Abstract
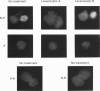
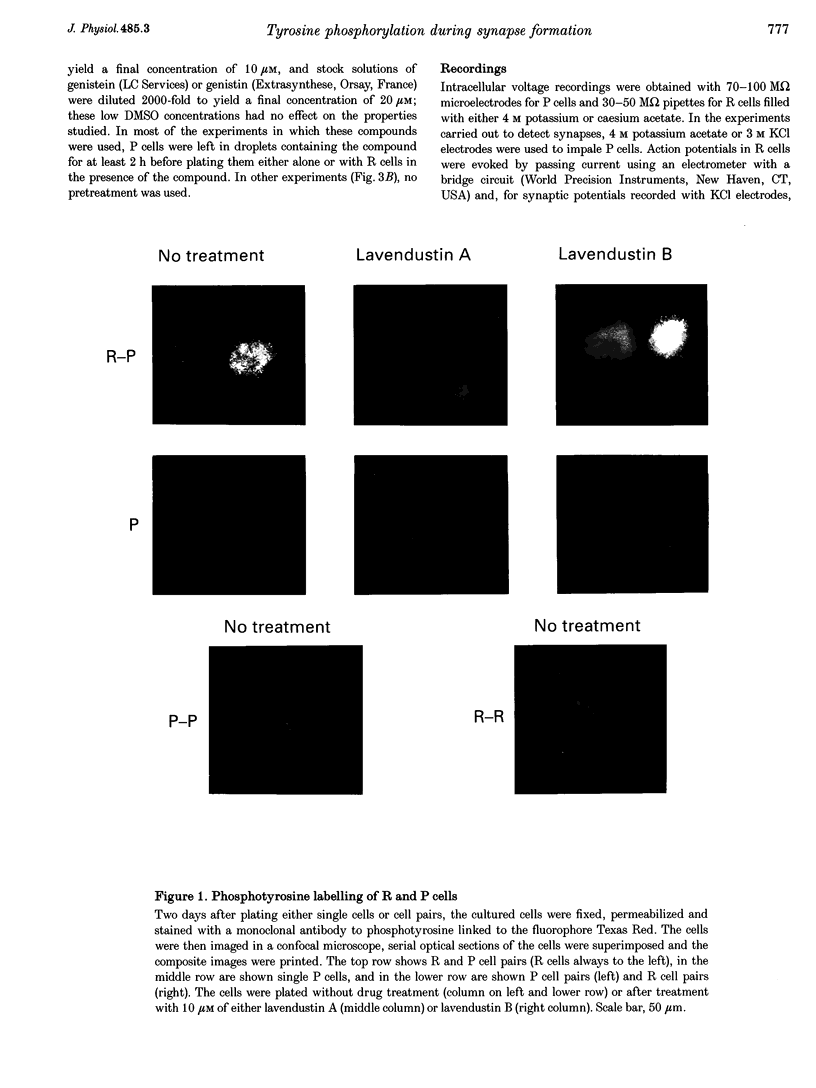
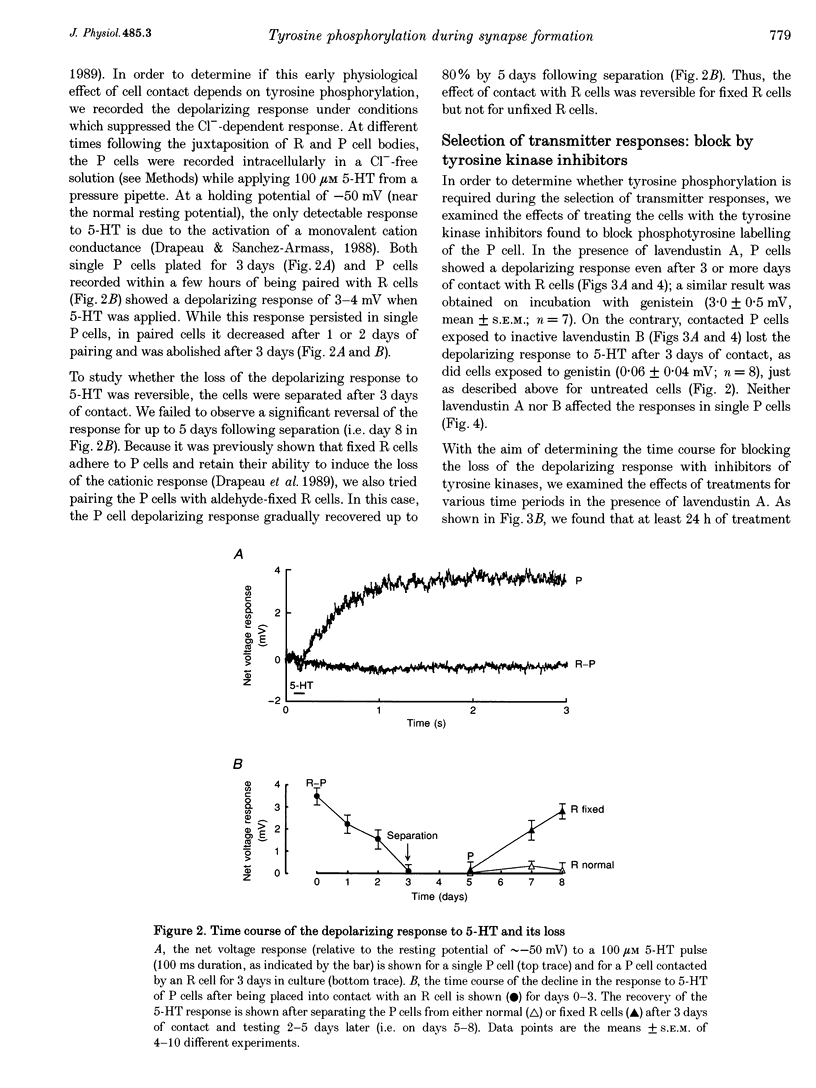
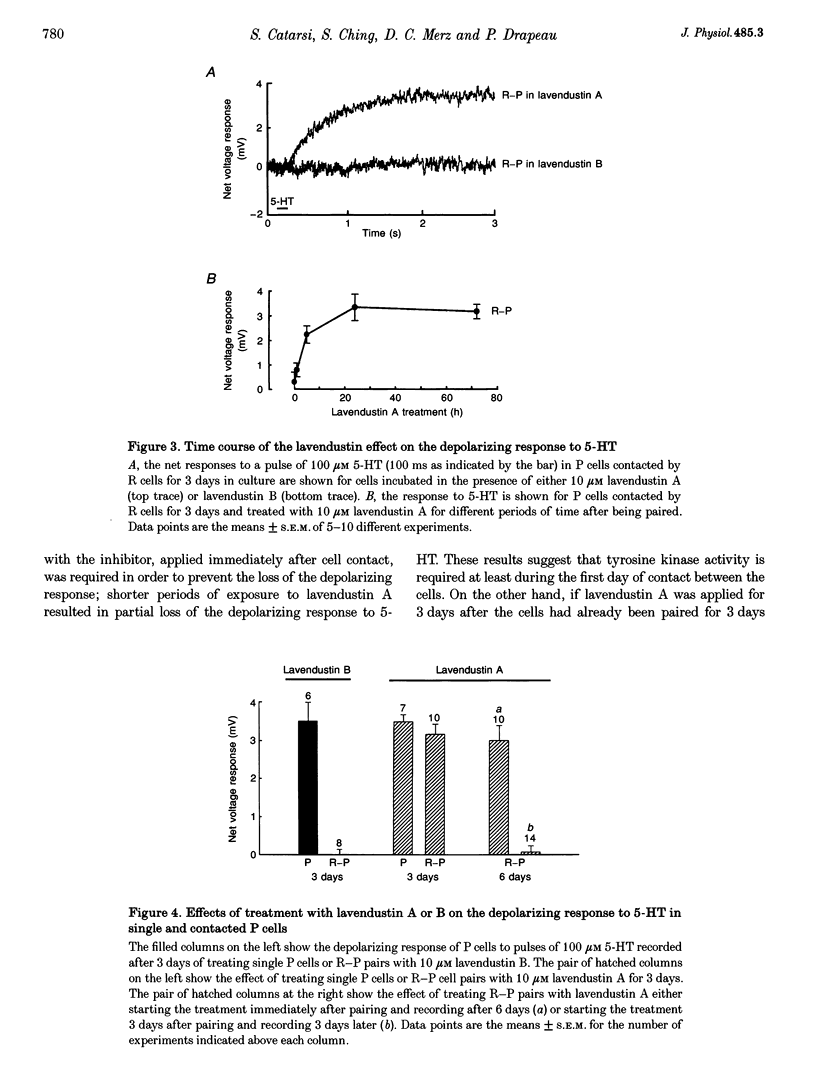
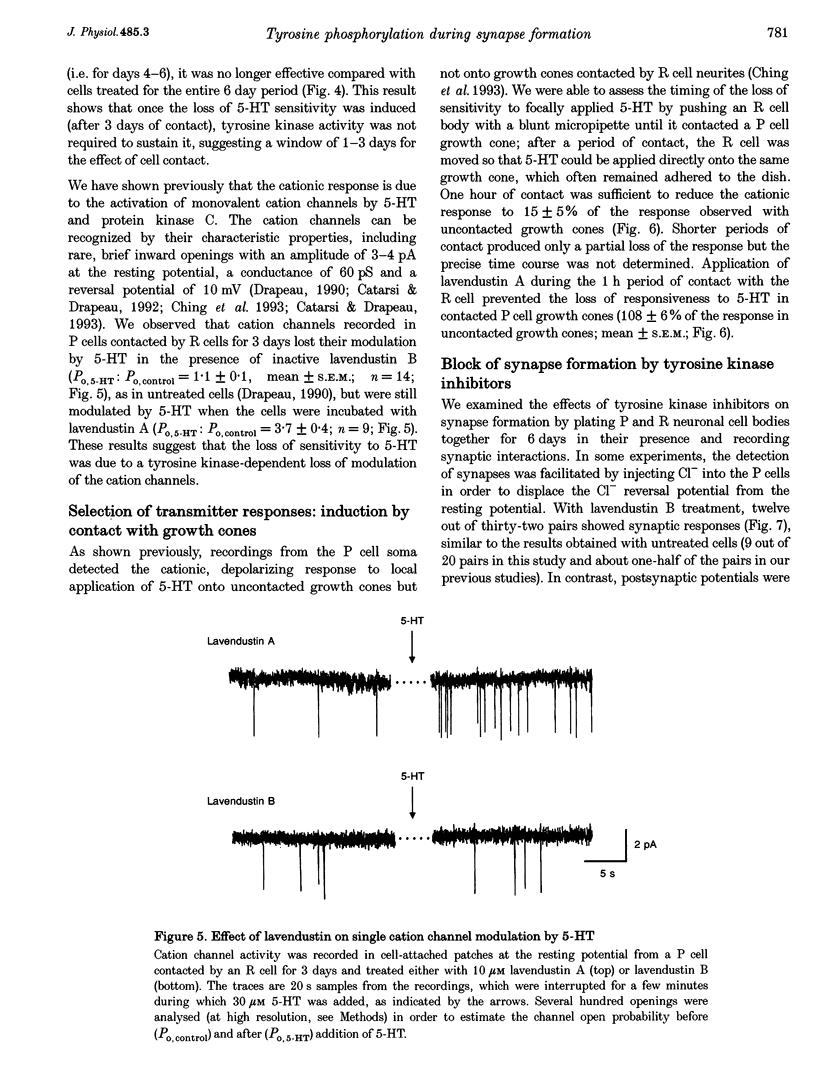
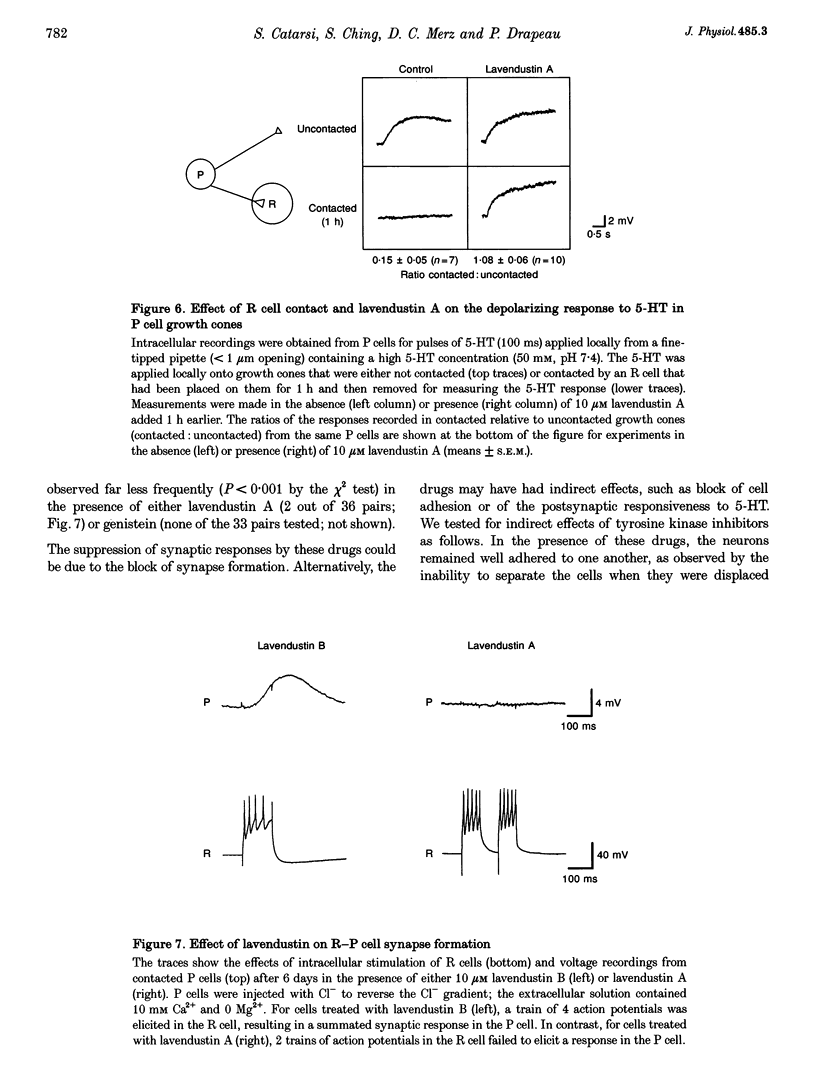
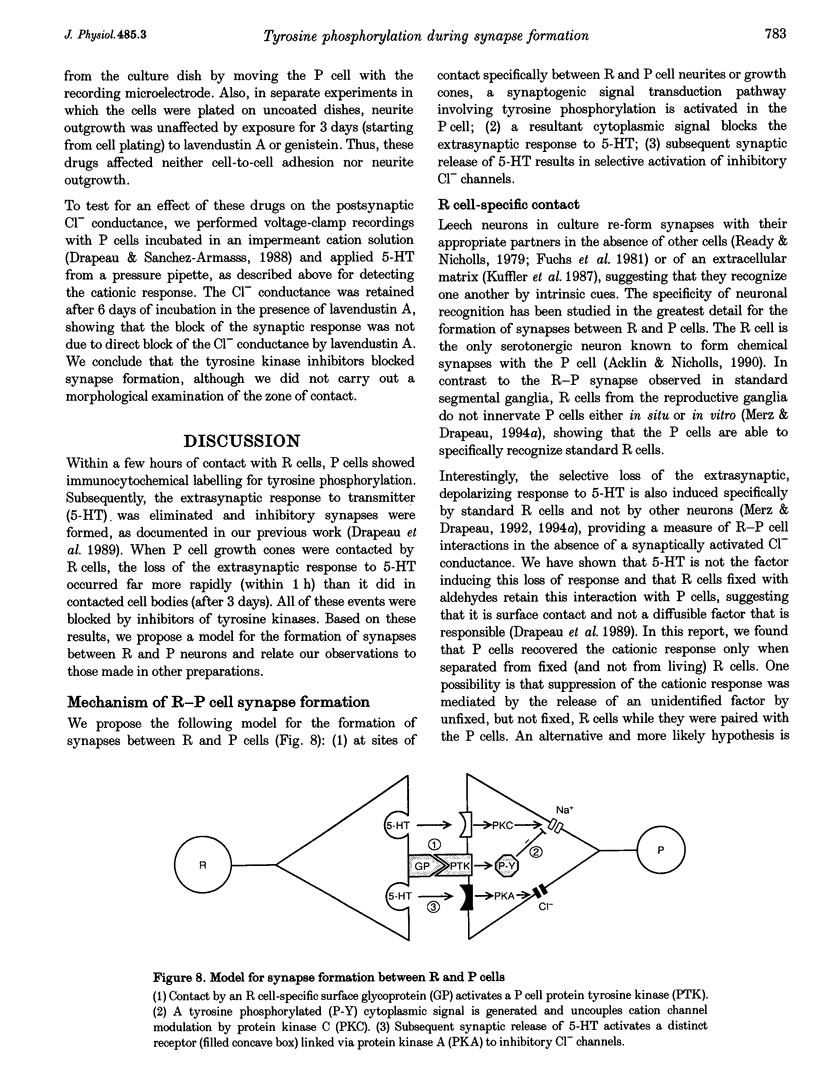
1. We have examined whether tyrosine phosphorylation is required for synapse formation between identified neurons from the central nervous system of the leech in culture. 2. Within a few hours of contact with the cell body of the serotonergic Retzius neuron (R cell), the soma of the postsynaptic pressure-sensitive neuron (P cell), but not the R cell, could be labelled intracellularly with an antibody against phosphotyrosine residues. The labelling seemed specific for P cells contacted by R cells, as it was greatly reduced in pairs of either R or P cells and in single cells. Genistein (20 microM) and lavendustin A (10 microM), selective inhibitors of tyrosine kinases, blocked the labelling of contacted P cells, whereas their ineffective analogues (genistein and lavendustin B) had no effect on labelling. 3. R cell contact also induced the loss of an extrasynaptic, depolarizing response (due to modulation of cation channels) to serotonin (5-HT) in the P cell within a few days of juxtaposing cell bodies and within an hour of contact with growth cones. Treatment of the neurons with the tyrosine kinase inhibitors (but not the ineffective analogues) prevented the loss of the depolarizing response and of single cation channel modulation by 5-HT. 4. R cells formed inhibitory, Cl(-)-dependent synapses with P cells. Synapse formation was prevented by the tyrosine kinase inhibitors but not by their ineffective analogues. These compounds had no obvious effect on neurite outgrowth or cell adhesion. We conclude that tyrosine phosphorylation is a signal during the formation of this synapse.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acklin S. E., Nicholls J. G. Intrinsic and extrinsic factors influencing properties and growth patterns of identified leech neurons in culture. J Neurosci. 1990 Apr;10(4):1082–1090. doi: 10.1523/JNEUROSCI.10-04-01082.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atashi J. R., Klinz S. G., Ingraham C. A., Matten W. T., Schachner M., Maness P. F. Neural cell adhesion molecules modulate tyrosine phosphorylation of tubulin in nerve growth cone membranes. Neuron. 1992 May;8(5):831–842. doi: 10.1016/0896-6273(92)90197-l. [DOI] [PubMed] [Google Scholar]
- Baker L. P., Peng H. B. Tyrosine phosphorylation and acetylcholine receptor cluster formation in cultured Xenopus muscle cells. J Cell Biol. 1993 Jan;120(1):185–195. doi: 10.1083/jcb.120.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnekow A., Jahn R., Schartl M. Synaptophysin: a substrate for the protein tyrosine kinase pp60c-src in intact synaptic vesicles. Oncogene. 1990 Jul;5(7):1019–1024. [PubMed] [Google Scholar]
- Belardetti F., Brunelli M., Demontis G., Sonetti D. Serotonin and Retzius cell depress the hyperpolarization following impulses of leech touch cell. Brain Res. 1984 May 21;300(1):91–102. doi: 10.1016/0006-8993(84)91343-x. [DOI] [PubMed] [Google Scholar]
- Bixby J. L., Jhabvala P. Tyrosine phosphorylation in early embryonic growth cones. J Neurosci. 1993 Aug;13(8):3421–3432. doi: 10.1523/JNEUROSCI.13-08-03421.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantley L. C., Auger K. R., Carpenter C., Duckworth B., Graziani A., Kapeller R., Soltoff S. Oncogenes and signal transduction. Cell. 1991 Jan 25;64(2):281–302. doi: 10.1016/0092-8674(91)90639-g. [DOI] [PubMed] [Google Scholar]
- Catarsi S., Drapeau P. Loss of extrasynaptic channel modulation by protein kinase C underlies the selection of serotonin responses in an identified leech neuron. Neuron. 1992 Feb;8(2):275–281. doi: 10.1016/0896-6273(92)90294-n. [DOI] [PubMed] [Google Scholar]
- Catarsi S., Drapeau P. Tyrosine kinase-dependent selection of transmitter responses induced by neuronal contact. Nature. 1993 May 27;363(6427):353–355. doi: 10.1038/363353a0. [DOI] [PubMed] [Google Scholar]
- Ching S., Catarsi S., Drapeau P. Selection of transmitter responses at sites of neurite contact during synapse formation between identified leech neurons. J Physiol. 1993 Aug;468:425–439. doi: 10.1113/jphysiol.1993.sp019780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiquet M., Nicholls J. G. Neurite outgrowth and synapse formation by identified leech neurones in culture. J Exp Biol. 1987 Sep;132:191–206. doi: 10.1242/jeb.132.1.191. [DOI] [PubMed] [Google Scholar]
- Dietzel I. D., Drapeau P., Nicholls J. G. Voltage dependence of 5-hydroxytryptamine release at a synapse between identified leech neurones in culture. J Physiol. 1986 Mar;372:191–205. doi: 10.1113/jphysiol.1986.sp016004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drapeau P. Loss of channel modulation by transmitter and protein kinase C during innervation of an identified leech neuron. Neuron. 1990 Jun;4(6):875–882. doi: 10.1016/0896-6273(90)90140-b. [DOI] [PubMed] [Google Scholar]
- Drapeau P., Melinyshyn E., Sanchez-Armass S. Contact-mediated loss of the nonsynaptic response to transmitter during reinnervation of an identified leech neuron in culture. J Neurosci. 1989 Jul;9(7):2502–2508. doi: 10.1523/JNEUROSCI.09-07-02502.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drapeau P., Sanchez-Armass S. Selection of postsynaptic serotonin receptors during reinnervation of an identified leech neuron in culture. J Neurosci. 1988 Dec;8(12):4718–4727. doi: 10.1523/JNEUROSCI.08-12-04718.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkins T., Zinn K., McAllister L., Hoffmann F. M., Goodman C. S. Genetic analysis of a Drosophila neural cell adhesion molecule: interaction of fasciclin I and Abelson tyrosine kinase mutations. Cell. 1990 Feb 23;60(4):565–575. doi: 10.1016/0092-8674(90)90660-7. [DOI] [PubMed] [Google Scholar]
- Falls D. L., Rosen K. M., Corfas G., Lane W. S., Fischbach G. D. ARIA, a protein that stimulates acetylcholine receptor synthesis, is a member of the neu ligand family. Cell. 1993 Mar 12;72(5):801–815. doi: 10.1016/0092-8674(93)90407-h. [DOI] [PubMed] [Google Scholar]
- Fuchs P. A., Henderson L. P., Nicholls J. G. Chemical transmission between individual Retzius and sensory neurones of the leech in culture. J Physiol. 1982 Feb;323:195–210. doi: 10.1113/jphysiol.1982.sp014068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs P. A., Nicholls J. G., Ready D. F. Membrane properties and selective connexions of identified leech neurones in culture. J Physiol. 1981 Jul;316:203–223. doi: 10.1113/jphysiol.1981.sp013783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant S. G., O'Dell T. J., Karl K. A., Stein P. L., Soriano P., Kandel E. R. Impaired long-term potentiation, spatial learning, and hippocampal development in fyn mutant mice. Science. 1992 Dec 18;258(5090):1903–1910. doi: 10.1126/science.1361685. [DOI] [PubMed] [Google Scholar]
- Greenwald I., Rubin G. M. Making a difference: the role of cell-cell interactions in establishing separate identities for equivalent cells. Cell. 1992 Jan 24;68(2):271–281. doi: 10.1016/0092-8674(92)90470-w. [DOI] [PubMed] [Google Scholar]
- Henderson L. P. The role of 5-hydroxytryptamine as a transmitter between identified leech neurones in culture. J Physiol. 1983 Jun;339:309–324. doi: 10.1113/jphysiol.1983.sp014718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidaka H., Kobayashi R. Pharmacology of protein kinase inhibitors. Annu Rev Pharmacol Toxicol. 1992;32:377–397. doi: 10.1146/annurev.pa.32.040192.002113. [DOI] [PubMed] [Google Scholar]
- Huganir R. L. Regulation of the nicotinic acetylcholine receptor by serine and tyrosine protein kinases. Adv Exp Med Biol. 1991;287:279–294. doi: 10.1007/978-1-4684-5907-4_23. [DOI] [PubMed] [Google Scholar]
- Keegan K., Halegoua S. Signal transduction pathways in neuronal differentiation. Curr Opin Neurobiol. 1993 Feb;3(1):14–19. doi: 10.1016/0959-4388(93)90029-x. [DOI] [PubMed] [Google Scholar]
- Kristan W. B., Jr, Nusbaum M. P. The dual role of serotonin in leech swimming. J Physiol (Paris) 1982;78(8):743–747. [PubMed] [Google Scholar]
- Kuffler D. P., Nicholls J., Drapeau P. Transmitter localization and vesicle turnover at a serotoninergic synapse between identified leech neurons in culture. J Comp Neurol. 1987 Feb 22;256(4):516–526. doi: 10.1002/cne.902560404. [DOI] [PubMed] [Google Scholar]
- Kuwada J. Y., Kramer A. P. Embryonic development of the leech nervous system: primary axon outgrowth of identified neurons. J Neurosci. 1983 Oct;3(10):2098–2111. doi: 10.1523/JNEUROSCI.03-10-02098.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Nicholls J. Steps in the development of chemical and electrical synapses by pairs of identified leech neurons in culture. Proc R Soc Lond B Biol Sci. 1989 Apr 22;236(1284):253–268. doi: 10.1098/rspb.1989.0023. [DOI] [PubMed] [Google Scholar]
- Merz D. C., Drapeau P. Cell surface contact mediates neuronal recognition and synapse formation between two identified leech neurons. J Neurobiol. 1994 Aug;25(8):1029–1037. doi: 10.1002/neu.480250811. [DOI] [PubMed] [Google Scholar]
- Merz D. C., Drapeau P. Cell-specific contact selects transmitter responses in an identified leech neuron. Proc Biol Sci. 1992 May 22;248(1322):129–133. doi: 10.1098/rspb.1992.0052. [DOI] [PubMed] [Google Scholar]
- Merz D. C., Drapeau P. Segmental specificity of neuronal recognition during synapse formation between identified leech neurons. J Neurosci. 1994 Jul;14(7):4125–4129. doi: 10.1523/JNEUROSCI.14-07-04125.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nairn A. C., Shenolikar S. The role of protein phosphatases in synaptic transmission, plasticity and neuronal development. Curr Opin Neurobiol. 1992 Jun;2(3):296–301. doi: 10.1016/0959-4388(92)90118-5. [DOI] [PubMed] [Google Scholar]
- O'Dell T. J., Kandel E. R., Grant S. G. Long-term potentiation in the hippocampus is blocked by tyrosine kinase inhibitors. Nature. 1991 Oct 10;353(6344):558–560. doi: 10.1038/353558a0. [DOI] [PubMed] [Google Scholar]
- Onoda T., Iinuma H., Sasaki Y., Hamada M., Isshiki K., Naganawa H., Takeuchi T., Tatsuta K., Umezawa K. Isolation of a novel tyrosine kinase inhibitor, lavendustin A, from Streptomyces griseolavendus. J Nat Prod. 1989 Nov-Dec;52(6):1252–1257. doi: 10.1021/np50066a009. [DOI] [PubMed] [Google Scholar]
- Peng H. B., Baker L. P., Dai Z. A role of tyrosine phosphorylation in the formation of acetylcholine receptor clusters induced by electric fields in cultured Xenopus muscle cells. J Cell Biol. 1993 Jan;120(1):197–204. doi: 10.1083/jcb.120.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ready D. F., Nicholls J. Identified neurones isolated from leech CNS make selective connections in culture. Nature. 1979 Sep 6;281(5726):67–69. doi: 10.1038/281067a0. [DOI] [PubMed] [Google Scholar]
- Sanchez-Armass S., Merz D. C., Drapeau P. Distinct receptors, second messengers and conductances underlying the dual responses to serotonin in an identified leech neurone. J Exp Biol. 1991 Jan;155:531–547. doi: 10.1242/jeb.155.1.531. [DOI] [PubMed] [Google Scholar]
- Schlessinger J., Ullrich A. Growth factor signaling by receptor tyrosine kinases. Neuron. 1992 Sep;9(3):383–391. doi: 10.1016/0896-6273(92)90177-f. [DOI] [PubMed] [Google Scholar]
- Wagner K. R., Mei L., Huganir R. L. Protein tyrosine kinases and phosphatases in the nervous system. Curr Opin Neurobiol. 1991 Jun;1(1):65–73. doi: 10.1016/0959-4388(91)90011-u. [DOI] [PubMed] [Google Scholar]
- Wallace B. G., Qu Z., Huganir R. L. Agrin induces phosphorylation of the nicotinic acetylcholine receptor. Neuron. 1991 Jun;6(6):869–878. doi: 10.1016/0896-6273(91)90227-q. [DOI] [PubMed] [Google Scholar]
- Wu D. Y., Goldberg D. J. Regulated tyrosine phosphorylation at the tips of growth cone filopodia. J Cell Biol. 1993 Nov;123(3):653–664. doi: 10.1083/jcb.123.3.653. [DOI] [PMC free article] [PubMed] [Google Scholar]