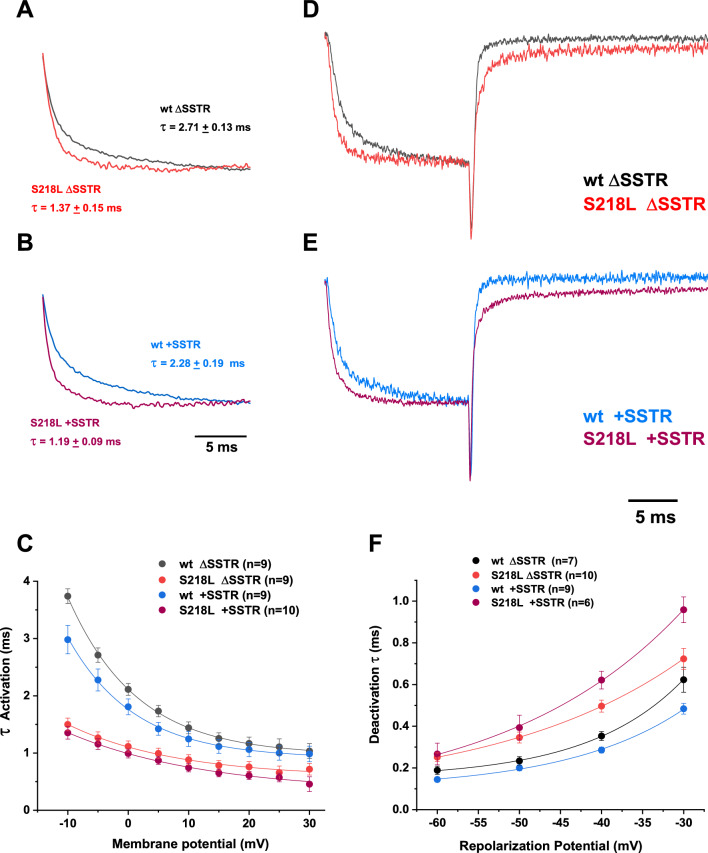
Fig. 3.
S218L FHM-1 mutation alters the kinetic properties of P/Q-type currents mediated by + /Δ SSTR isoforms. Superimposed normalized recordings of peak currents from SSTR (A) and +SSTR (B) isoforms show the difference in the activation time course between wild type and S218L channels, with the onset of activation distinctively faster in the mutant channels (S218L ΔSSTR, red trace in A; S218L +SSTR purple trace in B). The average activation time constant values at the peak of the I-V curve is shown next to the corresponding traces. Mean activation time constants were plotted against membrane potential (C), showing that mutant channels display a shallower voltage dependence than the wild types. To examine channel deactivation, currents were evoked by a brief depolarizing test pulse from a holding of −100 to −5 mV, and membrane potential was repolarized to various membrane potentials to record tail currents. Exemplary recordings corresponding to repolarization to −40 mV from each pair of wt (black trace, D; blue trace, E) vs mutant isoforms (red trace, D; purple trace, E) were normalized and overlapped, showing that both mutant channels not only activate faster, but also take longer to close when the membrane is repolarized, relative to wild type. The decay phase of the tail currents was fitted with a single exponential to obtain deactivation time constants, plotted as a function of the repolarization potential (F). Deactivation voltage dependence was more pronounced in the wild type isoforms (see text for details)