Abstract
Background
Inflammatory bowel disease (IBD) is a chronic disease in which macrophages play an important role in its pathogenesis. Platelet-derived growth factor-BB (PDGF-BB) secreted by macrophages is involved in the repair of vascular endothelial injury during inflammatory reactions.
Methods
The expression levels of M1 macrophages and PDGF-BB in serum and colonic mucosa of 30 patients with Crohn’s disease (CD) and 30 patients with ulcerative colitis (UC) were measured using enzyme-linked immunosorbent assays and immunohistochemistry. Logistic regression was used for univariate and multivariate analyses, and receiver operating characteristic curves were used to evaluate diagnostic value. Associations were evaluated using Spearman correlation analysis.
Results
The expression of serum PDGF-BB and M1 macrophages with positive CXCL9 expression in patients with active-stage IBD [206.55(160.41,262.90)and 337.30(217.73,472.28) pg/ml] was higher than that in patients with remission stage [153.42(107.02,219.68)and 218.37(144.49,347.33)pg/ml] and controls [156.19(91.16,216.08)and 191.20(121.42,311.76)pg/ml](P < 0.05). The expression of PDGF-BB, CD86, and CXCL9 in the colon of patients with active-stage IBD [0.380(0.266,0.542) 0.663(0.480,0.591) and 0.564(0.378,0.765) /µm2] was higher than that in the remission stage [0.308(0.214,0.420), 0.376(0.206,0.591) and 0.413(0.275,0.570) /µm2] and controls [0.265(0.185,0.384), 0.416(0.269,0.534) and 0.497(0.415,0.642) /µm2] (P < 0.05). A positive correlation was observed between CD86 and PDGF-BB, and CXCL9 and PDGF-BB levels in patients with IBD (P < 0.05). CD86 and PDGF-BB in the colonic mucosa were independent risk factors for active IBD, and the area under the curve for their combined diagnosis was 0.754 (95%CI: 0.654–0.852, P < 0.05).
Conclusions
PDGF-BB was associated with M1 macrophages and has a potential diagnostic value for active IBD.
Trial registration
Not applicable.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12876-024-03518-y.
Keywords: Inflammatory bowel disease, Crohn’s disease, Ulcerative colitis, Macrophage, Platelet-derived growth factor-BB
Highlights
A potential correlation was observed between PDGF-BB and M1 macrophages.
CD86 and PDGF-BB in colonic mucosa were independent risk factors for active IBD.
PDGF-BB and M1 macrophages with positive CXCL9 expression were significantly increased during active IBD.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12876-024-03518-y.
Background
Inflammatory bowel disease (IBD) mainly includes Crohn’s disease (CD) and ulcerative colitis (UC). This group of chronic nonspecific inflammatory diseases of the gastrointestinal tract is characterized by easy recurrence, imbalance in intestinal mucosal immune regulation, and lifelong duration [1]. The pathogenesis of IBD originates from the interaction between the host and external factors, including the intestinal microbial system, immune system, genetic composition of the host, and specific external factors [2]. In IBD, microvascular endothelial cells are a crucial barrier between the blood and tissue microenvironment [3]. Destruction of the intestinal barrier results in many unfavorable factors, including increased intestinal permeability, immune homeostasis disorders, and aggravation of intestinal inflammation, which can lead to intestinal diseases [4, 5].
In IBD, the mechanism involved in the regression of inflammatory reactions changes, leading to a persistent and highly inflammatory state in the intestine. A large number of cytokines and active substances used to kill antigens and invading microorganisms can cause intestinal damage, which aggravates intestinal infection and stimulates an autoimmune response [6]. Macrophages play an important role in innate immunity and exhibit strong plasticity. They can change their phenotype and function by integrating environmental stimuli, thereby responding quickly to various antigens [7]. When IBD occurs or other pathogens invade, chemokines, such as chemokine (C-C motif) ligand 2 and chemokine (C-C motif) ligand 7, secreted by intestinal tissue-colonized macrophages increase [8], and these chemokines induce monocyte chemotaxis from the periphery to the intestine, and the rapidly increasing monocytes mainly differentiate into M1 macrophages. The expression of M1 macrophages and intestinal mechanical barriers are at the core of intestinal homeostasis system [9].
M1 macrophages are the main producers of the C-X-C motif chemokine ligand 9 (CXCL9) [10]. CXCL9 and CXCL10 have been identified as potential early biomarkers to predict the response to immunoscreen inhibitors [11], and they participate in the chemotaxis of Th1 cells through overexpression. The secretion of interferon-γ (IFN-γ) after Th1 cells are recruited and activated induces macrophages to differentiate into M1 macrophages and enhance the expression of CXCL9, thus forming an amplification feedback pathway of IBD inflammation [12, 13] with resulting damage to the intestinal epithelium. However, the mechanisms underlying intestinal mucosal repair and microvascular dysfunction involving macrophages in IBD are poorly understood. Therefore, there is an urgent need to explore the molecular mechanism underlying the occurrence and progression of IBD to deal with the problems of inflammation and intestinal barrier destruction caused by these diseases.
Vascular endothelial injury also accelerates tissue necrosis and inflammation, with the self-repair mechanism of vascular endothelial harm likewise being triggered [14]. Platelet-derived growth factor (PDGF) comprises a family of cysteine growth factors and four closely similar polypeptide chains (A, B, C and D). During tissue regeneration, platelets and monocytes/macrophages gather in the injured area, stimulating fibroblasts to contract the collagen matrix, and fostering the development of granular tissue and tissue regeneration through the release of PDGF-BB. The role of PDGF-BB in IBD remains a subject of debate. PDGF-BB is highly expressed in the inflammatory activity of IBD, suggesting that PDGF-BB promotes an inflammatory response in IBD [15]. Some studies have suggested that PDGF-BB plays a protective role against intestinal inflammatory reaction [16]. In this study, we observed the dynamic changes in PDGF-BB expression in the serum and colon of patients with IBD at different disease stages and discussed the correlation between PDGF-BB and M1 macrophages in IBD to provide ideas for studying the targets regulating the IBD inflammatory response and mucosal repair.
Methods
Patients
From January 2021 to May 2023, 60 patients newly diagnosed with IBD, including 30 with CD and 30 with UC, were selected from the Department of Gastroenterology, Jiaxing Second Hospital. Thirty healthy controls were recruited from an outpatient clinic during the same period. All patients were divided into the IBD patient and control groups after medical history collection, physical examination, laboratory examination, imaging examination, colonoscopy, and pathological examination. Exclusion criteria included: (1) Patients with age below 14 or above 80 years old; (2) Patients with heart, lung, kidney, liver or other organic diseases; (3) Patients with tumor, blood system diseases or other autoimmune diseases; (4) Pregnancy and lactation.
Patients were divided into active and remission IBD groups (patients with active IBD entered remission after 12 weeks of standardized drug treatment, according to the guidelines). Crohn’s disease activity index(CDAI) was used to assess disease activity of CD. CDAI between 150 and 220 meant mild, between 220 and 450 meant moderate, and more than 450 meant severe CD disease. The Mayo score was used to assess disease activity of UC. The Mayo score ranged from 0 to 12, with higher scores indicating more severe disease. The disease sites of patients with CD and UC were classified according to the Montreal phenotypic classification mentioned in the consensus and were divided into L1/E1(CD terminal ileum /UC rectum), L2/E2(CD colon/UC left colon), and L3/E3(CD ileocolon/UC extensive colon). The disease behavior of the patients with CD was also classified according to the Montreal phenotypic classification, which was divided into B1 (non-stenosis and non-penetration), B2 (stenosis), and B3 (penetration) types.
The diagnosis of IBD, the stages of disease activity (active and remission stages), and the standardized drug treatment scheme all met the diagnostic criteria of the Consensus Opinion on Diagnosis and Treatment of IBD (Beijing, 2018) [17].
Sampling and preservation
Two milliliters of fasting elbow venous blood was collected from patients with active IBD and controls on day 1 of our hospital visit. Patients with active IBD were re-sampled when they entered remission. Blood samples were centrifuged at 3000 rpm for 10 min after self-coagulation in a blank vacuum tube, and serum was collected and stored at -20℃.
Patients and the control group provided biopsy samples during informed consent colonoscopy and stored them at -80℃. Biopsy samples were obtained from patients with active IBD at the edge of the ulcer from the colonic segment with the highest grade of colonic mucosal inflammation. Patients with IBD in remission who underwent colonoscopy obtained biopsy samples from the same sampling colonic segment. In the control group, colonic mucosa was biopsied from the middle of the sigmoid colon.
Measurement of cytokines by enzyme-linked immunosorbent assay
According to the guide provided by the enzyme-linked immunosorbent assay (ELISA) kit manufacturer, the concentration of cytokines in serum, including IFN-γ (Proteintech, #KE00146), CXCL9 (Proteintech, #KE00165), and PDGF-BB (Proteintech, #KE00161), was quantified. The concentration of each serum sample was measured twice with a full-wavelength enzyme-labeling instrument (Thermo Scientific) and averaged as the final concentration.
Immunohistochemistry assay
Colonic mucosa was embedded in paraffin, dewaxed, and hydrated routinely, treated with 0.01 mol/L citric acid buffer (pH 6.0) at 95℃ for 20 min, washed with 3% hydrogen peroxide for 10 min, and then dyed. The sample was dyed with drops of primary antibodies, including rabbit anti- CD86 (Abcam, #ab234401), rabbit anti- CXCL9 (Abcam, #ab202961), and rabbit anti-PDGF-BB (Abcam, # ab23914), incubated at 4℃ overnight. Then it was washed and combined with secondary antibody (Zhongshan Jinqiao Biotechnology, PV-8000).The slide and the secondary antibody were incubated at 37℃ for 30 min. Finally, the sections were stained with DAB and Harris hematoxylin, sequential water washing, dehydration, transparency, and film sealing were performed. Images were captured under a microscope (Nikon Eclipse 80i). The positive area is showed in brownish yellow. Carl Zeiss Imaging Systems software was used for image analysis. Positive index of each kind of cytokine was obtained: total optical density (∑ positive area of the site × average optical density of the site)/image area (µm [2]). The higher the yellowness of the photo taken, the greater the positive index value.
Statistical analysis
Data were analyzed using IBM SPSS Statistics 23 (IBM SPSS, Turkey). Frequencies and percentages were used to describe count data. The mean ± standard deviation (x ± s) was applied to describe the normally distributed continuous data, and between-group comparisons were made using an independent-samples t-test. If the data were skewed, the median (upper quartile Q1, lower quartile Q3) was used to describe the data, and ANOVA test was used for comparisons. The chi-square test was used for between-group comparisons of count data. The Wilcoxon signed-rank test was used to compare the different disease stages in each group. Spearman’s text was used for correlation analysis. Logistic regression analysis was used for univariate and multivariate analyses. Statistical significance was set at P < 0.05 for all comparisons.
Study approval
This trial was approved by the hospital ethics committee. All patients provided written informed consent before the first study procedure.
Results
Demographical and clinical parameters
Patients with IBD and healthy controls are shown in Table 1. Sixty patients (UC, n = 30; CD, n = 30) and 30 healthy controls participated in this study, among these 48 (53.3%) were male. Disease duration of IBD patients ranges from 0.5 to 10 years. The average age of the UC group (47.9 ± 17.1) was higher than that of the CD group (37.4 ± 12.5) and control group (43 ± 14.9) without statistical difference (P > 0.05). Non-significant differences were observed among the three cohorts in terms of body mass index (BMI) and smoking status, alcohol intake, triglyceride (TG), total cholesterol (TC), low-density lipoprotein (LDL), and hemoglobin (Hb) levels, with all P values exceeding 0.05. Similarly, no significant variations were identified in high-sensitivity C-reactive protein (hsCRP), erythrocyte sedimentation rate (ESR), prevalence of fecal calprotectin (FC) positivity, or distribution patterns of lesions within the patient groups (P > 0.05). However, it is of great importance to note that hsCRP concentrations in the patient group were significantly elevated compared with those in the control group.
Table 1.
Demographic and clinical data of IBD patients and controls
| CD(n = 30) | UC(n = 30) | controls (n = 30) | |
|---|---|---|---|
| Male (n, (%)) | 19 (63.3) | 16 (53.3) | 13 (43.3) |
Age(years,  ) ) |
37.4 ± 12.5 | 47.9 ± 17.1 | 43 ± 14.9 |
BMI (kg/m2, 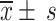 ) ) |
21.4 ± 3.6 | 20.6 ± 3.0 | 22.1 ± 2.7 |
| Smoking(n, (%)) | 4(13.3) | 4(13.3) | 5(16.7) |
| Alcohol(n, (%)) | 4(13.3) | 3(10.0) | 5(16.7) |
| hsCRP(mg/L, Me(Q1,Q3)) | 6.36 (2.02,12.45)* | 3.91 (0.61,18.83) * | 1.57 (0.00,3.24) |
| ESR(mm/h, Me(Q1,Q3)) | 14.5 (4.7,34.0) | 13.5(4.7,26.5) | - |
| TG(mmol/L, Me(Q1,Q3)) | 1.08 (0.84,1.22) | 0.98 (0.67,1.51) | 1.03 (0.93,1.41) |
| TC (mmol/L, Me(Q1,Q3)) | 3.79 (3.49,4.34) | 4.08 (3.46,4.76) | 3.28 (2.98,2.68) |
| LDL (mmol/L, Me(Q1,Q3)) | 2.20 (1.86,2.56) | 2.20 (1.78,2.75) | 2.22 (1.88,2.99) |
| Hb (g/L, Me(Q1,Q3)) | 131 (119,147) | 131 (117,145) | 139 (131,147) |
| FC-positive (n, (%)) | 13 (43.3) | 18 (60.0) | - |
| CDAI/Mayo score(Me(Q1,Q3)) | 217.8(200.7,235.0) | 9(7,10) | |
| Lesion sites (n, (%)) | |||
| L1/E1 | 4 (13.3) | 2 (6.7) | - |
| L2/E2 | 6 (20.0) | 12 (40.0) | - |
| L3/E3 | 20 (66.7) | 16 (53.3) | - |
| Disease behavior (n (%)) | |||
| B1 | 13 (43.3) | - | - |
| B2 | 15 (50.0) | - | - |
| B3 | 2 (6.7) | - | - |
* means p < 0.05
Difference in serum cytokine expression between different stages of IBD and controls
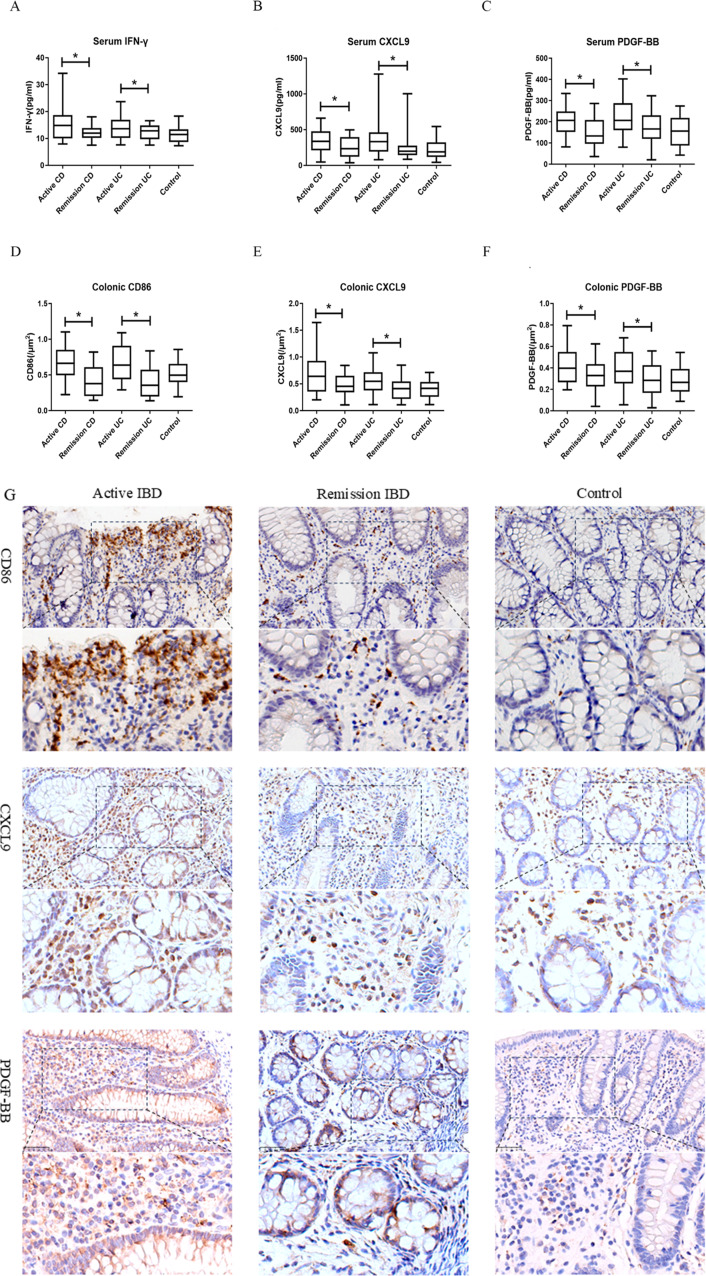
To study the characteristics of M1 macrophages and PDGF-BB in IBD, the abundance of macrophages and the levels of PDGF-BB in the serum and mucosa of patients with UC and CD were detected using ELISA and immunohistochemistry. The expression of IFN-γ, CXCL9, and PDGF-BB in the serum of the CD and UC groups was significantly higher than that in the remission period and the control group (P < 0.05) (Fig. 1A and C). Further immunohistochemical analysis demonstrated that CD86 and M1 macrophages with positive CXCL9 expression and PDGF-BB were significantly abundant in the colonic mucosa of the active IBD group (including the UC and CD groups) (P < 0.05) (Fig. 1D and G). The remission group was not significantly different from the control group (P > 0.05).
Fig. 1.
Expression of different factors in the serum and colonic tissue of patients with IBD at different stages (A-G) Comparison of serum IFN-γ (A), CXCL9 (B), and PDGF-BB (C) levels in patients with IBD at different stages of the disease and the control group. Comparison of the levels of CD86 (D), CXCL9 (E), and PDGF-BB (F) in colon tissues of patients with IBD at different disease stages and the control group. Pathological immunohistochemical analysis of CD86, CXCL9, and PDGF-BB in colonic tissues of patients with IBD at different stages and the control group (G)
Risk factors related to disease activity in patients with IBD
Univariate analysis showed that hsCRP, Hb, three factors in serum (IFN-γ, CXCL9, and PDGF-BB), and three factors in colonic mucosa (CD86, CXCL9, and PDGF-BB) were risk factors for patients with active IBD (P < 0.05). Furthermore, these factors were included in the multivariate analysis. The results suggested that colonic CD86 (OR: 1.345; 95%CI (1.005–1.799)) and PDGF-BB (OR: 1.643; 95%CI (1.005–2.688)) were independent risk factors for patients with active IBD (P < 0.05) (Table 2).
Table 2.
Univariate and multivariate analysis of related risk factors of active IBD
| univariate | multivariate | ||||
|---|---|---|---|---|---|
| OR(95%CI) | P | OR(95%CI) | P | ||
| Sex | 0.546(0.225–1.325) | > 0.05 | |||
| Age | 0.999(0.971–1.028) | > 0.05 | |||
| BMI | 0.899(0.781–1.036) | > 0.05 | |||
| hsCRP | 1.287(1.083–1.529) | < 0.05 | 1.152(0.967–1.373) | > 0.05 | |
| TC | 0.513(0.197–1.333) | > 0.05 | |||
| TG | 1.274(0.806,2.013) | > 0.05 | |||
| LDL | 0.820(0.471–1.428) | > 0.05 | |||
| Hb | 0.974(0.950–0.999) | < 0.05 | 0.987(0.944–1.033) | > 0.05 | |
| serum | IFN-γ | 1.176(1.038–1.333) | < 0.05 | 1.145(0.959–1.369) | > 0.05 |
| CXCL9 | 1.004(1.001–1.007) | < 0.05 | 1.002(0.998–1.006) | > 0.05 | |
| PDGF-BB | 1.010(1.003–1.016) | < 0.05 | 1.009(1.000-1.018) | > 0.05 | |
|
colonic mucosa |
CD86 | 1.319(1.066–1.631) | < 0.05 | 1.345(1.005–1.799) | < 0.05 |
| CXCL9 | 1.366(1.103–1.691) | < 0.05 | 1.342(0.995–1.810) | > 0.05 | |
| PDGF-BB | 1.761(1.249–2.482) | < 0.05 | 1.643(1.005–2.688) | < 0.05 | |
Correlation analysis between M1 macrophage and PDGF-BB in patients with IBD
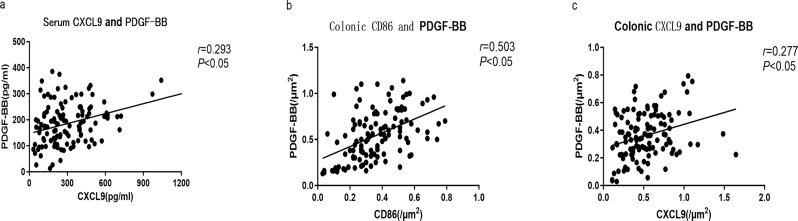
M1 macrophages with positive CXCL9 expression were positively correlated with PDGF-BB levels in patients with IBD (Spearman’s rho = 0.293, P < 0.05). A positive correlation was observed between CD86 and PDGF-BB expression in the colon (r = 0.503, P < 0.05), and M1 macrophages with positive CXCL9 expression showed positive correlation with PDGF-BB expression in the colon. (r = 0.277, P < 0.05) (Fig. 2A and C).
Fig. 2.
Spearman correlation analysis between CD86, CXCL9, and PDGF-BB in patients with IBD (A-C). (A) CXCL9 and PDGF-BB in serum. (B) CD86 and PDGF-BB in colonic tissue. (C) CXCL9 and PDGF-BB in colonic tissue
Receiver operating characteristic curve analysis of colon CD86 and PDGF-BB in patients with IBD
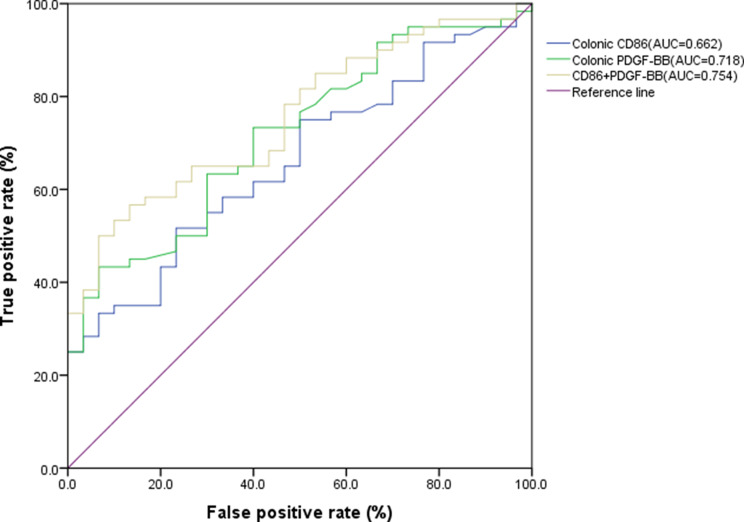
Colonic CD86 and PDGF-BB levels are independent risk factors for active IBD. Receiver operating characteristic (ROC) curve analysis showed that colonic CD86, PDGF-BB, or their combination could be used to diagnose active IBD (P < 0.05). The area under the curve (AUC) was 0.662 (95% CI: 0.549–0.775), 0.718 (95% CI: 0.611–0.824), and 0.754(95% CI: 0.654–0.852), and the sensitivity was 51.7% and 43.3%, respectively. The optimal critical values of CD86 and PDGF-BB for diagnosing active IBD were 0.652/µm2 and 0.440/µm2, respectively (Fig. 3).
Fig. 3.
ROC curve of active IBD
Discussion
This study provides evidence for a correlation between M1 macrophages and PDGF-BB in active IBD. The expression of macrophage biomarkers and PDGF-BB of serum and colonic mucosa in the UC and CD groups was significantly higher than that in the remission and control groups. In this study, CD86 and PDGF-BB levels in mucosal tissue were found to be independent risk factors for active IBD through univariate and multivariate analyses. Biomarkers of M1 macrophages were significantly correlated with PDGF-BB in both the serum and colonic mucosa of patients with IBD.
In the present study, significantly increase of the expression of M1-type macrophages and its related factors was observed in patients with IBD during the disease activity stage. M1-type macrophages, as an independent risk factor for IBD activation, had a clear diagnostic value for active IBD, which demonstrated the important role of M1-type macrophages in IBD disease activation. Through the study of patients with IBD, it has been found that M1 macrophages are involved and play multiple roles. Immune effector cells against antigens in the innate immune system include M1 macrophages, dendritic cells, and intestinal epithelial cells, which present antigens and activate adaptive immunity, resulting in intestinal tissue damage [18, 19].
Typically, activated M1 macrophages and alternately activated M2 macrophages play important roles in the progression of IBD. In intestines of general persons, M2 macrophages, which have anti-inflammatory effects, are commonly found. However, in patients with IBD, the expression of M1 macrophages changes significantly [20–22]. Obvious changes were observed in the recruitment and number of macrophages in patients with IBD. These cells produce a large number of proinflammatory factors such as tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), IL-6, IL-12, and IL-23 [23, 24]. Additionally, they express triggering receptor expressed on myeloid cells-1 (TREM-1), which effectively amplifies inflammatory reactions in the intestine. The secretion of pro-inflammatory cytokines IL-6, IL-12, TNF-α, and IL-1β in M1 macrophages pretreated with lupeol decreased significantly, whereas the secretion of anti-inflammatory cytokine IL-10 increased significantly, which was related to the downregulation of the typical M1 macrophage marker CD86 and the upregulation of the typical M2 macrophage marker CD206 [25]. Our study highlighted the important role of M1-type macrophages in IBD inflammatory response, which participated in IBD inflammatory activities together with other IBD pro-inflammatory factors, such as IFN-γ and CXCL9.
In addition, our study showed that in addition to M1-type macrophages, M1-related proinflammatory factor IFN-γ and chemokine CXCL9 were significantly increased in IBD, with the expression levels significantly higher in the active IBD than in the remission stage, which suggested that M1-related cytokines IFN-γ and CXCL9 were correlated with IBD inflammatory activation, both in serum and colonic mucosa. Based on univariate analysis, both IFN-γ and CXCL9 were risk factors for IBD, which supported the important role of the inflammatory pathway with IFN-γ, M1-type macrophages and CXCL9 in IBD. Chen et al. confirmed the causal relationship between CXCL9, CXCL11, Casp8, and UC through a Mendelian randomized analysis. Further analysis found that increased expression of CXCL9, CXCL11, and Casp8 was of great importance in UC [26]. A retrospective study demonstrated significant differences in the serum concentrations of nine cytokines/chemokines between patients with IBD in the active and remission stages. The serum concentration of CXCL9 in patients with active-stage IBD was higher than that in patients with IBD in the remission stage [27]. Similar results were found in the serum and colon tissues, indicating that CXCL9 is a potential risk factor for IBD. Increased expression of key factors secreted by M1 macrophages (CXCL9, CXCL10, CXCL11, and CD40) in IBD recruits and activates T cells [12]. This provided a perspective for clinical research and treatment. Doctors can alleviate inflammatory symptoms and T-cell infiltration in patients by regulating the expression of key factors in M1 macrophages. The results of the present study demonstrate that M1 macrophages and its related cytokines play a critical role in active IBD, which provided a potential target for clinical treatment of IBD.
Interestingly, the results of the current study found that PDGF-BB was highly expressed in serum and colonic mucosa during the active stage of IBD disease, suggesting that PDGF-BB also played an important role in the inflammatory activation of IBD. In addition, PDGF-BB, as an independent risk factor for active IBD, has diagnostic value for IBD disease activation as well as M1. This makes us wonder how PDGF-BB, which is usually involved in mucosal repair, is involved in the inflammatory response in IBD. The involvement of PDGF-BB in inflammatory responses in IBD is uncommon in previous studies. PDGF is a double-stranded polypeptide originally found in platelets [28, 29], and consists of five polypeptide subtypes [30]. PDGF-BB is an effective mitogen of many cell types (31–32) with angiogenesis ability and is considered a key regulator of tissue repair and regeneration [33]. The relationship between PDGF-BB and inflammatory reactions has gradually emerged in many disease fields, including respiratory diseases [34], ophthalmology [35], and orthopedics [36], but is lacking in IBD. Margarita et al. found that PDGF-BB was positively correlated with the expression of inflammatory factors such as IL-4, IL-8, IL-15, and IL-17 in IBD serum, suggesting that PDGF-BB is involved in the progression of IBD. Activated inflammatory cells would release PDGF-BB, which acts as a chemical inducer and participates in the recruitment and activation of inflammatory cells, eventually leading to the continuation of the disease [15]. Myeloid PDGF receptor alpha protects the intestinal flora and maintains intestinal homeostasis by activating macrophages to polarize into anti-inflammatory phenotype [16]. M1 macrophages have the main pro-inflammatory and anti-angiogenic effects [37, 38], and were positively correlated with the expression of PDGF-BB in the present study, suggesting that M1 macrophages were also involved in the high expression of PDGF-BB. In systemic sclerosis, CXCL4 activates macrophages and leads to an increase in PDGF-BB release, thus promoting an increase in pro-inflammatory cytokines expression and fibrosis formation. PDGF-BB also leads to an increase in chemokine expression, such as that of CXCL9 [39]. The present study demonstrated that the expression of PDGF-BB was positively correlated with that of CXCL9, suggesting that the correlation between PDGF-BB and chemokines should be considered. In univariate and multivariate analyses, CD86 and PDGF-BB were independent risk factors for patients with IBD (P < 0.05). The results of the multivariate analysis showed that CXCL9 was not significant, which may result from the insufficient sample size; future studies should be performed in in vivo and vitro with larger clinical sample sizes to validate these results. Besides, further studies remain to be conducted on which specific signaling pathway PDGF-BB participates to play its dual role in different stages of IBD disease, so as to find therapeutic targets for regulating IBD inflammatory activity and promoting mucosal repair in the early stage of the disease.
This study has some limitations, which are mainly reflected in the small sample size, single-center research, lack of research on the correlation between other factors related to M1 macrophages and PDGF-BB, except chemokines, lack of quantitative detection of fecal calprotectin, comparison to adjustment “normal” mucosa in active IBD, and further animal model verification and exploration of cell pathways.
Conclusions
In summary, active IBD was accompanied by an increase in the expression of PDGF-BB, CXCL9, and M1 macrophages, among which the colonic PDGF-BB and M1 macrophage marker CD86 were independent risk factors for disease activity and had diagnostic value. CXCL9 was a potential risk factor for IBD. In addition, a potential correlation was observed between colon PDGF-BB and M1 macrophages.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We gratefully acknowledge the central laboratory of the Second Hospital of Jiaxing for providing the necessary equipment for this study. We would also like to thank Editage (Cactus Communications) for the paper language polishing service.
Abbreviations
- IBD
Inflammatory bowel disease
- CD
Chron’s disease
- UC
Ulcerative colitis
- PDGF-BB
Platelet-derived growth factor-BB
- ELISA
Enzyme-linked immunosorbent assay
- ROC
Receiver operating characteristic
- AUC
Area under the curve
- BMI
Body mass index
- ESR
Erythrocyte sedimentation rate
- FC
Fecal calprotectin
- Hb
Hemoglobin
- hsCRP
High-sensitivity C-reactive protein
- IFN-γ
Interferon-γ
- LDL
Low-density lipoprotein
- TC
Total cholesterol
- TG
Triglyceride
- TNF-α
Tumor necrosis factor-α
- IL-1β
Interleukin-1β
- PI3Kγ
Phosphoinositide 3-kinase γ
- NF-κB
Nuclear factor kappa-B
Author contributions
ZYF participated in research design, research conduct, data analysis and major paper writing. SWQ collected clinical data and serological samples, as well as analyzed the data. XJ made contributions to collect clinical data and give academic guidance to the study. CWZ collected clinical data and pathological specimen. JFM was in charge of serological tests and analysis. JQX managed pathological examination and analysis. JMZ contributed to research design, research conduct and article review. HYS was responsible for the whole study, who participated in data collection, data analysis, article review and research guidance. All authors read and approved the final manuscript.
Funding
This work was financially supported by the Zhejiang Provincial Medicine and Health Technology Project (No. 2021KY1116) and the Science and Technology Bureau of Jiaxing city, Zhejiang, China (No. 2022AD30007).
Data availability
The original data of the study is available in the “Supplementary files” (Supplementary Tables 1-2).
Declarations
Ethics approval and consent to participate
The study had been performed in accordance with the Declaration of Helsinki and had been approved by the medical ethics committee of The Second Hospital of Jiaxing (No. JXEY-2021SW095). Informed consent to participate in the study had all been obtained from participants.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jinming Zhang, Email: zjm100123@126.com.
Haiyan Shen, Email: shymaomao1980@163.com.
References
- 1.Atreya I, Atreya R, Neurath MF. NF-kappaB in inflammatory bowel disease. [J] J Intern Med. 2008;263(6):591–6. [DOI] [PubMed] [Google Scholar]
- 2.Kaser A, Zeissig S, Blumberg RS. Inflammatory bowel disease. [J] Annual Rev Immunol. 2010;28:573–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cromer WE, Mathis JM, Granger DN, et al. Role of the endothelium in inflammatory bowel diseases. [J] World J Gastroenterol. 2011;17(5):578–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Binion DG, West GA, Ina K, et al. Enhanced leukocyte binding by intestinal microvascular endothelial cells in inflammatory bowel disease. [J] Gastroenterol. 1997;112(6):1895–907. [DOI] [PubMed] [Google Scholar]
- 5.Park JS, Cresci GA. M. Dysfunctional intestinal microvascular endothelial cells: insights and therapeutic implications in gastrointestinal inflammation. [J] Immunometabolism (Cobham Surrey). 2024;6(2):e00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hunter P. The inflammation theory of disease. The growing realization that chronic inflammation is crucial in many diseases opens new avenues for treatment. [J] EMBO Rep. 2012;13(11):968–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bain CC, Mowat AM. Macrophages in intestinal homeostasis and inflammation. [J] Immunological Reviews. 2014;260(1):102–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pull SL, Doherty JM, Mills JC, et al. Activated macrophages are an adaptive element of the colonic epithelial progenitor niche necessary for regenerative responses to injury. [J] Proc Natl Acad Sci United States Am. 2005;102(1):99–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu Y, Chen H, Ouyang W, et al. Unraveling the role of M1 macrophage and CXCL9 in predicting immune checkpoint inhibitor efficacy through multicohort analysis and single-cell RNA sequencing. [J] MedComm. 2024;5(3):e471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.House IG, Savas P, Lai J et al. Macrophage-derived CXCL9 and CXCL10 are required for Antitumor Immune responses following Immune Checkpoint Blockade. [J] Clin Cancer Res. 2020;26 (2):487–504. [DOI] [PubMed]
- 11.Chow MT, Ozga AJ, Servis RL, et al. Intratumoral Activity of the CXCR3 chemokine system is required for the efficacy of Anti-PD-1 therapy. [J] Immunity. 2019;50(6):1498-1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dharmasiri S, Garrido-Martin EM, Harris RJ, et al. Human intestinal macrophages are involved in the Pathology of both Ulcerative Colitis and Crohn Disease. [J] Inflamm Bowel Dis. 2021;27(10):1641–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seyedizade SS, Afshari K, Bayat S et al. Current status of M1 and M2 macrophages pathway as drug targets for inflammatory bowel disease. [J] Arch Immunol Ther Exp (Warsz). 2020;68 (2):10. [DOI] [PubMed]
- 14.Szabo S, Tolstanova G. New molecules as drug candidates for the treatment of upper and lower GI tract ulcers. [J] Curr Pharm Des. 2015;21(21):2993–3001. [DOI] [PubMed] [Google Scholar]
- 15.Martinez-Fierro ML, Garza-Veloz I, Rocha-Pizaña MR, et al. Serum cytokine, chemokine, and growth factor profiles and their modulation in inflammatory bowel disease. [J] Med. 2019;98(38):e17208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dörk R, Pelczar P, Shiri AM, et al. Myeloid cell-specific deletion of PDGFR-α promotes dysbiotic intestinal microbiota and thus increased colitis susceptibility. [J]. J Crohn’s Colitis. 2023;17(11):1858–69. [DOI] [PubMed] [Google Scholar]
- 17.Wu K, Liang J, Ran Z, et al. Consensus on diagnosis and treatment of inflammatory bowel disease (Beijing, 2018). [J] Chin J Practical Intern Med. 2018;38(09):796–813. [Google Scholar]
- 18.Marks DJ, Segal AW. Innate immunity in inflammatory bowel disease: a disease hypothesis. [J]. J Pathol. 2008;214(2):260–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marks DJ, Rahman FZ, Sewell GW, et al. Crohn’s disease: an immune deficiency state. [J] Clin Reviews Allergy Immunol. 2010;38(1):20–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu T, Dang S, Zhu R, et al. Adamts18 deficiency promotes colon carcinogenesis by enhancing β-catenin and p38MAPK/ERK1/2 signaling in the mouse model of AOM/DSS-induced colitis-associated colorectal cancer. [J] Oncotarget. 2017;8(12):18979–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tian L, Li W, Yang L et al. Cannabinoid receptor 1 participates in liver inflammation by promoting M1 macrophage polarization via RhoA/NF-κB p65 and ERK1/2 pathways, Respectively, in Mouse Liver Fibrogenesis. [J] Front Immunol.. 2017;8 1214. [DOI] [PMC free article] [PubMed]
- 22.Zhang L, Liu P, Wen W, et al. IL-17A contributes to myocardial ischemic injury by activating NLRP3 inflammasome in macrophages through AMPKα/p38MAPK/ERK1/2 signal pathway in mice. [J] Mol Immunol. 2019;105:240–50. [DOI] [PubMed] [Google Scholar]
- 23.Rugtveit J, Nilsen EM, Bakka A, et al. Cytokine profiles differ in newly recruited and resident subsets of mucosal macrophages from inflammatory bowel disease. [J] Gastroenterol. 1997;112(5):1493–505. [DOI] [PubMed] [Google Scholar]
- 24.Schenk M, Bouchon A, Seibold F, et al. TREM-1–expressing intestinal macrophages crucially amplify chronic inflammation in experimental colitis and inflammatory bowel diseases. [J] J Clin Invest. 2007;117(10):3097–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cao Y, Xu Y, Auchoybur ML, et al. Regulatory role of IKKɑ in myocardial ischemia/reperfusion injury by the determination of M1 versus M2 polarization of macrophages. [J] J Mol Cell Cardiol. 2018;123:1–12. [DOI] [PubMed] [Google Scholar]
- 26.Chen J, Zhou Y, Sun Y, et al. Bidirectional mendelian randomisation analysis provides evidence for the Causal involvement of Dysregulation of CXCL9, CCL11 and CASP8 in the pathogenesis of Ulcerative Colitis. [J]. J Crohn’s Colitis. 2023;17(5):777–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kessel C, Lavric M, Weinhage T, et al. Serum biomarkers confirming stable remission in inflammatory bowel disease. [J] Sci Rep. 2021;11(1):6690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tallquist M, Kazlauskas A. PDGF signaling in cells and mice. [J] Cytokine Growth Factor Reviews. 2004;15(4):205–13. [DOI] [PubMed] [Google Scholar]
- 29.Manzat Saplacan RM, Balacescu L, Gherman C, et al. The role of PDGFs and PDGFRs in Colorectal Cancer. [J] Mediators Inflamm. 2017;2017:4708076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fredriksson L, Li H, Eriksson U. The PDGF family: four gene products form five dimeric isoforms. [J] Cytokine Growth Factor Reviews. 2004;15(4):197–204. [DOI] [PubMed] [Google Scholar]
- 31.Gruber R, Karreth F, Frommlet F, et al. Platelets are mitogenic for periosteum-derived cells. [J] J Orthop Research: Official Publication Orthop Res Soc. 2003;21(5):941–8. [DOI] [PubMed] [Google Scholar]
- 32.Lee MH, Kwon BJ, Koo MA et al. Mitogenesis of vascular smooth muscle cell stimulated by platelet-derived growth factor-bb is inhibited by blocking of intracellular signaling by epigallocatechin-3-O-gallate. [J] Oxidative medicine and cellular longevity. 2013;2013:827905. [DOI] [PMC free article] [PubMed]
- 33.Ozaki Y, Nishimura M, Sekiya K, et al. Comprehensive analysis of chemotactic factors for bone marrow mesenchymal stem cells. [J] Stem Cells Dev. 2007;16(1):119–29. [DOI] [PubMed] [Google Scholar]
- 34.Heldin CH, Westermark B. Mechanism of action and in vivo role of platelet-derived growth factor. [J] Physiological Reviews. 1999;79(4):1283–316. [DOI] [PubMed] [Google Scholar]
- 35.Phipps MC, Xu Y, Bellis SL. Delivery of platelet-derived growth factor as a chemotactic factor for mesenchymal stem cells by bone-mimetic electrospun scaffolds. [J] PloS One. 2012;7(7):e40831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li J, Wang X, Su Y, et al. TRIM33 modulates inflammation and Airway Remodeling of PDGF-BB-Induced Airway smooth-muscle cells by the Wnt/β-Catenin pathway. [J] Int Archives Allergy Immunol. 2022;183(10):1127–36. [DOI] [PubMed] [Google Scholar]
- 37.Namba H, Kashiwagi Y, Nishitsuka K, et al. Association of PDGF-BB-induced thrombomodulin with the regulation of inflammation in the corneal and scleral stroma. [J] Invest Ophthalmol Visual Sci. 2010;51(11):5460–9. [DOI] [PubMed] [Google Scholar]
- 38.Cai Y, Wang Z, Liao B et al. Anti-inflammatory and Chondroprotective effects of platelet-derived growth Factor-BB on Osteoarthritis Rat models. [J] J Gerontol A Biol Sci Med Sci. 2023;78 (1):51–9. [DOI] [PubMed]
- 39.Fujii J, Osaki T. Involvement of nitric oxide in protecting against Radical Species and Autoregulation of M1-Polarized macrophages through metabolic remodeling. [J] Molecules. 2023;28 (2):814. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original data of the study is available in the “Supplementary files” (Supplementary Tables 1-2).