Abstract
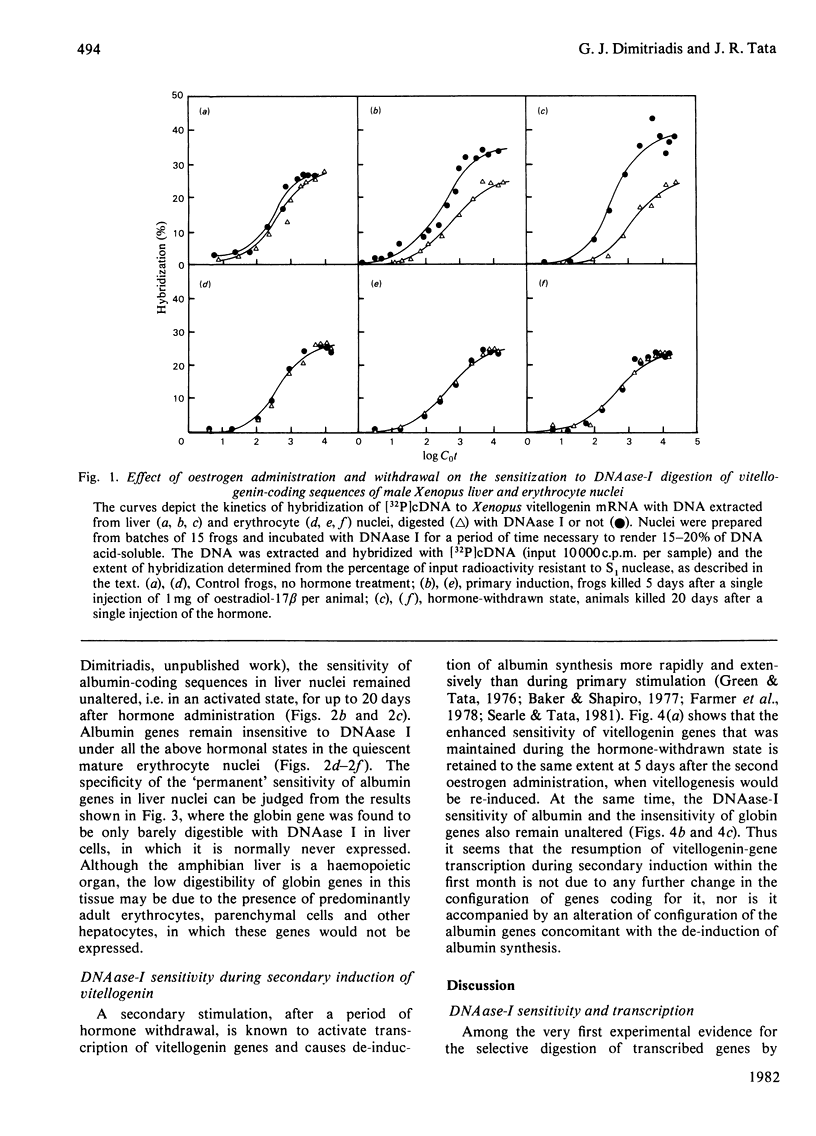
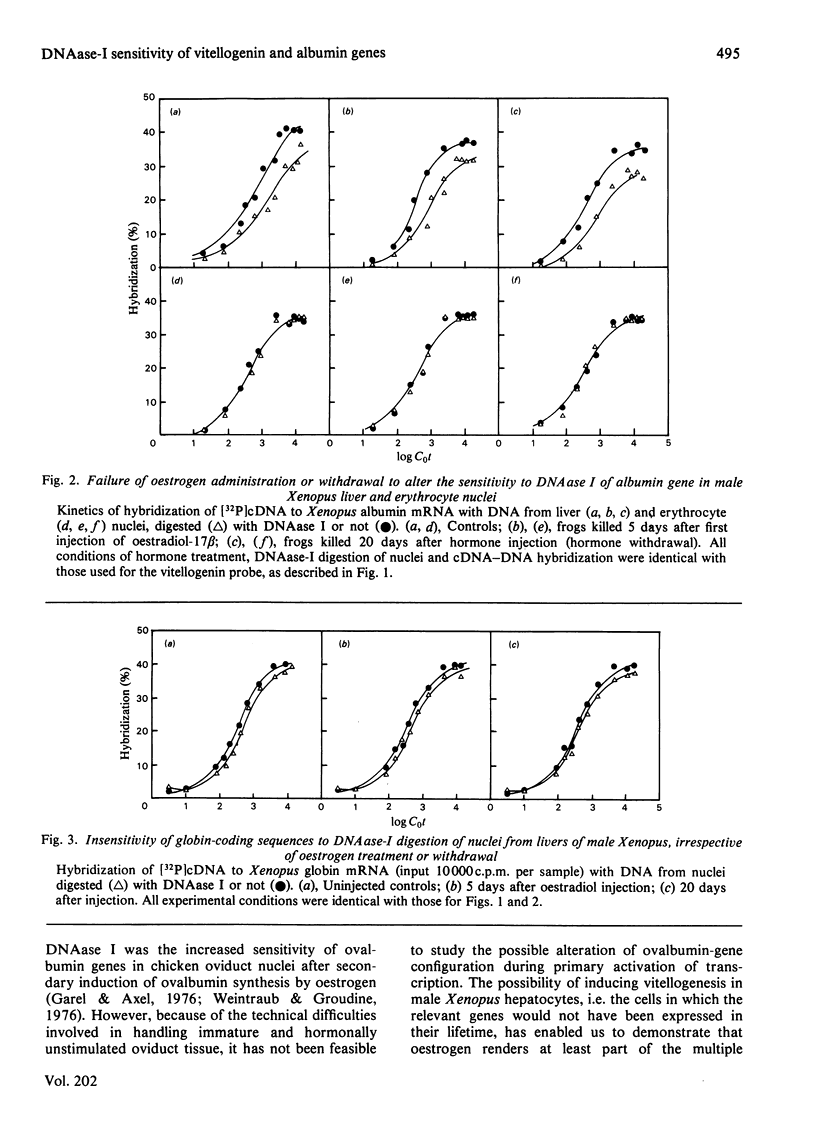
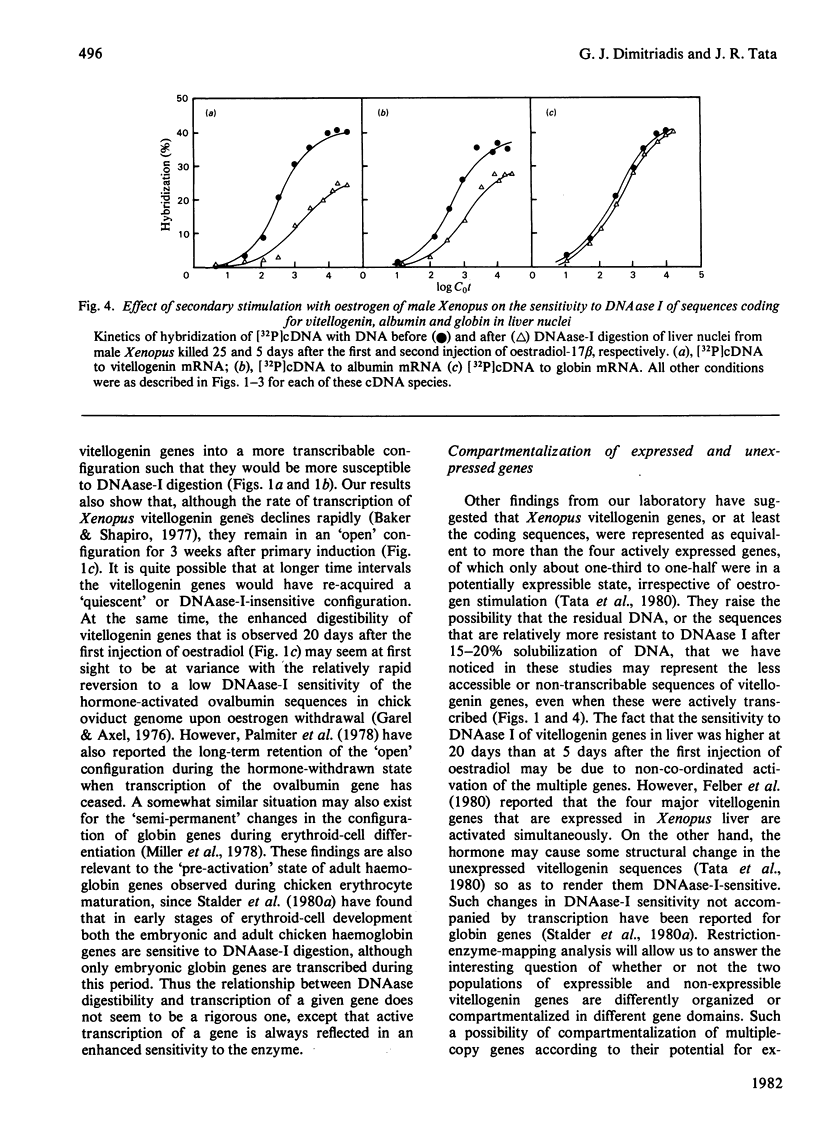
The sensitivity to DNAase (deoxyribonuclease) I (which preferentially digests transcribed sequences) of vitellogenin and albumin genes in liver and erythrocytes of male Xenopus after primary and secondary induction of vitellogenesis by oestrogen was measured by hybridization to cDNA (complementary DNA) of the residual DNA after enzymic digestion of isolated nuclei. Vitellogenin sequences were rendered selectively more sensitive to limited DNAase-I digestion (15-20% of DNA rendered acid-soluble) during primary hormonal activation (5 days) of vitellogenin genes in liver, but not erythrocyte, nuclei. Hormone withdrawal (25 days after first injection) did not result in reversion to a pre-activation gene configuration, nor did secondary hormonal stimulation (5 days after second and 25 days after first injection) augment the sensitivity of the genes to digestion by the nuclease. Similar hormone treatment did not affect the sensitivity of the constitutively expressed albumin genes in liver nuclei, nor their insensitivity in erythrocyte nuclei. Under the same conditions, globin genes remained indigestible in liver nuclei. It is concluded that primary induction of vitellogenesis in male Xenopus liver is accompanied by relatively long-lasting (3-4 weeks) change in the configuration of vitellogenin genes in hepatic nuclei which is not reversed or further modified during short-term oestrogen withdrawal or upon secondary stimulation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker H. J., Shapiro D. J. Kinetics of estrogen induction of Xenopus laevis vitellogenin messenger RNA as measured by hybridization to complementary DNA. J Biol Chem. 1977 Dec 10;252(23):8428–8434. [PubMed] [Google Scholar]
- Bellard M., Gannon F., Chambon P. Nucleosome structure III: the structure and transcriptional activity of the chromatin containing the ovalbumin and globin genes in chick oviduct nuclei. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 2):779–791. doi: 10.1101/sqb.1978.042.01.078. [DOI] [PubMed] [Google Scholar]
- Britten R. J., Graham D. E., Neufeld B. R. Analysis of repeating DNA sequences by reassociation. Methods Enzymol. 1974;29:363–418. doi: 10.1016/0076-6879(74)29033-5. [DOI] [PubMed] [Google Scholar]
- Dawid I. B., Wahli W. Application of recombinant DNA technology to questions of developmental biology: a review. Dev Biol. 1979 Mar;69(1):305–328. doi: 10.1016/0012-1606(79)90294-x. [DOI] [PubMed] [Google Scholar]
- Deeley R. G., Udell D. S., Burns A. T., Gordon J. I., Goldberger R. F. Kinetics of avian vitellogenin messenger RNA induction. Comparison between primary and secondary response to estrogen. J Biol Chem. 1977 Nov 25;252(22):7913–7915. [PubMed] [Google Scholar]
- Dimitriadis G. J. Isolation and characterization of xenopus laevis albumin mRNA. Eur J Biochem. 1981 Aug;118(2):255–260. doi: 10.1111/j.1432-1033.1981.tb06394.x. [DOI] [PubMed] [Google Scholar]
- Dimitriadis G. J., Tata J. R. Subnuclear fractionation by mild micrococcal-nuclease treatment of nuclei of different transcriptional activities causes a partition of expressed and non-expressed genes. Biochem J. 1980 May 1;187(2):467–477. doi: 10.1042/bj1870467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer S. R., Henshaw E. C., Berridge M. V., Tata J. R. Translation of Xenopus vitellogenin mRNA during primary and secondary induction. Nature. 1978 Jun 1;273(5661):401–403. doi: 10.1038/273401a0. [DOI] [PubMed] [Google Scholar]
- Felber B. K., Maurhofer S., Jaggi R. B., Wyler T., Wahli W., Ryffel G. U., Weber R. Isolation and translation in vitro of four related vitellogenin mRNAs of estrogen-stimulated Xenopus laevis. Eur J Biochem. 1980 Mar;105(1):17–24. doi: 10.1111/j.1432-1033.1980.tb04469.x. [DOI] [PubMed] [Google Scholar]
- Felsenfeld G. Chromatin. Nature. 1978 Jan 12;271(5641):115–122. doi: 10.1038/271115a0. [DOI] [PubMed] [Google Scholar]
- Flint S. J., Weintraub H. M. An altered subunit configuration associated with the actively transcribed DNA of integrated adenovirus genes. Cell. 1977 Nov;12(3):783–794. doi: 10.1016/0092-8674(77)90277-x. [DOI] [PubMed] [Google Scholar]
- Garel A., Axel R. Selective digestion of transcriptionally active ovalbumin genes from oviduct nuclei. Proc Natl Acad Sci U S A. 1976 Nov;73(11):3966–3970. doi: 10.1073/pnas.73.11.3966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garel A., Zolan M., Axel R. Genes transcribed at diverse rates have a similar conformation in chromatin. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4867–4871. doi: 10.1073/pnas.74.11.4867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber-Huber S., Felber B. K., Weber R., Ryffel G. U. Estrogen induces tissue specific changes in the chromatin conformation of the vitellogenin genes in Xenopus. Nucleic Acids Res. 1981 Jun 11;9(11):2475–2494. doi: 10.1093/nar/9.11.2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon J. I., Burns A. T., Christmann J. L., Deeley R. G. Cloning of a double-stranded cDNA that codes for a portion of chicken preproalbumin. A general method for isolating a specific DNA sequence from partially purified mRNA. J Biol Chem. 1978 Dec 10;253(23):8629–8639. [PubMed] [Google Scholar]
- Gross-Bellard M., Oudet P., Chambon P. Isolation of high-molecular-weight DNA from mammalian cells. Eur J Biochem. 1973 Jul 2;36(1):32–38. doi: 10.1111/j.1432-1033.1973.tb02881.x. [DOI] [PubMed] [Google Scholar]
- Groudine M., Eisenman R., Weintraub H. Chromatin structure of endogenous retroviral genes and activation by an inhibitor of DNA methylation. Nature. 1981 Jul 23;292(5821):311–317. doi: 10.1038/292311a0. [DOI] [PubMed] [Google Scholar]
- Hayward M. A., Mitchell T. A., Shapiro D. J. Induction of estrogen receptor and reversal of the nuclear/cytoplasmic receptor ratio during vitellogenin synthesis and withdrawal in Xenopus laevis. J Biol Chem. 1980 Dec 10;255(23):11308–11312. [PubMed] [Google Scholar]
- McMaster G. K., Carmichael G. G. Analysis of single- and double-stranded nucleic acids on polyacrylamide and agarose gels by using glyoxal and acridine orange. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4835–4838. doi: 10.1073/pnas.74.11.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller D. M., Turner P., Nienhuis A. W., Axelrod D. E., Gopalakrishnan T. V. Active conformation of the globin genes in uninduced and induced mouse erythroleukemia cells. Cell. 1978 Jul;14(3):511–521. doi: 10.1016/0092-8674(78)90237-4. [DOI] [PubMed] [Google Scholar]
- Noll M., Kornberg R. D. Action of micrococcal nuclease on chromatin and the location of histone H1. J Mol Biol. 1977 Jan 25;109(3):393–404. doi: 10.1016/s0022-2836(77)80019-3. [DOI] [PubMed] [Google Scholar]
- O'Brien R. L., Parker J. W. Oxidation-induced lymphocyte transformation. Cell. 1976 Jan;7(1):13–20. doi: 10.1016/0092-8674(76)90250-6. [DOI] [PubMed] [Google Scholar]
- Palmiter R. D., Mulvihill E. R., McKnight G. S., Senear A. W. Regulation of gene expression in the chick oviduct by steroid hormones. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 2):639–647. doi: 10.1101/sqb.1978.042.01.066. [DOI] [PubMed] [Google Scholar]
- Panet A., Cedar H. Selective degradation of integrated murine leukemia proviral DNA by deoxyribonucleases. Cell. 1977 Aug;11(4):933–940. doi: 10.1016/0092-8674(77)90304-x. [DOI] [PubMed] [Google Scholar]
- Searle P. F., Tata J. R. Vitellogenin gene expression in male Xenopus hepatocytes during primary and secondary stimulation with estrogen in cell cultures. Cell. 1981 Mar;23(3):741–746. doi: 10.1016/0092-8674(81)90437-2. [DOI] [PubMed] [Google Scholar]
- Stalder J., Groudine M., Dodgson J. B., Engel J. D., Weintraub H. Hb switching in chickens. Cell. 1980 Apr;19(4):973–980. doi: 10.1016/0092-8674(80)90088-4. [DOI] [PubMed] [Google Scholar]
- Stalder J., Larsen A., Engel J. D., Dolan M., Groudine M., Weintraub H. Tissue-specific DNA cleavages in the globin chromatin domain introduced by DNAase I. Cell. 1980 Jun;20(2):451–460. doi: 10.1016/0092-8674(80)90631-5. [DOI] [PubMed] [Google Scholar]
- Tata J. R., Baker B. S., Deeley J. V. Vitellogenin as a multigene family. Not all Xenopus vitellogenin genes may be in an "expressible" configuration. J Biol Chem. 1980 Jul 25;255(14):6721–6726. [PubMed] [Google Scholar]
- Tata J. R., Smith D. F. Vitellogenesis: a versatile model for hormonal regulation of gene expression. Recent Prog Horm Res. 1979;35:47–95. doi: 10.1016/b978-0-12-571135-7.50006-0. [DOI] [PubMed] [Google Scholar]
- Wachsmuth E. D., Jost J. P. Localization of vitellogenin and serum albumin in hepatic parenchymal cells of normal and estradiol-treated immature chickens. Biochim Biophys Acta. 1976 Jul 21;437(2):454–461. doi: 10.1016/0304-4165(76)90014-3. [DOI] [PubMed] [Google Scholar]
- Wahli W., Dawid I. B., Ryffel G. U., Weber R. Vitellogenesis and the vitellogenin gene family. Science. 1981 Apr 17;212(4492):298–304. doi: 10.1126/science.7209528. [DOI] [PubMed] [Google Scholar]
- Wahli W., Dawid I. B., Wyler T., Jaggi R. B., Weber R., Ryffel G. U. Vitellogenin in Xenopus laevis is encoded in a small family of genes. Cell. 1979 Mar;16(3):535–549. doi: 10.1016/0092-8674(79)90028-x. [DOI] [PubMed] [Google Scholar]
- Wangh L. J., Osborne J. A., Hentschel C. C., Tilly R. Parenchymal cells purified from Xenopus liver and maintained in primary culture synthesize vitellogenin in response to estradiol-17 beta and serum albumin in response to dexamethasone. Dev Biol. 1979 Jun;70(2):479–499. doi: 10.1016/0012-1606(79)90040-x. [DOI] [PubMed] [Google Scholar]
- Weintraub H., Groudine M. Chromosomal subunits in active genes have an altered conformation. Science. 1976 Sep 3;193(4256):848–856. doi: 10.1126/science.948749. [DOI] [PubMed] [Google Scholar]
- Weintraub H., Larsen A., Groudine M. Alpha-Globin-gene switching during the development of chicken embryos: expression and chromosome structure. Cell. 1981 May;24(2):333–344. doi: 10.1016/0092-8674(81)90323-8. [DOI] [PubMed] [Google Scholar]
- Westley B., Knowland J. Estrogen causes a rapid, large and prolonged rise in the level of nuclear estrogen receptor in Xenopus laevis liver. Biochem Biophys Res Commun. 1979 Jun 13;88(3):1167–1172. doi: 10.1016/0006-291x(79)91531-6. [DOI] [PubMed] [Google Scholar]


