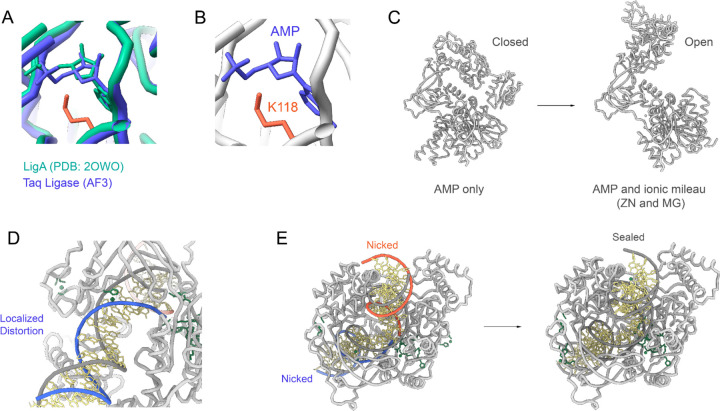
Figure 3. Conformational Changes and DNA Interactions in Taq Ligase Structural Ensemble.
(A) Closed conformation of Taq ligase with NAD, showing AMP moiety adjacent to catalytic lysine (K118) in the ensemble. (B) Zoomed view of the catalytic site in the closed state, highlighting AMP’s position next to K118. (C) Closed-to-Open conformation transition of Taq ligase in the adenylate-intermediate state, allowing an accessible surface for DNA binding. (D) Closed conformation with nicked DNA, depicting the formation of a ternary complex for efficient phosphodiester bond catalysis. (E) DNA adopts an RNA-like A-form helix near the nick in the LigA complex, reflecting localized structural distortions during repair.