Abstract
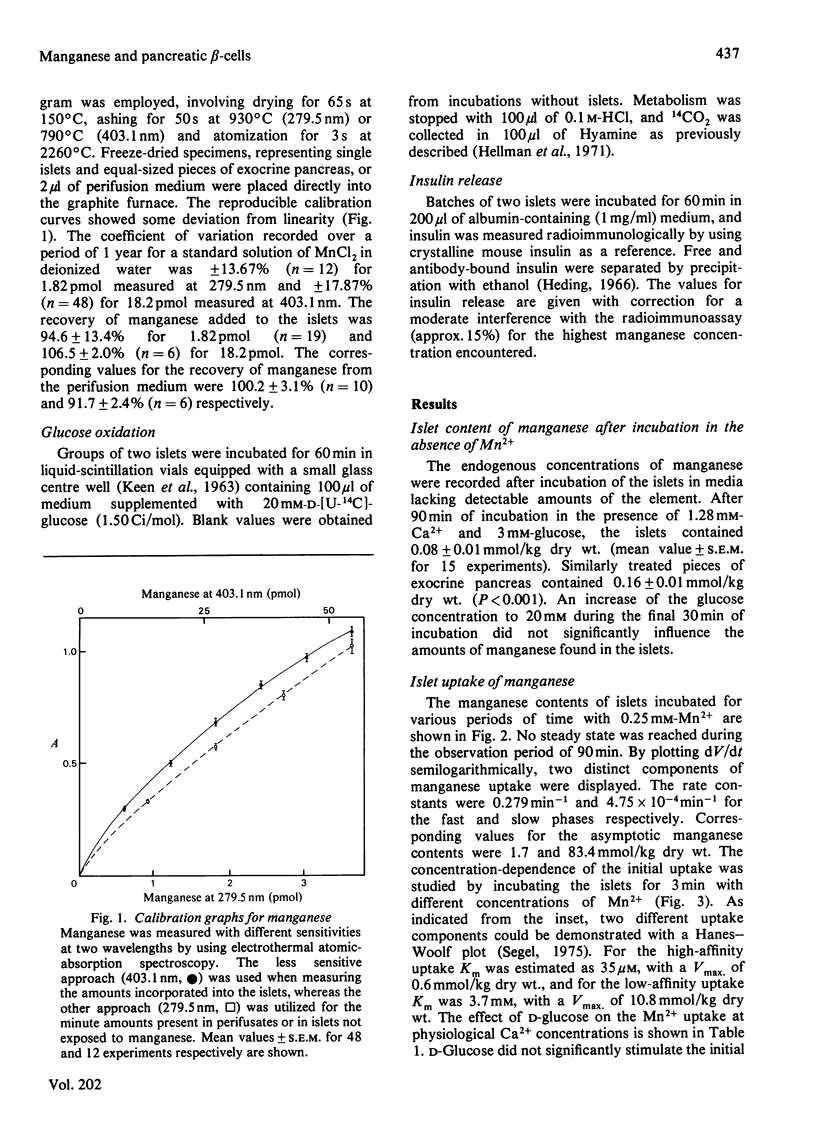
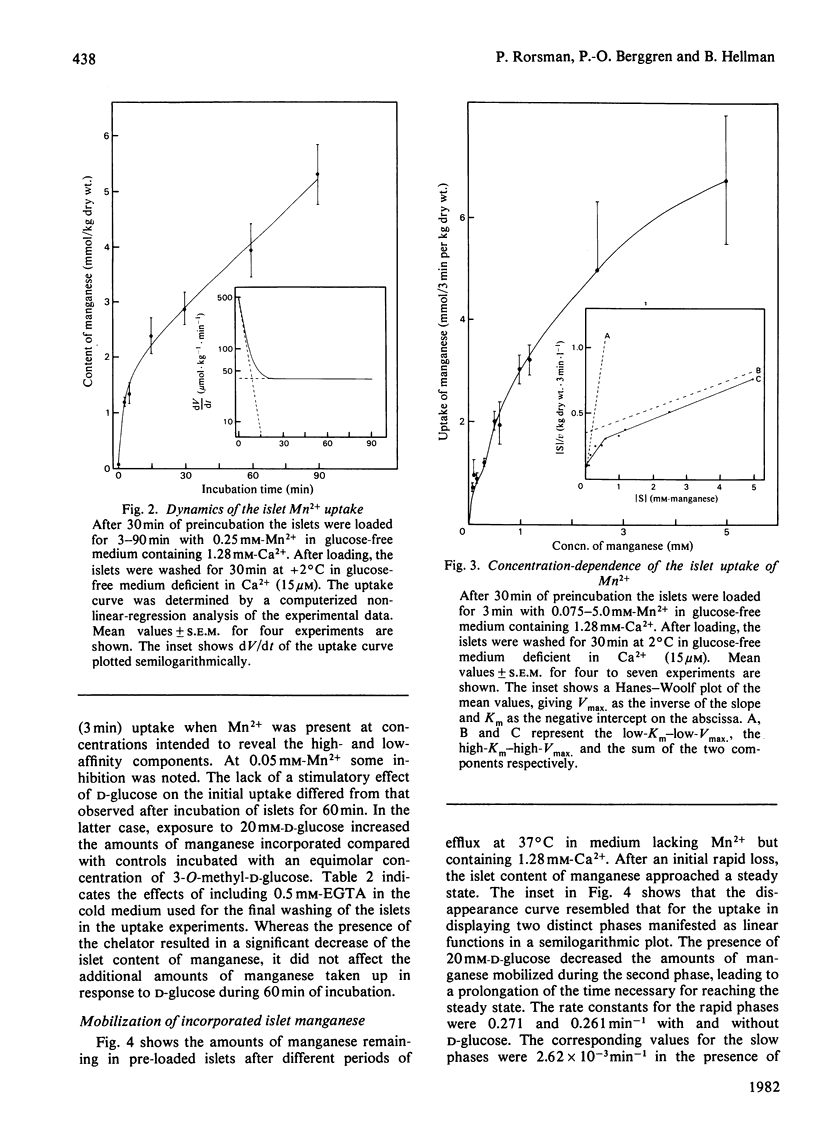
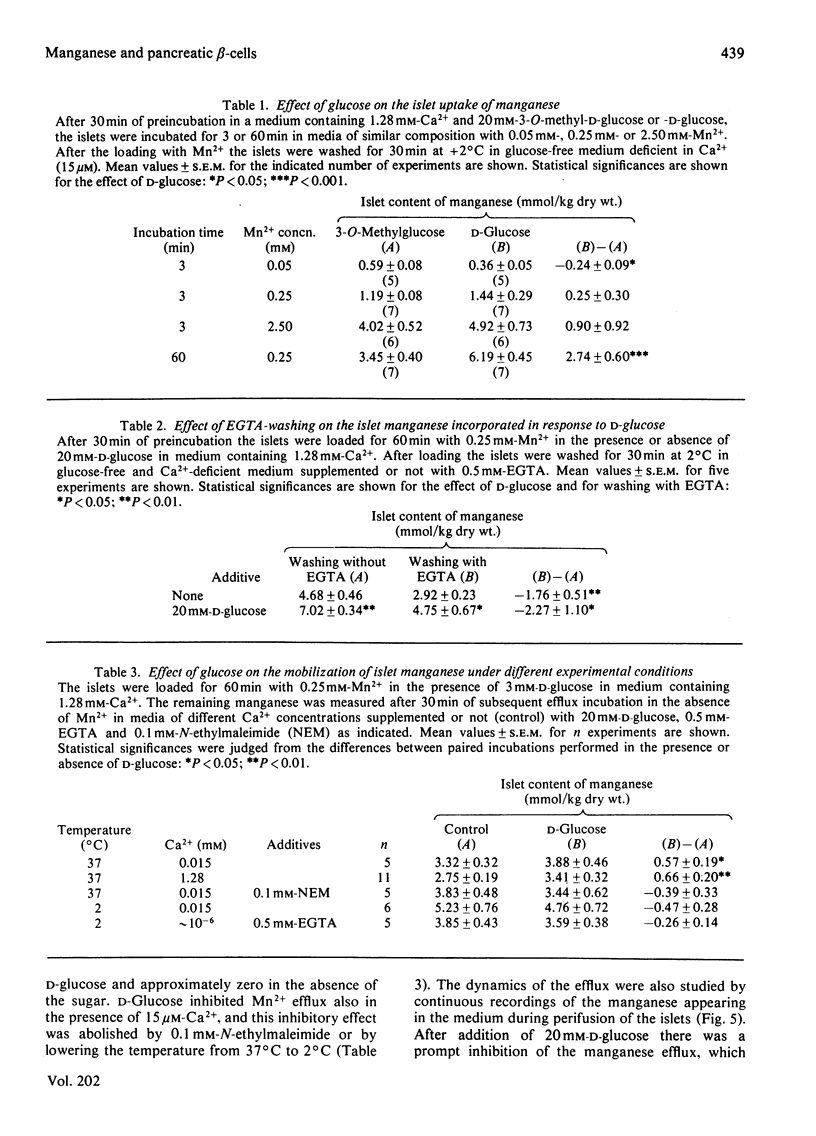
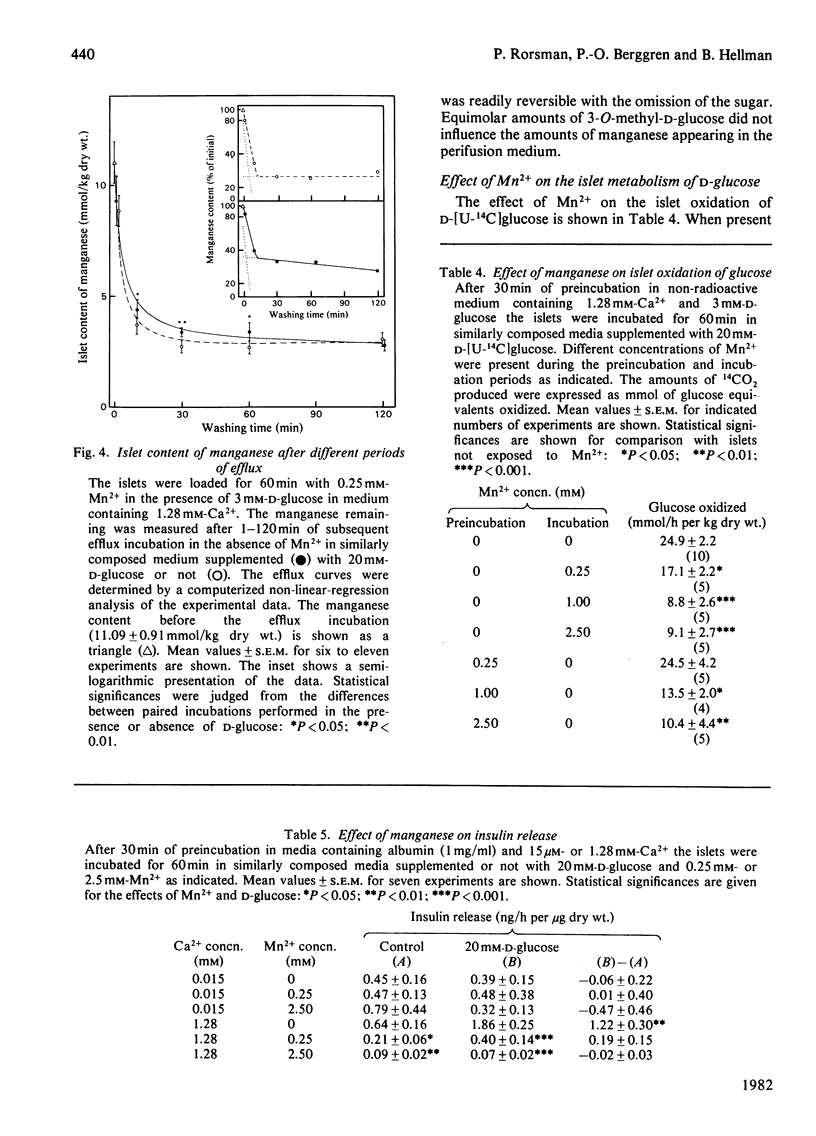
Electrothermal atomic-absorption spectroscopy was employed for measuring manganese in beta-cell-rich pancreatic islets microdissected from ob/ob mice. The islet content of endogenous manganese was 80 mumol/kg dry wt., which is about half as much as found in the exocrine pancreas. The initial uptake was characterized by two components, with approximate Km values of 35 microM and 3.7 microM respectively. After 60 min of incubation with 0.25 mM-Mn2+, the intracellular concentration of manganese corresponded to an almost 25-fold accumulation compared with that of the extracellular medium. When exposed to 20 mM-D-glucose, the islets retained more manganese, owing to suppression of its mobilization. The glucose inhibition of efflux was prompt and reversible, as indicated from direct recordings of manganese in a perifusion medium. D-Glucose was an equally potent inhibitor of efflux in the presence of 15 microM- and 1.28 mM-Ca2+. The inhibitory action disappeared when metabolism was suppressed by adding 0.1 mM-N-ethylmaleimide or by lowering the temperature from 37 degrees C to 2 degrees C. At a concentration of 0.25 mM, Mn2+ abolished the insulin-releasing action of D-glucose, exerting only moderate suppression of its metabolism. The addition of Mn2+ resulted in inhibition of basal insulin release in the presence of 1.28 mM-Ca2+, but not in a Ca2+-deficient medium. The studies indicate that the previously observed phenomenon of glucose inhibition of 45Ca efflux has a counterpart in the suppression of manganese mobilization from the pancreatic islets. With the demonstration of a pronounced glucose inhibition of manganese efflux, it is evident that Mn2+ may represent a useful tool for exploring the mechanism of glucose-induced retention of calcium in the pancreatic beta-cells.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrahamsson H., Gylfe E., Hellman B. Influence of external calcium ions on labelled calcium efflux from pancreatic beta-cells and insulin granules in mice. J Physiol. 1981 Feb;311:541–550. doi: 10.1113/jphysiol.1981.sp013603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arqueros L., Daniels A. J. Manganese as agonist and antagonist of calcium ions: dual effect upon catecholamine release from adrenal medulla. Life Sci. 1981 Mar 30;28(13):1535–1540. doi: 10.1016/0024-3205(81)90387-8. [DOI] [PubMed] [Google Scholar]
- Baker P. F., Glitsch H. G. Voltage-dependent changes in the permeability of nerve membranes to calcium and other divalent cations. Philos Trans R Soc Lond B Biol Sci. 1975 Jun 10;270(908):389–409. doi: 10.1098/rstb.1975.0018. [DOI] [PubMed] [Google Scholar]
- Borle A. B. Control, Modulation, and regulation of cell calcium. Rev Physiol Biochem Pharmacol. 1981;90:13–153. doi: 10.1007/BFb0034078. [DOI] [PubMed] [Google Scholar]
- Borle A. B. Kinetic analyses of calcium movements in HeLa cell cultures. I. Calcium influx. J Gen Physiol. 1969 Jan;53(1):43–56. doi: 10.1085/jgp.53.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels A. J., Johnson L. N., Williams R. J. Uptake of manganese by chromaffin granules in vitro. J Neurochem. 1979 Oct;33(4):923–929. doi: 10.1111/j.1471-4159.1979.tb09922.x. [DOI] [PubMed] [Google Scholar]
- Dean P. M., Matthews E. K. Electrical activity in pancreatic islet cells: effect of ions. J Physiol. 1970 Sep;210(2):265–275. doi: 10.1113/jphysiol.1970.sp009208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delahayes J. F. Depolarization-induced movement of Mn 2 cations across the cell membrane in the guinea pig myocardium. Circ Res. 1975 Jun;36(6):713–718. doi: 10.1161/01.res.36.6.713. [DOI] [PubMed] [Google Scholar]
- Donatsch P., Lowe D. A., Richardson B. P., Taylor P. The functional significance of sodium channels in pancreatic beta-cell membranes. J Physiol. 1977 May;267(2):357–376. doi: 10.1113/jphysiol.1977.sp011817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FATT P., GINSBORG B. L. The ionic requirements for the production of action potentials in crustacean muscle fibres. J Physiol. 1958 Aug 6;142(3):516–543. doi: 10.1113/jphysiol.1958.sp006034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flatt P. R., Berggren P. O., Gylfe E., Hellman B. Calcium and pancreatic beta-cell function. IX. Demonstration of lanthanide-induced inhibition of insulin secretion independent of modifications in transmembrane Ca2+ fluxes. Endocrinology. 1980 Oct;107(4):1007–1013. doi: 10.1210/endo-107-4-1007. [DOI] [PubMed] [Google Scholar]
- Flatt P. R., Gylfe E., Hellman B. Thulium binding to the pancreatic beta-cell membrane. Endocrinology. 1981 Jun;108(6):2258–2263. doi: 10.1210/endo-108-6-2258. [DOI] [PubMed] [Google Scholar]
- Frankel B. J., Gylfe E., Hellman B., Idahl L. A., Landström U., Løvtrup S., Sehlin J. Metabolism of cold-stored pancreatic islets. Diabetologia. 1978 Sep;15(3):187–190. doi: 10.1007/BF00421237. [DOI] [PubMed] [Google Scholar]
- Fukuda J., Kawa K. Permeation of manganese, cadmium, zinc, and beryllium through calcium channels of an insect muscle membrane. Science. 1977 Apr 15;196(4287):309–311. doi: 10.1126/science.847472. [DOI] [PubMed] [Google Scholar]
- Gunter T. E., Gunter K. K., Puskin J. S., Russell P. R. Efflux of Ca2+ and Mn2+ from rat liver mitochondria. Biochemistry. 1978 Jan 24;17(2):339–345. doi: 10.1021/bi00595a023. [DOI] [PubMed] [Google Scholar]
- Gylfe E., Buitrago A., Berggren P. O., Hammarström K., Hellman B. Glucose inhibition of 45Ca efflux from pancreatic islets. Am J Physiol. 1978 Aug;235(2):E191–E196. doi: 10.1152/ajpendo.1978.235.2.E191. [DOI] [PubMed] [Google Scholar]
- Gylfe E., Hellman B. Calcium and pancreatic beta-cell function. 2. Mobilisation of glucose-sensitive 45Ca from perifused islets rich in beta-cells. Biochim Biophys Acta. 1978 Jan 18;538(2):249–257. doi: 10.1016/0304-4165(78)90353-7. [DOI] [PubMed] [Google Scholar]
- Hahn H. J., Hellman B., Lernmark A., Sehlin J., Täljedal I. B. The pancreatic beta-cell recognition of insulin secretogogues. Influence of neuraminidase treatment on the release of insulin and the islet content of insulin, sialic acid, and cyclic adenosine 3':5'-monophosphate. J Biol Chem. 1974 Aug 25;249(16):5275–5284. [PubMed] [Google Scholar]
- Havu N., Lundgren G., Falkmer S. Microchemical assays of glutathione, zinc, cobalt and manganese in micro-dissected areas of the endocrine pancreas in the hagfish, Myxine glutinosa. Acta Endocrinol (Copenh) 1977 Nov;86(3):561–569. doi: 10.1530/acta.0.0860561. [DOI] [PubMed] [Google Scholar]
- Hellman B. Calcium and pancreatic beta-cell function. 5. Mobilisation of a glucose-stimulated pool of intracellular 45Ca by metabolic inhibitors and the ionophore A-23187. Acta Endocrinol (Copenh) 1979 Apr;90(4):624–636. [PubMed] [Google Scholar]
- Hellman B. Methodological approaches to studies on the pancreatic islets. Diabetologia. 1970 Apr;6(2):110–120. doi: 10.1007/BF00421438. [DOI] [PubMed] [Google Scholar]
- Hellman B., Sehlin J., Täljedal I. B. Calcium and secretion: distinction between two pools of glucose-sensitive calcium in pancreatic islets. Science. 1976 Dec 24;194(4272):1421–1423. doi: 10.1126/science.795030. [DOI] [PubMed] [Google Scholar]
- Hellman B., Sehlin J., Täljedal I. B. Effects of glucose and other modifiers of insulin release on the oxidative metabolism of amino acids in micro-dissected pancreatic islets. Biochem J. 1971 Jul;123(4):513–521. doi: 10.1042/bj1230513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellman B. Studies in obese-hyperglycemic mice. Ann N Y Acad Sci. 1965 Oct 8;131(1):541–558. doi: 10.1111/j.1749-6632.1965.tb34819.x. [DOI] [PubMed] [Google Scholar]
- Hellman B. The significance of calcium for glucose stimulation of insulin release. Endocrinology. 1975 Aug;97(2):392–398. doi: 10.1210/endo-97-2-392. [DOI] [PubMed] [Google Scholar]
- Hermansen K., Iversen J. Dual action of mn++ upon the secretion of insulin and glucagon from the isolated, perfused canine pancreas. Possible interactions with ca++. Diabetologia. 1978 Dec;15(6):475–479. doi: 10.1007/BF02342873. [DOI] [PubMed] [Google Scholar]
- Impraim C. C., Micklem K. J., Pasternak C. A. Calcium, cells and virus--alterations caused by paramyxoviruses. Biochem Pharmacol. 1979 Jun 15;28(12):1963–1969. doi: 10.1016/0006-2952(79)90652-x. [DOI] [PubMed] [Google Scholar]
- KEEN H., FIELD J. B., PASTAN I. H. A simple method for in vitro metabolic studies using small volumes of tissue and medium. Metabolism. 1963 Feb;12:143–147. [PubMed] [Google Scholar]
- Kikuchi M., Wollheim C. B., Cuendet G. S., Renold A. E., Sharp G. W. Studies on the dual effects of glucose on 45Ca++ efflux from isolated rat islets. Endocrinology. 1978 May;102(5):1339–1349. doi: 10.1210/endo-102-5-1339. [DOI] [PubMed] [Google Scholar]
- Lin Y. M., Liu Y. P., Cheung W. Y. Cyclic 3':5'-nucleotide phosphodiesterase. Purification, characterization, and active form of the protein activator from bovine brain. J Biol Chem. 1974 Aug 10;249(15):4943–4954. [PubMed] [Google Scholar]
- Malaisse W. J., Brisson G. R., Baird L. E. Stimulus-secretion coupling of glucose-induced insulin release. X. Effect of glucose on 45 Ca efflux from perifused islets. Am J Physiol. 1973 Feb;224(2):389–394. doi: 10.1152/ajplegacy.1973.224.2.389. [DOI] [PubMed] [Google Scholar]
- Naber S. P., McDaniel M. L., Lacy P. E. The effect of glucose on the acute uptake and efflux of calcium-45 in isolated rat islets. Endocrinology. 1977 Sep;101(3):686–693. doi: 10.1210/endo-101-3-686. [DOI] [PubMed] [Google Scholar]
- Ochi R. Manganese-dependent propagated action potentials and their depression by electrical stimulation in guinea-pig myocardium perfused by sodium-free media. J Physiol. 1976 Dec;263(2):139–156. doi: 10.1113/jphysiol.1976.sp011625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochi R. The slow inward current and the action of manganese ions in guinea-pig's myocardium. Pflugers Arch. 1970;316(1):81–94. doi: 10.1007/BF00587898. [DOI] [PubMed] [Google Scholar]
- Pfeiffer D. R., Kauffman R. F., Lardy H. A. Effects of N-ethylmaleimide on the limited uptake of Ca2+, Mn2+, and Sr2+ by rat liver mitochondria. J Biol Chem. 1978 Jun 25;253(12):4165–4171. [PubMed] [Google Scholar]
- Spears G., Sneyd J. G., Loten E. G. A method for deriving kinetic constants for two enzymes acting on the same substrate. Biochem J. 1971 Dec;125(4):1149–1151. doi: 10.1042/bj1251149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teo T. S., Wang J. H. Mechanism of activation of a cyclic adenosine 3':5'-monophosphate phosphodiesterase from bovine heart by calcium ions. Identification of the protein activator as a Ca2+ binding protein. J Biol Chem. 1973 Sep 10;248(17):5950–5955. [PubMed] [Google Scholar]
- Vainio H., Mela L., Chance B. Energy dependent bivalent cation translocation in rat liver mitochondria. Eur J Biochem. 1970 Feb;12(2):387–391. doi: 10.1111/j.1432-1033.1970.tb00863.x. [DOI] [PubMed] [Google Scholar]
- Wolff D. J., Poirier P. G., Brostrom C. O., Brostrom M. A. Divalent cation binding properties of bovine brain Ca2+-dependent regulator protein. J Biol Chem. 1977 Jun 25;252(12):4108–4117. [PubMed] [Google Scholar]


