Abstract
Minimal change disease (MCD) is a universal primary glomerular disease contributing to nephrotic syndrome. Lon peptidase 1 (LONP1) has been suggested to protect podocytes from damage during the progression of MCD. Accordingly, our research further explored the specific mechanisms of LONP1. Initially, the expressions of TRPC6, p-ERK1/2, and LONP1 in the kidney tissues of MCD patients were detected by immunohistochemistry and Western blot. Human podocytes AB8/13 were serially subjected to transfection with shTRPC6/shNC, and 48-h treatment with 30 µg/ml puromycin aminonucleoside (PAN). The viability, apoptosis, and migration of AB8/13 cells were assessed by cell counting kit-8, flow cytometry, and transwell assays. The mRNA and protein expressions of LONP1 were downregulated while those of TRPC6 were upregulated in the kidney tissues of MCD patients. PAN induced podocyte injury and migration and inhibited LONP1 expression, whereas TRPC6 silencing did oppositely. The phosphorylation level of ERK1/2 was reduced in MCD samples, which was negatively associated with TRPC6 expression and positively associated with LONP1 expression. Furthermore, ERK phosphorylation agonist offset the effects of TRPC6 silencing on mitigating podocyte injury and migration as well as upregulating LONP1 expression. Collectively, TRPC6 knockdown-induced ERK1/2 inactivation can ameliorate podocyte injury in MCD by increasing the expression of LONP1.
Keywords: Transient receptor potential cation channel subfamily C member 6, extracellular signal-regulated kinase 1/2, podocyte injury, Lon peptidase 1
Introduction
Minimal change disease (MCD) is one of the most common pathological types of nephrotic syndrome [1]. MCD is pathologically characterized by the disappearance of diffuse podocyte foot process, and the appearance of flat cell body, visible vacuoles and microvilli under the electron microscope, with the clinical manifestation of massive proteinuria (the protein content exceeding the normal range in urine) and hypoalbuminemia (plasma albumin concentration below normal range) [2,3]. Under the light microscope, the structure of glomerulus is generally normal, and the epithelial cells of renal proximal convoluted tubules show glassy droplets and a large number of lipids [3]. The exact pathogenesis of MCD has not yet been clarified, but humoral immunity, podocyte damage, respiratory virus and immune dysfunction have been evidenced as contributors for MCD [4–6]. Glucocorticoids are the cornerstone of MCD treatment; however, MCD patients receiving hormone therapy may experience repeated relapse, hormone dependence or even drug resistance, and finally develop end-stage renal disease despite high treatment efficacy [7]. Therefore, effective and complete treatment of MCD cannot be guaranteed under traditional hormone therapy (mainly through supplementing the missing glucocorticoids in the body) [8], necessitating the exploration on new therapeutic targets.
At present, some studies have explored immunosuppressive regimens to treat MCD. For instance, cyclophosphamide has been confirmed to help hormone-dependent MCD patients withdraw from glucocorticoids [9]. Calcineurin inhibitors such as oral cyclosporine and FK506 can mitigate and reduce recurrence of MCD in patients [10]. Given the limited evidence for the treatment of MCD with mycophenolate mofetil, it is only recommended for use in cases of intolerance to glucocorticoids, cyclophosphamide, and calcineurin inhibitors [11]. In addition to the previously known T cells, B cells are also involved in the pathogenesis of MCD, and anti-CD20 monoclonal antibodies have been applied to treat frequently recurrent MCD [12]. In view of limited studies reporting the efficacy of immunosuppressants on MCD, further verification by studies with a larger sample size is needed.
In recent years, with the in-depth study of podocytes, the pathogenesis of MCD has been proven to be inextricably related to podocyte injury [13,14]. Besides, Gabriel Cara-Fuentes et al. unveiled that the occurrence and development of MCD are closely associated with cytoskeletal protein, CD80 and angiopoietin in podocytes [15]. Accordingly, we inferred that the key to treating MCD lies in protecting the structure and function of renal podocytes in patients.
The mitochondrial protease Lon protease 1 (LONP1) is an adenosine triphosphate (ATP)-dependent protease that maintains mitochondrial metabolism by clearing abnormal proteins and repairing mitochondrial DNA [16], and its ability to protect podocyte from injury in MCD has recently been confirmed [17]. Therefore, our study further explored the specific mechanism of LONP1 in MCD. According to the study of Ji-Eun Kim et al. transient receptor potential cation channel subfamily C member 6 (TRPC6)-mediated extracellular signal-regulated kinase 1/2 (ERK1/2) activation potentiates the resistance of dentate granule cells to status epilepticus via regulating LONP1 expression and mitochondrial dynamics [18]. Furthermore, multiple factors including angiotensin II and reactive oxygen species (ROS) in the diabetic nephropathy milieu contribute to a dramatic increase in calcium influx through TRPC6 channels, leading to podocyte hypertrophy and foot process effacement [19,20]. It is well established that TRPC family members play a role in the progression of glomerular disease and the pathogenesis of chronic kidney disease (CKD) [21]. Taken together, we speculated that TRPC6 is involved in the pathogenesis of MCD by regulating LONP1 expression to hamper ERK1/2 activation.
Materials and methods
Ethical statement
Kidney tissue samples were collected from MCD patients in the First Affiliated Hospital of Ningbo University from August 2023 to November 2023, and adjacent kidney tissues (excluding the tissue infiltrated by cancer histiocytes) from patients with renal tumors undergoing surgical resection were used as controls. The experimental protocols were approved by the Ethics Committee of the First Affiliated Hospital of Ningbo University (Ethics Approval of the First Affiliated Hospital of Ningbo University No. 087 A, 2023), and the written informed consent was signed by all participants.
Immunohistochemistry
The expressions of TRPC6, ERK1/2 and LONP1 in the kidney tissues were assessed by immunohistochemistry. In short, the collected kidney tissues were fixed in 4% paraformaldehyde (NBS0135, NoninBio, China) at 4 °C for 24 h, embedded in paraffin, and sliced to serial coronal sections (thickness: 4 μm) in sequence. Thereafter, tissue sections were dewaxed, and hydrated with xylene (C0430530223, Nanjing Reagent, China) and gradient ethanol. Prior to immunohistochemistry staining, the heat-mediated antigen retrieval was performed. The sections were soaked in citrate buffer (pH 6.0), heated to 95 °C for 15 min by microwave and later cooled to room temperature for 20 min. Post 5-min washing twice in distilled water and 1-h blockage with 5% goat serum (C0265, Beyotime, China), incubation of the sections with anti-TRPC6 (1:200, ab233413, abcam, UK), anti-p-ERK1/2 (1:100, ab201015, abcam, UK) or anti-LONP1 (1:25, ab224316, abcam, UK) primary antibody at 4 °C overnight was carried out. Afterwards, the sections serially experienced washing thrice with phosphate buffered saline (PBS, BUF036A, Bio-Rad, USA), 1-h incubation with the goat anti-rabbit (ab6721, 1:1000, abcam, UK) secondary antibody at 37 °C, and washing with PBS again. Following 3,3′-diaminobenzidine (DAB) color development (P0202, Beyotime, China) and hematoxylin (C0107, Beyotime, China) counterstaining, the sections were subjected to differentiation in hydrochloric acid and alcohol, dehydration, transparentization and mounting. Finally, observation of sections was conducted under the microscope (VHX-7000N, Keyence, China) at the magnification of ×100.
Cell culture and treatment
As per a previous study [22], human podocytes AB8/13 from American Type Culture Collection (ATCC, USA) were proliferated in RPMI 1640 medium (12633012, Thermo Fisher, USA) containing 10% fetal bovine serum (FBS, 04-010-1B, Novobio, China), 100 U/mL penicillin-streptomycin (03-031-1B, Novobio, China) as well as 1% Insulin-Transferrin-Selenium (ITS, 214505, Novobio, China) at 33 °C with 5% CO2. Next, AB8/13 cells were incubated with 30 µg/ml puromycin aminonucleoside (PAN, an amino nucleoside antibiotic that can induce cell apoptosis, HY-15695, MedChemExpress, China) for 48 h to mimic MCD in vitro [23]. The cells were treated with 200 nM phosphorylated ERK (p-ERK) agonist Ro 67-7476 (HY-100403, MedChemExpress, China) for 1 h [24].
Transfection
The short hairpin RNA (shRNA) targeting TRPC6 (shTRPC6, CCCCCTTAAGTGGTGACTTTTCC) and its negative control (shNC) were purchasable from Genepharma (China). As for transfection, AB8/13 cells were cultured in 6-well plates at a density of 1 × 105 cells/well until reaching 80% confluence. Next, 5 µL of Escort III transfection reagent (L3037, Sigma-Aldrich, USA) and shTRPC6/shNC (2 µg) were separately diluted in serum-free RPMI 1640 medium and later mixed. The formed stable complex was cultured with AB8/13 cells for 48 h, and the specificity of shTRPC6-mediated gene silencing was determined by quantitative real-time PCR (qRT-PCR).
qRT-PCR
Total RNA extraction from AB8/13 cells was performed using Trizol reagent (KGA1202, KeyGEN BioTECH, China), followed by cDNA generation with an Advantage RT-for-PCR Kit (639505, Clontech, USA). The qPCR was conducted employing EmeraldAmp MAX PCR Master Mix (RR320Q, Takara, Japan) in a SmartChip Real-Time PCR System (640022, Clontech, USA), with the reaction condition of predenaturation (2 min at 95 °C) and 45 thermal cycles (15 s at 95 °C, 10 s at 60 °C and 10 s at 72 °C). The expression of TRPC6 was standardized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and calculated by 2-ΔΔCT method [25]. The primer information was listed as follows: TRPC6, (forward) TACAGGACAGGCTTTGGCTG, (reverse) AGAGACTCTCCAGCCCTCAG; GAPDH, (forward) GGTCACCAGGGCTGCTTTTA, (reverse) CCCGTTCTCAGCCATGTAGT.
Cell counting kit-8 (CCK-8) assay
Following the transfection, AB8/13 cells (5 × 103 per well) were introduced into each well of 96-well plates and cultured for 24 h. Later, 20 μL CCK-8 solution (E606335, Sangon, China) was added for 4 h of culture. The absorbance was tested at 450 nm via the microplate reader (SpectraMax 190, Molecular Devices, China).
Flow cytometry
To analyze the apoptosis rate of AB8/13 cells, 5 × 104 cells were collected, washed twice with PBS, and suspended in 195 μL binding buffer (C1062S, Beyotime, China). Next, cell suspension was cultivated with 5 μL Annexin V-FITC and 10 μL propidium iodide (C1062S, Beyotime) in the dark for 20 min, and the percentage of apoptotic cells was evaluated by an Accuri C6 plus flow cytometer (Becton Dickinson, USA).
Transwell assay
A Transwell chamber (351184) was purchased from Corning Incorporation (USA), of which the upper chamber was full of serum-free medium and the lower chamber was filled with medium containing 10% FBS. After being seeded to the upper chamber, cells were cultured for 24 h at 37 °C. Later, cells migrating into the lower chamber were fixed in 4% paraformaldehyde (P1110, Solarbio, China), stained by crystal violet (V5265, Sigma, USA), photographed with the microscope (magnification: ×250) and counted for migration analysis.
Western blot
Total protein was isolated from AB8/13 cells by RIPA buffer (P0013B, Beyotime, China), and the protein concentration was quantitated with Bradford assay kit (23236, Thermo Fisher, USA). Next, proteins were subjected to electrophoresis with SDS-PAGE gels (R21449, Yuanye, China), transferred onto polyvinylidene fluoride (PVDF) membranes (G6015-1, Servicebio, USA), and then blocked in 3% nonfat dry milk in TBS with Tween-20 (TBST, ST673, Beyotime, China). Subsequently, the membranes were probed with primary antibodies against TRPC6 (1:1000, ab105845, 106 kDa, abcam, UK), LONP1 (1:10000, ab224316, 106 kDa, abcam, UK), ERK1/2 (1:10000, ab184699, 42, 44 kDa, abcam, UK), p-ERK1/2 (1:1000, 42, 44 kDa, #9101, Cell Signaling Technology, USA), and the loading control GAPDH (1:1000, 37 kDa, #5174, Cell Signaling Technology, USA) at 4 °C for 12 h. HRP-conjugated goat anti-rabbit IgG H&L (1:10000, AS014, ABclonal, China) or goat anti-mouse IgG H&L (1:10000, ab6789, Abcam) was then incubated with the membranes. Thereafter, the membranes were exposed to ECL basic kit (RM00020, ABclonal, China) for 1 min, and the images were captured by ImageQuant LAS 4000MINI Ultra-Sensitive Chemiluminescence Imager (Sinopharm Chemical Reagent Co. Ltd, China), followed by digitization under ImageJ software (National Institutes of Health, USA).
Statistical analysis
Measurement data were described as the means ± standard deviation. The comparison among multiple groups was conducted by one-way ANOVA, and GraphPad Prism 8.0 was utilized to analyze statistical data. p < 0.05 was regarded to be statistically different.
Results
TRPC6 expression was upregulated while ERK phosphorylation and LONP1 expression were downregulated in the kidney tissues of MCD patients
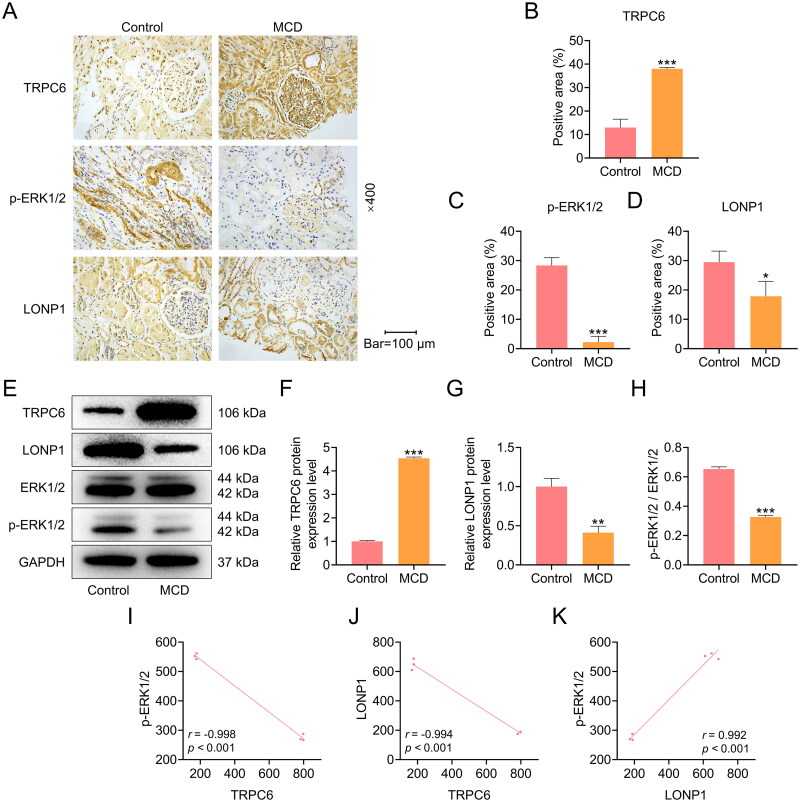
The expressions of TRPC6-ERK1/2-LONP1 axis in the glomeruli of clinical kidney tissues were examined by immunohistochemistry and Western blot. As displayed in Figures 1A to 1H, the expression of TRPC6 was elevated in the kidney tissues of MCD patients in comparison with adjacent normal kidney tissues from patients with renal tumors (p < 0.001). In addition, levels of ERK phosphorylation, p-ERK/ERK and LONP1 were reduced in the kidney tissues of MCD group relative to Control group (p < 0.05, Figure 1A-1H). Through expression correlation analysis, the negative correlation between TRPC6 with p-ERK1/2 (r = −0.998, p < 0.001, Figure 1I) and LONP1 (r = −0.994, p < 0.001, Figure 1J), and a positive correlation between p-ERK1/2 with LONP1 were detected (r = 0.992, p < 0.001, Figure 1K).
Figure 1.
The protein expressions of TRPC6, LONP1 and p-ERK1/2 in the kidney tissues of MCD patients.
(A-D) The expressions of TRPC6, p-ERK1/2, and LONP1 in the kidney tissues of MCD patients were detected by immunohistochemistry (magnification: ×400, scale bar = 100 µm). (E-H) The protein expressions of TRPC6, p-ERK1/2, ERK1/2 and LONP1 in the kidney tissues were measured by Western blot. GAPDH was used as a loading control. (I) The correlation between TRPC6 and p-ERK1/2 (r = -0.998, p < 0.001). (J) The correlation between TRPC6 and LONP1 (r = -0.994, p < 0.001). (K) The correlation between LONP1 and p-ERK1/2 (r = 0.992, p < 0.001). The data are presented as the mean ± standard deviation of three independent experiments; **p < 0.01, ***p < 0.001 vs. Control. Abbreviation: TRPC6, transient receptor potential cation channel subfamily C member 6; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; LONP1, Lon peptidase 1; ERK, extracellular signal-regulated kinase; p-ERK, phosphorylated ERK.
TRPC6 silencing reversed the effect of PAN on the viability, apoptosis, and migration of AB8/13 podocytes via regulating ERK phosphorylation and LONP1 expression
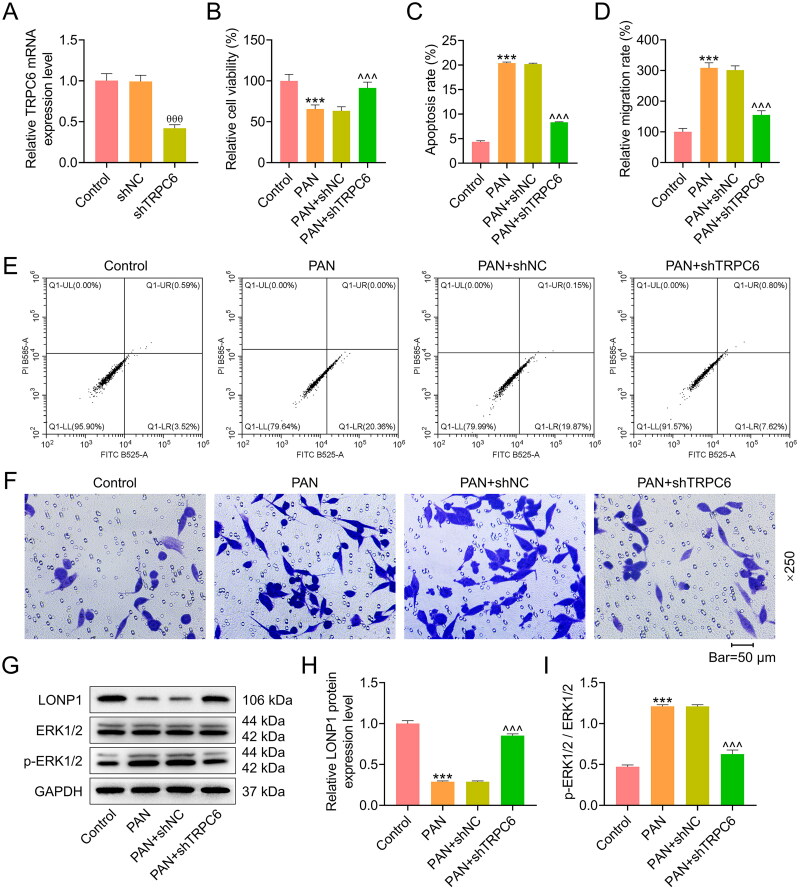
To further clarify the role of TRPC6-ERK1/2-LONP1 axis in MCD, AB8/13 podocytes were transfected with shTRPC6 and then treated with PAN. Following the transfection, we observed that TRPC6 mRNA expression was remarkably diminished in the shTRPC6 group (p < 0.001, Figure 2A), indicating the successful transfection. The cell viability was weakened, and the apoptosis and migration rates were increased in the PAN group (p < 0.001, Figure 2B-2F); however, such effects of PAN were all reversed by shTRPC6 (p < 0.001, Figure 2B-2F). Besides, PAN treatment caused a reduction in the protein expression of LONP1 and an increase in the ratio of p-ERK1/2 to ERK1/2 (p < 0.001, Figure 2G-2I). Conversely, AB8/13 podocytes after PAN treatment and shTRPC6 transfection exhibited a higher LONP1 protein level and a lower p-ERK1/2 to ERK1/2 ratio (p < 0.001, Figure 2G-2I).
Figure 2.
Effects of TRPC6 silencing on viability, apoptosis, migration, ERK phosphorylation and LONP1 expression in PAN-treated podocytes.
AB8/13 cells were transfected with shTRPC6 or shNC, and then treated with 30 µg/ml PAN for 48 h. (A) The efficiency of TRPC6 knockdown was determined by qRT-PCR. GAPDH was used as a housekeeping gene control. (B) The viability of AB8/13 cells in the Control, PAN, PAN+shNC, and PAN+shTRPC6 groups was assessed by cell counting kit-8 assay. (C, E) The apoptosis rate of AB8/13 cells in each group was detected by flow cytometry. (D, F) The percentage of migrating cells in each group was determined by transwell assay (magnification: ×250, scale bar = 50 µm). (G-I) The protein expression level of LONP1 and the ratio of p-ERK1/2/ERK1/2 were measured by Western blot. GAPDH was taken as the loading control. The data are presented as the mean ± standard deviation of three independent experiments; ***p < 0.001 vs. Control; θθθp < 0.001 vs. shNC; ^^^p < 0.001 vs. PAN+shNC. Abbreviation: TRPC6, transient receptor potential cation channel subfamily C member 6; shTRPC6, short hairpin RNA targeting TRPC6; shNC, shRNA negative control; PAN, puromycin aminonucleoside; qRT-PCR, quantitative real-time PCR; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; LONP1, Lon peptidase 1; ERK, extracellular signal-regulated kinase; p-ERK, phosphorylated ERK.
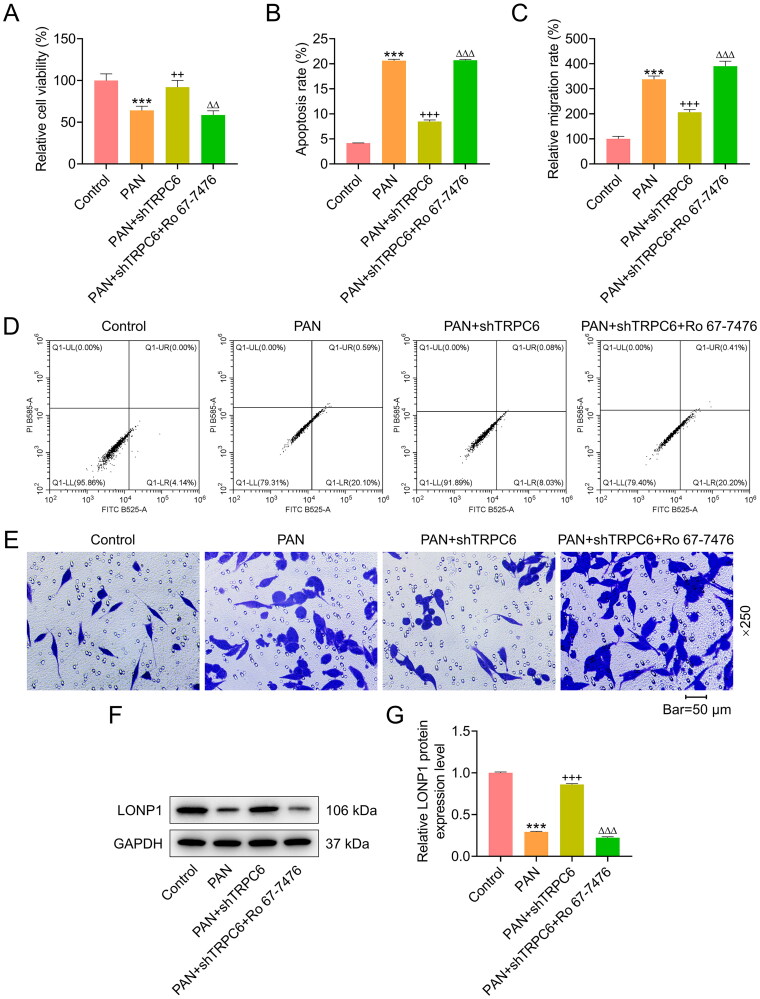
ERK phosphorylation agonist Ro 67-7476 offset the effect of TRPC6 silencing on the viability, apoptosis and migration of PAN-treated podocytes
In an attempt to verify whether ERK phosphorylation makes profound impacts upon podocytes transfected with shTRPC6 and then exposed to PAN, ERK phosphorylation agonist Ro 67-7476 was utilized to treat AB8/13 podocytes. According to CCK-8 assay results, Ro 67-7476 attenuated the influences of PAN and TRPC6 silencing on increasing podocyte viability (p < 0.01, Figure 3A). Ro 67-7476 also offset the inhibiting effect of TRPC6 silencing on the apoptotic and migratory abilities of PAN-treated podocytes (p < 0.001, Figure 3B-3E). Furthermore, the protein level of LONP1 was markedly lower in the PAN+shTRPC6 + Ro 67-7476 group than the PAN+shTRPC6 group (p < 0.001, Figure 3F-3G).
Figure 3.
Effects of ERK phosphorylation agonist Ro 67-7476 on viability, apoptosis, migration, and LONP1 expression in podocytes co-treated with PAN and shTRPC6.
AB8/13 cells were transfected with shTRPC6, and then treated with 30 µg/ml PAN for 48 h and Ro 67-7476 for 1 h. (A) The viability of AB8/13 cells in the Control, PAN, PAN+shTRPC6, and PAN+shTRPC6 + Ro 67-7476 groups was detected by cell counting kit-8 assay. (B, D) The apoptosis rate of AB8/13 cells in each group was evaluated by flow cytometry. (C, E) The migration rate of AB8/13 cells in each group was determined by transwell assay (magnification: ×250, scale bar = 50 µm). (F-G) LONP1 protein expression in each group was quantified by Western blot. GAPDH was taken as the loading control. The data are presented as the mean ± standard deviation of three independent experiments; ***p < 0.001 vs. Control; ++p < 0.01, +++p < 0.001 vs. PAN; △△p < 0.01, △△△p < 0.001 vs. PAN+shTRPC6. Abbreviation: TRPC6, transient receptor potential cation channel subfamily C member 6; shTRPC6, short hairpin RNA targeting TRPC6; PAN, puromycin aminonucleoside; LONP1, Lon peptidase 1; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Discussion
The primary function of renal podocytes is to maintain the integrity of the glomerular filtration barrier [4], for which a large amount of energy is required [4]. The stabilization of mitochondria, as the main energy source, is extremely important for podocyte structure and function [26]. Ample studies have shown that podocyte injury and renal dysfunction are induced after podocyte mitochondrial dysfunction, but are significantly improved when podocyte mitochondrial function is restored [27,28]. In addition, it has been evidenced that the expression of LONP1 is essential for maintaining mitochondrial proteostasis and function [29,30]. Deletion or mutation of LONP1 can cause mitochondrial malformation as well as a reduction in mitochondrial DNA expression [31,32]. When LONP1 is deleted in podocytes, mice may suffer from severe proteinuria, impaired renal function, and even a high mortality rate [17]. Therefore, we inferred that upregulation of LONP1 in MCD patients could effectively protect podocytes and alleviate MCD. In this study, we found that the mRNA and protein expressions of LONP1 were diminished in the kidney tissues of MCD patients. However, the specific mechanism of LONP1 at the molecular level is still unclear, and there is no consensus on how to actively and effectively elevate the expression of LONP1 within podocytes.
In a previous study, the expression or activity of LONP1 could be regulated by ERK1/2 [33]. However, there is a knowledge gap regarding the involvement of TRPC6, the upstream regulatory factor of ERK1/2, in the regulation of LONP1. Ji-Eun Kim et al. have reported that activation of TRPC6 can promote ERK1/2 phosphorylation and increase the expression of LONP1, thus improving the mitochondrial function of neuron dentate granule cells [18]. TRPC6 is one of the main members of the transient receptor potential family, possessing a similar expression distribution with LONP1 [34]. TRPC6 is widely expressed in kidney tissues, with the strongest expression in podocytes, especially in the primary process of podocytes close to the fissure membrane, which is one of the structural proteins encoding podocytes [34]. Recent studies have proved that TRPC6 is involved in the pathogenesis of MCD. Clemens C Möller et al. found that TRPC6 is highly expressed in patients with MCD, and is distributed in segments in dense groups [35]. Shengyou Yu et al. unveiled that in the rat model of purinomycin nephropathy, podocyte injury is aggravated with the increase of TRPC6 expression [36]. Inhibition of TRPC6 signal pathway has been evidenced to mitigate podocyte injury [37,38]. In the present study, TRPC6 level was upregulated in MCD samples, which had a negative association with LONP1 expression. Besides, silencing of TRPC6 abrogated PAN-induced podocyte injury, migration and low expression of LONP1. Therefore, our study evidenced for the first time that there was a correlation between the expressions of TRPC6 and LONP1 in patients with MCD, and the regulatory mechanism of TRPC6 on LONP1 expression was further explored.
By reviewing previous literature, we identified that insulin decreases the expression of mouse mitochondrial LONP1 through the activation of ERK signaling pathway [39], while TRPC6 can regulate renal ischemia-reperfusion injury, oxidative stress, as well as autophagic response through the ERK signaling pathway [40], indicating the correlation between LONP1/TRPC6 and ERK signaling pathway. ERK is a serine/threonine kinase belonging to the mitogen activated protein kinases (MAPKs) family, which is implicated in cellular responses including proliferation, differentiation, migration and apoptosis by mediating polypeptide growth factor signaling [41]. Two major isoforms of ERK, ERK1 and ERK2, are proteins encoded by two splice variants of the same gene [42]. A study confirmed the involvement of ERK in the migration, invasion, and epithelial mesenchymal transition of kidney carcinoma cells [43]. In addition, Jie Lei et al. found that ERK1/2 can activate mTOR signaling to participate in high glucose-induced podocyte apoptosis [44]. In the present study, we discovered that the phosphorylation level of ERK1/2 was declined in MCD samples, which was negatively associated with TRPC6 expression and was positively associated with LONP1 expression. ERK phosphorylation agonist could reverse the effect of silenced TRPC6 on mitigating podocyte injury and migration as well as upregulating LONP1 expression. In addition, LONP1 has been reported to protect mitochondrial function and attenuate chronic kidney disease [45]. Due to the important role of LONP1 in regulating mitochondrial gene expression and maintaining mitochondrial stability, the ameliorative effect of TRPC6 on podocyte injury may stem from its regulation on mitochondrial function; however, the necessity for further validation of this relationship remains a priority for future research.
In summary, TRPC6 knockdown-induced ERK1/2 inactivation ameliorates podocyte injury in MCD by increasing the expression of LONP1, indicating its potential in targeted treatment for MCD. Of note, in the future, the in vivo verification experiments will be conducted, and the expression differences of TRPC6-ERK1/2-LONP1 axis in children and adult MCD patients will be explored.
Supplementary Material
Funding Statement
This work was supported by Zhejiang Medicine and Health Science and Technology Project under Grant [No. 2022KY309].
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The analyzed data sets generated during the study are available from the corresponding author on reasonable request.
References
- 1.Vivarelli M, Massella L, Ruggiero B, et al. Minimal change disease. Clin J Am Soc Nephrol. 2017;12(2):332–345. doi: 10.2215/CJN.05000516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dias CB, Pinheiro CC, Silva Vdos S, et al. Proteinuria predicts relapse in adolescent and adult minimal change disease. Clinics (Sao Paulo). 2012;67(11):1271–1274. doi: 10.6061/clinics/2012(11)08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maas RJ, Deegens JK, Smeets B, et al. Minimal change disease and idiopathic FSGS: manifestations of the same disease. Nat Rev Nephrol. 2016;12(12):768–776. doi: 10.1038/nrneph.2016.147. [DOI] [PubMed] [Google Scholar]
- 4.Garg P. A review of podocyte biology. Am J Nephrol. 2018;47(Suppl 1):3–13. doi: 10.1159/000481633. [DOI] [PubMed] [Google Scholar]
- 5.Schönenberger E, Ehrich JH, Haller H, et al. The podocyte as a direct target of immunosuppressive agents. Nephrol Dial Transplant. 2011;26(1):18–24. doi: 10.1093/ndt/gfq617. [DOI] [PubMed] [Google Scholar]
- 6.Zhang H, Wang Z, Dong L, et al. New insight into the pathogenesis of minimal change nephrotic syndrome: role of the persistence of respiratory tract virus in immune disorders. Autoimmun Rev. 2016;15(7):632–637. doi: 10.1016/j.autrev.2016.02.007. [DOI] [PubMed] [Google Scholar]
- 7.Azukaitis K, Palmer SC, Strippoli GF, et al. Interventions for minimal change disease in adults with nephrotic syndrome. Cochrane Database Syst Rev. 2022;3(3):Cd001537. doi: 10.1002/14651858.CD001537.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sim JJ, Bhandari SK, Batech M, et al. End-stage renal disease and mortality outcomes across different glomerulonephropathies in a large diverse US population. Mayo Clin Proc. 2018;93(2):167–178. doi: 10.1016/j.mayocp.2017.10.021. [DOI] [PubMed] [Google Scholar]
- 9.Ponticelli C, Edefonti A, Ghio L, et al. Cyclosporin versus cyclophosphamide for patients with steroid-dependent and frequently relapsing idiopathic nephrotic syndrome: a multicentre randomized controlled trial. Nephrol Dial Transplant. 1993;8(12):1326–1332. [PubMed] [Google Scholar]
- 10.Li X, Li H, Chen J, et al. Tacrolimus as a steroid-sparing agent for adults with steroid-dependent minimal change nephrotic syndrome. Nephrol Dial Transplant. 2008;23(6):1919–1925. doi: 10.1093/ndt/gfm637. [DOI] [PubMed] [Google Scholar]
- 11.Fujinaga S, Ohtomo Y, Hirano D, et al. Mycophenolate mofetil therapy for childhood-onset steroid dependent nephrotic syndrome after long-term cyclosporine: extended experience in a single center. Clin Nephrol. 2009;72(4):268–273. [PubMed] [Google Scholar]
- 12.Fujinaga S, Hirano D, Nishizaki N, et al. Single infusion of rituximab for persistent steroid-dependent minimal-change nephrotic syndrome after long-term cyclosporine. Pediatr Nephrol. 2010;25(3):539–544. doi: 10.1007/s00467-009-1377-5. [DOI] [PubMed] [Google Scholar]
- 13.Li J-S, Chen X, Peng L, et al. Angiopoietin-like-4, a potential target of tacrolimus, predicts earlier podocyte injury in minimal change disease. PLoS One. 2015;10(9):e0137049. doi: 10.1371/journal.pone.0137049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang K, Sun W, Zhang L, et al. miR-499 ameliorates podocyte injury by targeting calcineurin in minimal change disease. Am J Nephrol. 2018;47(2):94–102. doi: 10.1159/000486967. [DOI] [PubMed] [Google Scholar]
- 15.Cara-Fuentes G, Venkatareddy M, Verma R, et al. Glomerular endothelial cells and podocytes can express CD80 in patients with minimal change disease during relapse. Pediatr Nephrol. 2020;35(10):1887–1896. doi: 10.1007/s00467-020-04541-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gibellini L, De Gaetano A, Mandrioli M, et al. The biology of Lonp1: more than a mitochondrial protease. Int Rev Cell Mol Biol. 2020;354:1–61. doi: 10.1016/bs.ircmb.2020.02.005. [DOI] [PubMed] [Google Scholar]
- 17.Gong W, Song J, Liang J, et al. Reduced Lon protease 1 expression in podocytes contributes to the pathogenesis of podocytopathy. Kidney Int. 2021;99(4):854–869. doi: 10.1016/j.kint.2020.10.025. [DOI] [PubMed] [Google Scholar]
- 18.Kim JE, Park H, Choi SH, et al. TRPC6-mediated ERK1/2 activation increases dentate granule cell resistance to status epilepticus via regulating lon protease-1 expression and mitochondrial dynamics. Cells. 2019;8(11):1376. doi: 10.3390/cells8111376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eckel J, Lavin PJ, Finch EA, et al. TRPC6 enhances angiotensin II-induced albuminuria. J Am Soc Nephrol. 2011;22(3):526–535. doi: 10.1681/ASN.2010050522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ilatovskaya DV, Palygin O, Chubinskiy-Nadezhdin V, et al. Angiotensin II has acute effects on TRPC6 channels in podocytes of freshly isolated glomeruli. Kidney Int. 2014;86(3):506–514. doi: 10.1038/ki.2014.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dryer SE, Roshanravan H, Kim EY.. TRPC channels: regulation, dysregulation and contributions to chronic kidney disease. Biochim Biophys Acta Mol Basis Dis. 2019;1865(6):1041–1066. doi: 10.1016/j.bbadis.2019.04.001. [DOI] [PubMed] [Google Scholar]
- 22.Liu D, Liu F, Wang X, et al. MiR-130a-5p prevents angiotensin II-induced podocyte apoptosis by modulating M-type phospholipase A2 receptor. Cell Cycle. 2018;17(21–22):2484–2495. doi: 10.1080/15384101.2018.1542901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kang YL, Saleem MA, Chan KW, et al. Trehalose, an mTOR independent autophagy inducer, alleviates human podocyte injury after puromycin aminonucleoside treatment. PLoS One. 2014;9(11):e113520. doi: 10.1371/journal.pone.0113520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen Y, Goudet C, Pin JP, et al. N-{4-Chloro-2-[(1,3-dioxo-1,3-dihydro-2H-isoindol-2-yl)methyl]phenyl}-2-hydroxybenzamide (CPPHA) acts through a novel site as a positive allosteric modulator of group 1 metabotropic glutamate receptors. Mol Pharmacol. 2008;73(3):909–918. doi: 10.1124/mol.107.040097. [DOI] [PubMed] [Google Scholar]
- 25.Livak KJ, Schmittgen TD.. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 26.Long J, Badal SS, Ye Z, et al. Long noncoding RNA Tug1 regulates mitochondrial bioenergetics in diabetic nephropathy. J Clin Invest. 2016;126(11):4205–4218. doi: 10.1172/JCI87927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kawakami T, Gomez IG, Ren S, et al. Deficient autophagy results in mitochondrial dysfunction and FSGS. J Am Soc Nephrol. 2015;26(5):1040–1052. doi: 10.1681/ASN.2013111202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Assady S, Wanner N, Skorecki KL, et al. New insights into podocyte biology in glomerular health and disease. J Am Soc Nephrol. 2017;28(6):1707–1715. doi: 10.1681/ASN.2017010027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li C, Chen W, Zheng L, et al. Ameliorative effect of ursolic acid on ochratoxin A-induced renal cytotoxicity mediated by Lonp1/Aco2/Hsp75. Toxicon. 2019;168:141–146. doi: 10.1016/j.toxicon.2019.07.014. [DOI] [PubMed] [Google Scholar]
- 30.Zhang B, Shen XL, Liang R, et al. Protective role of the mitochondrial Lon protease 1 in ochratoxin A-induced cytotoxicity in HEK293 cells. J Proteomics. 2014;101:154–168. doi: 10.1016/j.jprot.2014.02.017. [DOI] [PubMed] [Google Scholar]
- 31.Peter B, Waddington CL, Oláhová M, et al. Defective mitochondrial protease LonP1 can cause classical mitochondrial disease. Hum Mol Genet. 2018;27(10):1743–1753. doi: 10.1093/hmg/ddy080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Strauss KA, Jinks RN, Puffenberger EG, et al. CODAS syndrome is associated with mutations of LONP1, encoding mitochondrial AAA+ Lon protease. Am J Hum Genet. 2015;96(1):121–135. doi: 10.1016/j.ajhg.2014.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim JE, Park H, Choi SH, et al. CDDO-me selectively attenuates CA1 neuronal death induced by status epilepticus via facilitating mitochondrial fission independent of LONP1. Cells. 2019;8(8):833. doi: 10.3390/cells8080833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dietrich A, Gudermann T.. TRPC6: physiological function and pathophysiological relevance. Handb Exp Pharmacol. 2014;222:157–188. doi: 10.1007/978-3-642-54215-2_7. [DOI] [PubMed] [Google Scholar]
- 35.Möller CC, Wei C, Altintas MM, et al. Induction of TRPC6 channel in acquired forms of proteinuric kidney disease. J Am Soc Nephrol. 2007;18(1):29–36. doi: 10.1681/ASN.2006091010. [DOI] [PubMed] [Google Scholar]
- 36.Yu S, Yu L.. Dexamethasone resisted podocyte injury via stabilizing TRPC6 expression and distribution. Evid Based Complement Alternat Med. 2012;2012:652059. doi: 10.1155/2012/652059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang H, You Y, Lin X, et al. Inhibition of TRPC6 signal pathway alleviates podocyte injury induced by TGF-β1. Cell Physiol Biochem. 2017;41(1):163–172. doi: 10.1159/000455985. [DOI] [PubMed] [Google Scholar]
- 38.Verheijden KAT, Sonneveld R, Bakker-van Bebber M, et al. The calcium-dependent protease calpain-1 links TRPC6 activity to podocyte injury. J Am Soc Nephrol. 2018;29(8):2099–2109. doi: 10.1681/ASN.2016111248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wardelmann K, Blümel S, Rath M, et al. Insulin action in the brain regulates mitochondrial stress responses and reduces diet-induced weight gain. Mol Metab. 2019;21:68–81. doi: 10.1016/j.molmet.2019.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Farmer LK, Rollason R, Whitcomb DJ, et al. TRPC6 binds to and activates calpain, independent of its channel activity, and regulates podocyte cytoskeleton, cell adhesion, and motility. J Am Soc Nephrol. 2019;30(10):1910–1924. doi: 10.1681/ASN.2018070729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guo YJ, Pan WW, Liu SB, et al. ERK/MAPK signalling pathway and tumorigenesis. Exp Ther Med. 2020;19(3):1997–2007. doi: 10.3892/etm.2020.8454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roskoski R. Jr. ERK1/2 MAP kinases: structure, function, and regulation. Pharmacol Res. 2012;66(2):105–143. doi: 10.1016/j.phrs.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 43.Yang J, Zhang N, Gao R, et al. TGF-β1 induced fascin1 expression facilitates the migration and invasion of kidney carcinoma cells through ERK and JNK signaling pathways. Biochem Biophys Res Commun. 2018;501(4):913–919. doi: 10.1016/j.bbrc.2018.05.081. [DOI] [PubMed] [Google Scholar]
- 44.Lei J, Zhao L, Zhang Y, et al. High glucose-induced podocyte injury involves activation of mammalian target of rapamycin (mTOR)-induced endoplasmic reticulum (ER) stress. Cell Physiol Biochem. 2018;45(6):2431–2443. doi: 10.1159/000488231. [DOI] [PubMed] [Google Scholar]
- 45.Bai M, Wu M, Jiang M, et al. LONP1 targets HMGCS2 to protect mitochondrial function and attenuate chronic kidney disease. EMBO Mol Med. 2023;15(2):e16581. doi: 10.15252/emmm.202216581. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The analyzed data sets generated during the study are available from the corresponding author on reasonable request.