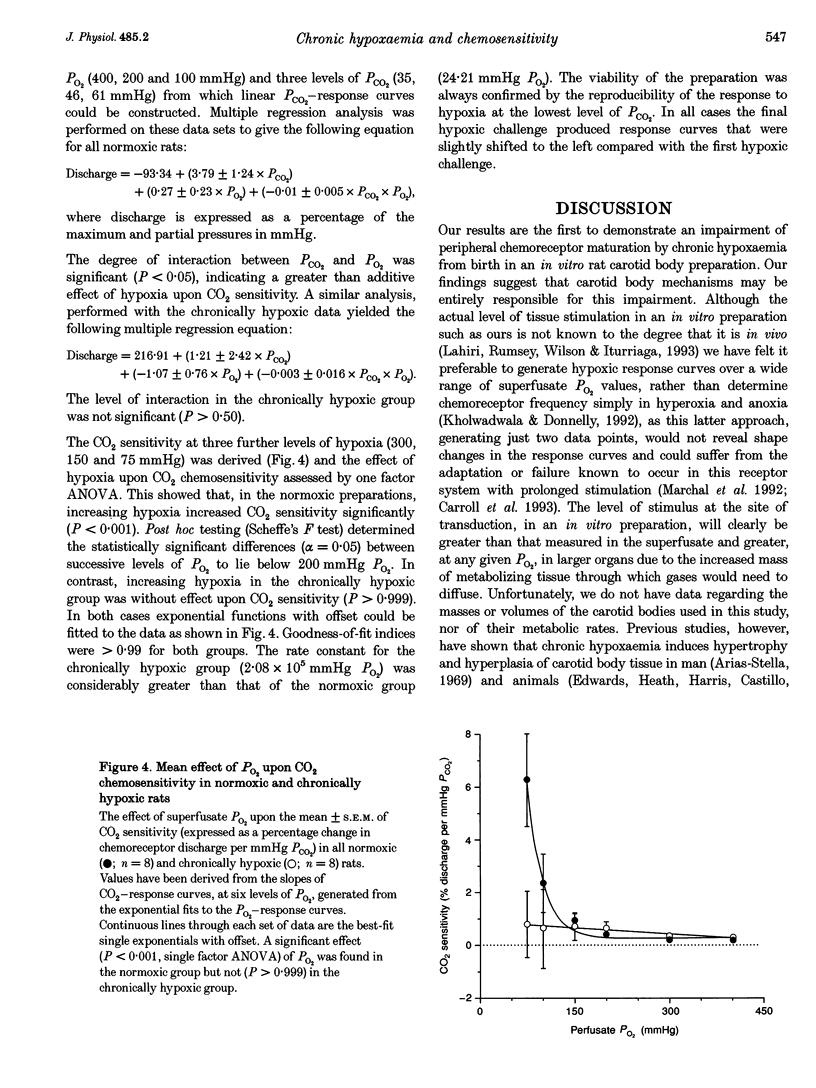
1. The effect of chronic hypoxaemia upon in vitro carotid body chemosensitivity was observed in eight rats > 5 weeks of age born and reared in 12% oxygen. Comparisons were made with eight age-matched normoxic rats. 2. Single exponential functions with offset were fitted to the normalized (percentage of maximum) discharge responses to ramp decreases in PO2 at three steady levels of PCO2. CO2 sensitivity was derived from these functions. 3. Increasing hypercapnia increased the horizontal asymptote of the exponential functions in the normoxic (0.15 +/- 0.03% discharge per mmHg PCO2; P < 0.001) and chronically hypoxic (0.13 +/- 0.04% discharge per mmHg PCO2; P < 0.005) animals but was without effect upon the rate constants in both groups (-0.04 +/- 0.18 mmHg PO2 per mmHg PCO2, P > 0.50 and 0.63 +/- 0.48 mmHg PO2 per mmHg PCO2, P > 0.20, respectively). Rate constants were greater in the chronically hypoxic animals (P < 0.05) compared with the normoxic animals. 4. CO2 chemosensitivity increased with decreasing PO2 in normoxic (P < 0.05) but not in chronically hypoxic (P > 0.50) rats. 5. Our results show that chronic hypoxaemia from birth attenuates the maturation of CO2-O2 interaction at the carotid body.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acker H., Dufau E., Huber J., Sylvester D. Indications to an NADPH oxidase as a possible pO2 sensor in the rat carotid body. FEBS Lett. 1989 Oct 9;256(1-2):75–78. doi: 10.1016/0014-5793(89)81721-1. [DOI] [PubMed] [Google Scholar]
- Barnard P., Andronikou S., Pokorski M., Smatresk N., Mokashi A., Lahiri S. Time-dependent effect of hypoxia on carotid body chemosensory function. J Appl Physiol (1985) 1987 Aug;63(2):685–691. doi: 10.1152/jappl.1987.63.2.685. [DOI] [PubMed] [Google Scholar]
- Biscoe T. J., Duchen M. R. Cellular basis of transduction in carotid chemoreceptors. Am J Physiol. 1990 Jun;258(6 Pt 1):L271–L278. doi: 10.1152/ajplung.1990.258.6.L271. [DOI] [PubMed] [Google Scholar]
- Blanco C. E., Dawes G. S., Hanson M. A., McCooke H. B. The response to hypoxia of arterial chemoreceptors in fetal sheep and new-born lambs. J Physiol. 1984 Jun;351:25–37. doi: 10.1113/jphysiol.1984.sp015229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco C. E., Hanson M. A., McCooke H. B. Effects on carotid chemoreceptor resetting of pulmonary ventilation in the fetal lamb in utero. J Dev Physiol. 1988 Apr;10(2):167–174. [PubMed] [Google Scholar]
- Buckler K. J., Vaughan-Jones R. D. Effects of hypercapnia on membrane potential and intracellular calcium in rat carotid body type I cells. J Physiol. 1994 Jul 1;478(Pt 1):157–171. doi: 10.1113/jphysiol.1994.sp020239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckler K. J., Vaughan-Jones R. D. Effects of hypoxia on membrane potential and intracellular calcium in rat neonatal carotid body type I cells. J Physiol. 1994 May 1;476(3):423–428. doi: 10.1113/jphysiol.1994.sp020143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calder N. A., Williams B. A., Smyth J., Boon A. W., Kumar P., Hanson M. A. Absence of ventilatory responses to alternating breaths of mild hypoxia and air in infants who have had bronchopulmonary dysplasia: implications for the risk of sudden infant death. Pediatr Res. 1994 Jun;35(6):677–681. doi: 10.1203/00006450-199406000-00011. [DOI] [PubMed] [Google Scholar]
- Carroll J. L., Bamford O. S., Fitzgerald R. S. Postnatal maturation of carotid chemoreceptor responses to O2 and CO2 in the cat. J Appl Physiol (1985) 1993 Dec;75(6):2383–2391. doi: 10.1152/jappl.1993.75.6.2383. [DOI] [PubMed] [Google Scholar]
- Delpiano M. A., Hescheler J. Evidence for a PO2-sensitive K+ channel in the type-I cell of the rabbit carotid body. FEBS Lett. 1989 Jun 5;249(2):195–198. doi: 10.1016/0014-5793(89)80623-4. [DOI] [PubMed] [Google Scholar]
- Eden G. J., Hanson M. A. Maturation of the respiratory response to acute hypoxia in the newborn rat. J Physiol. 1987 Nov;392:1–9. doi: 10.1113/jphysiol.1987.sp016765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards C., Heath D., Harris P., Castillo Y., Krüger H., Arias-Stella J. The carotid body in animals at high altitude. J Pathol. 1971 Aug;104(4):231–238. doi: 10.1002/path.1711040404. [DOI] [PubMed] [Google Scholar]
- Hanson M. A., Kumar P., Williams B. A. The effect of chronic hypoxia upon the development of respiratory chemoreflexes in the newborn kitten. J Physiol. 1989 Apr;411:563–574. doi: 10.1113/jphysiol.1989.sp017590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt C. E., McCulloch K., Brouillette R. T. Diminished hypoxic ventilatory responses in near-miss sudden infant death syndrome. J Appl Physiol Respir Environ Exerc Physiol. 1981 Jun;50(6):1313–1317. doi: 10.1152/jappl.1981.50.6.1313. [DOI] [PubMed] [Google Scholar]
- Kholwadwala D., Donnelly D. F. Maturation of carotid chemoreceptor sensitivity to hypoxia: in vitro studies in the newborn rat. J Physiol. 1992;453:461–473. doi: 10.1113/jphysiol.1992.sp019239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch G., Wendel H. Adjustment of arterial blood gases and acid base balance in the normal newborn infant during the first week of life. Biol Neonat. 1968;12(3):136–161. doi: 10.1159/000240100. [DOI] [PubMed] [Google Scholar]
- Lahiri S., Brody J. S., Motoyama E. K., Velasquez T. M. Regulation of breathing in newborns at high altitude. J Appl Physiol Respir Environ Exerc Physiol. 1978 May;44(5):673–678. doi: 10.1152/jappl.1978.44.5.673. [DOI] [PubMed] [Google Scholar]
- Lahiri S., DeLaney R. G. Stimulus interaction in the responses of carotid body chemoreceptor single afferent fibers. Respir Physiol. 1975 Sep;24(3):249–266. doi: 10.1016/0034-5687(75)90017-1. [DOI] [PubMed] [Google Scholar]
- Lahiri S., Rumsey W. L., Wilson D. F., Iturriaga R. Contribution of in vivo microvascular PO2 in the cat carotid body chemotransduction. J Appl Physiol (1985) 1993 Sep;75(3):1035–1043. doi: 10.1152/jappl.1993.75.3.1035. [DOI] [PubMed] [Google Scholar]
- López-López J., González C., Ureña J., López-Barneo J. Low pO2 selectively inhibits K channel activity in chemoreceptor cells of the mammalian carotid body. J Gen Physiol. 1989 May;93(5):1001–1015. doi: 10.1085/jgp.93.5.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchal F., Bairam A., Haouzi P., Crance J. P., Di Giulio C., Vert P., Lahiri S. Carotid chemoreceptor response to natural stimuli in the newborn kitten. Respir Physiol. 1992 Feb;87(2):183–193. doi: 10.1016/0034-5687(92)90058-5. [DOI] [PubMed] [Google Scholar]
- Mills L., Nurse C. Chronic hypoxia in vitro increases volume of dissociated carotid body chemoreceptors. Neuroreport. 1993 Jun;4(6):619–622. doi: 10.1097/00001756-199306000-00004. [DOI] [PubMed] [Google Scholar]
- Mortola J. P., Morgan C. A., Virgona V. Respiratory adaptation to chronic hypoxia in newborn rats. J Appl Physiol (1985) 1986 Oct;61(4):1329–1336. doi: 10.1152/jappl.1986.61.4.1329. [DOI] [PubMed] [Google Scholar]
- Mulligan E., Lahiri S., Storey B. T. Carotid body O2 chemoreception and mitochondrial oxidative phosphorylation. J Appl Physiol Respir Environ Exerc Physiol. 1981 Aug;51(2):438–446. doi: 10.1152/jappl.1981.51.2.438. [DOI] [PubMed] [Google Scholar]
- Okubo S., Mortola J. P. Control of ventilation in adult rats hypoxic in the neonatal period. Am J Physiol. 1990 Oct;259(4 Pt 2):R836–R841. doi: 10.1152/ajpregu.1990.259.4.R836. [DOI] [PubMed] [Google Scholar]
- Peers C. Hypoxic suppression of K+ currents in type I carotid body cells: selective effect on the Ca2(+)-activated K+ current. Neurosci Lett. 1990 Nov 13;119(2):253–256. doi: 10.1016/0304-3940(90)90846-2. [DOI] [PubMed] [Google Scholar]
- Sorensen S. C., Severinghaus J. W. Respiratory insensitivity to acute hypoxia persisting after correction of tetralogy of Fallot. J Appl Physiol. 1968 Sep;25(3):221–223. doi: 10.1152/jappl.1968.25.3.221. [DOI] [PubMed] [Google Scholar]
- Stea A., Jackson A., Nurse C. A. Hypoxia and N6,O2'-dibutyryladenosine 3',5'-cyclic monophosphate, but not nerve growth factor, induce Na+ channels and hypertrophy in chromaffin-like arterial chemoreceptors. Proc Natl Acad Sci U S A. 1992 Oct 15;89(20):9469–9473. doi: 10.1073/pnas.89.20.9469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatsumi K., Pickett C. K., Weil J. V. Attenuated carotid body hypoxic sensitivity after prolonged hypoxic exposure. J Appl Physiol (1985) 1991 Feb;70(2):748–755. doi: 10.1152/jappl.1991.70.2.748. [DOI] [PubMed] [Google Scholar]
- Williams B. A., Smyth J., Boon A. W., Hanson M. A., Kumar P., Blanco C. E. Development of respiratory chemoreflexes in response to alternations of fractional inspired oxygen in the newborn infant. J Physiol. 1991 Oct;442:81–90. doi: 10.1113/jphysiol.1991.sp018783. [DOI] [PMC free article] [PubMed] [Google Scholar]


