ABSTRACT
Peritoneal dialysis (PD), long established as the leading form of home dialysis, has comparatively good 5-year outcomes and cost-utility analyses have consistently demonstrated benefits to both patients and payers. Future improvements should still be sought, such as the further development of promising technologies designed to limit PD-associated harm, but given the physical and anatomical constraints of PD, these are unlikely to be transformational through the dialysis process itself. Rather, future focus should be on interventions that are effective across the whole dialysis population, such as mitigating the rate of loss in residual kidney function, pharmacological interventions for symptoms of kidney failure and suppressing inflammation. The greatest future challenge for the modality is inequity of access. In Europe, variation in PD uptake is >10-fold across the continent, with several contributing factors: differing economic drivers, variation in the empowerment of patients, physician attitudes and bias, small centre size, lack of experience, a nursing staff crisis, poor organizational culture and a lack of motivation and educational opportunities. It is time for a collective effort to address this and recently EuroPD convened a policy forum to initiate a multistakeholder approach to the problem, which extends to home haemodialysis. Use of PD worldwide is also highly variable, for some of the same reasons listed above, but with the additional challenges of the high cost of PD fluid and the lack of universal healthcare coverage. In the future, PD could and should play an important part in providing equitable access to dialysis worldwide, but to achieve this—and for the sake of the planet—point-of-care dialysis fluid generation would be transformative.
Keywords: cost-effectiveness, equity, inflammation, survival, technology
INTRODUCTION
It is sometimes said that to predict the future we have to learn from the past. Peritoneal dialysis (PD), at least its use for chronic kidney failure, is now well established, being approximately 48 years of age, and my personal experience with its use includes 40 of these years. During that time I have had more than one opportunity to consider how the treatment modality is progressing and it is informative to look back on these when considering future challenges and opportunities [1–3]. I will start this review with a brief summary of the current state of the therapy, describing the main advances that have been made before making the argument that PD has now more than ‘come of age’ and that the main challenges ahead are those related to equity of access, scaling up, addressing the green agenda and, perhaps most of all, challenging the profession to address issues of institutional culture and self-interest that are clearly barriers to its optimal use.
PD: THE STATE OF THE ART
For a good part of those 48 years there were genuine concerns that PD was associated with inferior survival. Some of these concerns were real [4, 5], while others were a product of poor-quality epidemiological studies [6], but what is now clear is that this is not now a concern. All of the major international registries tell the same story, that survival over time on both haemodialysis (HD) and PD has gradually improved, but the rate of improvement has been faster for PD such that outcomes at 5 years are now equivalent; if anything they are possibly a little better for PD early on, especially in those patients who are younger, less comorbid and likely to be transplanted [7–10]. Previous concerns related to specific patient groups using PD are no longer justified [11]. There are still concerns that the rate of transfer from PD to HD is unsatisfactorily high, with PD-related infection being the main cause [12], but there is now substantial evidence as to how this can be improved. Research from the Australia–New Zealand (ANZDATA) registry, Peritoneal Dialysis Outcomes and Practice Patterns Study (PDOPPS) and Standardizing Care to Improve Outcomes in Pediatric Endstage Kidney Disease Paediatric Dialysis Collaborative have led the way in showing us that this is primarily a dialysis-unit performance issue and collaborative quality improvement initiatives and ‘calls to action’ have clearly shown that this can be tackled successfully [13–17].
There has also been substantial progress in clarifying the role of PD in the overall trajectory of an individual's lifetime living with kidney failure. As already implied, PD has consistently been shown to be an excellent choice for individuals bridging to transplantation and, for many for whom a transplant is not feasible due to age and comorbidity, a suitable anticipated lifetime treatment choice [11]. For a relatively small group for whom transplantation would be the preferred option but for some reason is found not to be feasible, PD should not be considered a treatment for life [18]. This group runs a risk of burn-out or developing peritoneal membrane damage that leads to insufficient ultrafiltration, for which there is now a clearer causal classification [19], necessitating transfer to HD and of course the concerning potential of getting encapsulating peritoneal sclerosis [20]. A switch to home HD is a good option for this group and should be made available and encouraged [21].
Led by the International Society of Peritoneal Dialysis, the concept of goal-directed dialysis prescription has also been an important development [22, 23]. This approach recognises the very significant limitations of the unidimensional, one-size-fits-all approach of a small solute clearance threshold when prescribing dialysis. Indeed, it rejects the notion of a ‘dialysis adequacy’ solute clearance–based metric, taking a more holistic view of what dialysis is trying to achieve. This should include the goals of symptom management [24] (often poorly addressed by dialysis), well-being, maintenance of nutrition, fluid management and lifestyle. It pays considerable attention to the value and maintenance of residual kidney function, important to both PD and HD, opening up the possibility of various incremental approaches to dialysis initiation [25].
FUTURE CHALLENGES AND OPPORTUNITIES
Improving outcomes
There is plenty of evidence that people on dialysis do not necessarily consider how long they survive as their most important concern—they want to live well, and this is affected by many things, including treatment burden, symptom management, lifestyle preferences and physical and cognitive functioning, to name a few [26–28]. However, these are not mutually exclusive, and it can be argued that further improvements are not in the realm of how dialysis is currently practiced in many settings. Dialysis only does three things: removes (some) uraemic toxins, controls salt and water balance and assists with acid–base balance. Of course it could do these better, but the constraints of how dialysis is typically delivered, be it three weekly sessions for HD or 24 hours/day, 7 days/week for PD is limited by human physiology, anatomy and the laws of physics. The quest to increase these further is one of diminishing returns, potentially harmful and associated with ever-increasing treatment burden. For PD in particular, this does not seem the right way forward and interventions that look beyond the amount of dialysis delivery are more likely to lead to better outcomes, e.g. novel technologies that diminish treatment-associated harm, prolonging the preservation of residual kidney function, developing pharmacological interventions that address the dialysis symptom burden (e.g. cramps, restless legs) and, in particular, tackling kidney failure–associated inflammation.
Novel technologies designed to limit treatment-associated harm
This decade has seen a welcome renewed interest in the development of new technologies and novel dialysis fluids that are designed to reduce the adverse effects of PD. Broadly, these can be divided into two categories—those designed to enhance the early, point-of-care detection of peritonitis and those intended to reduce damage to the peritoneal membrane caused by excessive long-term exposure to glucose. Both these approaches, if successful, have the potential to extend the treatment period on PD by preventing transfer to HD.
Approaches to point-of-care testing for peritonitis include the early detection of infection-mediated release of cytokines such as matrix metalloproteinase-8 and interleukin-6 (IL-6) using the PERiPLEX system [29], the early detection of turbidity in the drained dialysate using the CloudCath system [30] and the use of the portable QuickCheck device [31] to make accurate measurements of the dialysate leukocyte count. All of these approaches have been evaluated in the clinical setting and found to be of clinical value, potentially identifying infection earlier [30] and benefitting patients through more responsive management of their infection [31]. It remains to be seen whether they will be widely adopted, but further development of this approach is likely and very promising.
Potential approaches to minimising the harmful effects of peritoneal glucose exposure include a device designed to deliver a constant infusion of glucose at a lower concentration, thus achieving more ultrafiltration for a given exposure to glucose [32], and alternatives to glucose such as xylitol [33] or the use of protective additives such as alanyl-glutamine [34] or carnitine [35]. Proving that these approaches protect the membrane from injury is very challenging, as long-term, very expensive trials will be needed. The observation that alanyl-glutamine was associated with less peritonitis is encouraging [34], but it is often easier to demonstrate the benefits of reduced glucose exposure by assessing the metabolic benefits [36] or simply demonstrating that it is safe and effective in delivery dialysis, as is planned in the ongoing ELIXIR study (NCT03994471).
Maximising preservation of residual kidney function
For a long time one of the cited benefits of starting renal replacement with PD has been the relative preservation of residual kidney function (RKF) [37]. This benefit has been attributed to less haemodynamic stress and is facilitated by the use of diuretics [38] and biocompatible dialysis fluids [39]. However, increasingly, most recently with the BISTRO trial in which efforts were made to avoid post-dialytic volume depletion, the difference in the rate of RKF decline has been less marked [40, 41]. Importantly it is the slower rate of decline in RKF that is associated with better survival in both dialysis modalities, and efforts to slow this further should be a focus our research efforts [42, 43]. This may be through the implementation of incremental dialysis (as already mentioned [25]) or extending the use of drugs recently demonstrated to slow the rate of kidney function decline, such as the sodium–glucose co-transporter 2 inhibitors, with their apparent diuretic effect [44], in the dialysis population.
Supressing inflammation
Inflammation is also a problem for people on dialysis, regardless of their modality of treatment. Our recent meta-analysis of 60 studies confirms this, showing a quantifiable relationship between circulating IL-6 levels and survival that remains after adjustment for the usual predictors such as age and comorbidity [45]. Supressing this inflammation may well have benefits beyond survival. The loss of muscle mass contributing to frailty, a particular problem in dialysis patients, where it is also associated with survival and overhydration, is driven, at least in part, by systemic inflammation [46]. Furthermore, in PD patients there is the additional problem of intraperitoneal inflammation, which is independent of systemic inflammation and is associated with membrane function, causing reduced ultrafiltration [47], increased peritoneal protein losses, hypoalbuminaemia and potentially progressive membrane injury [48, 49]. Trials involving IL-6 suppression are now under way and clearly offer the hope of improving a number of outcomes in the PD population.
Equity of access to PD
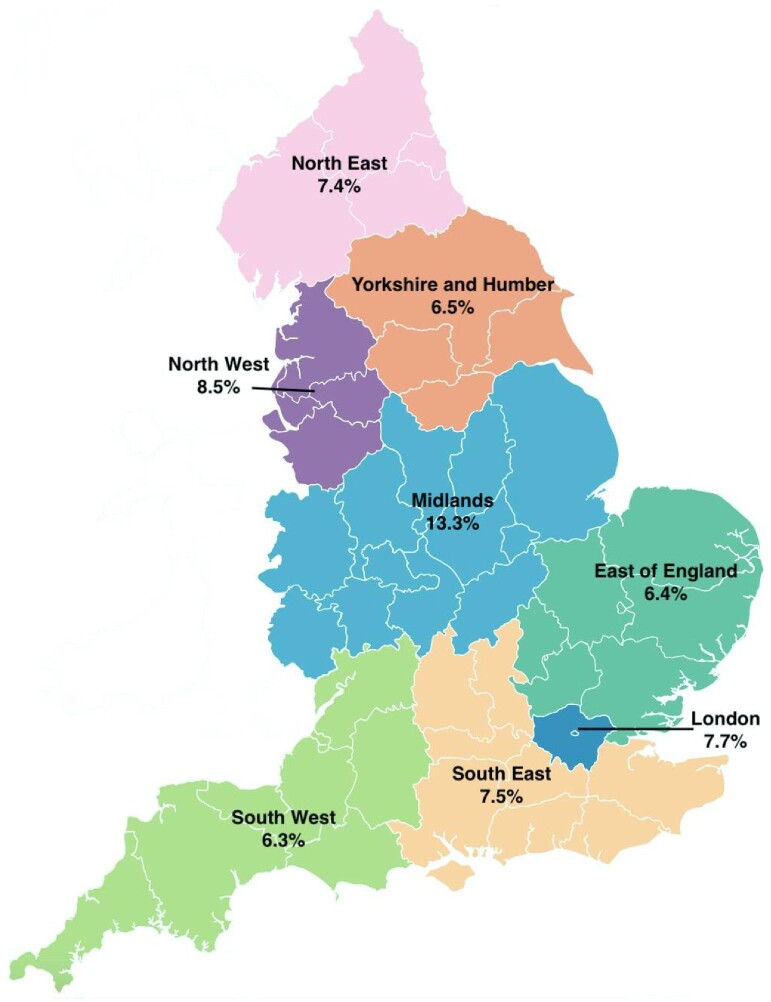
Despite the equivalence of outcomes, relative cost-effectiveness [50, 51] and lifestyle benefits to a significant proportion of people with kidney failure, there continues to be very significant inequity of access to PD at the national, European and global level. As elaborated previously [52], these barriers are at multiple levels, ranging from lack of patient empowerment to lack of universal healthcare coverage (see Table 1). Breaking down these barriers—and they all need to be tackled, as if any persist the problem remains—is the main challenge facing the future of PD. It is convenient to consider these at the country, European and global level, starting with my own country, England, which still demonstrates unwarranted variation in the use of home dialysis, despite universal healthcare coverage, treatment that is free at the point of care and medical and nursing professions whose salaries are not dependent on which treatment their patients prefer (see Figure 2).
Table 1:
Overview of the tiered barriers to preventing access to PD. To achieve equitable access it is necessary to tackle every level where a problem exists.
| Level | Nature of barrier | Potential solutions |
|---|---|---|
| High level (region/country/jurisdiction) | Lack of universal healthcare coverage for dialysis | Government to negotiate private–public partnerships that specify a proportion of PD use, or with PD manufacturers to develop PD-first or PD-preferred approaches. Avoid creating perception of PD as a second-class treatment |
| High cost of PD solutions | Within-country production Development of low-cost point-of-care dialysis fluid generation |
|
| Reimbursement models that favour private in-centre HD provision over community PD (or home HD) | Payers to negotiate reimbursement models that reflect the cost–utility benefits of PD | |
| No opportunity for the collective patient voice to be heard | Develop national patient organisations with the confidence and ability to influence development | |
| Lack of knowledge and education or lack of training opportunities | Implementation of national training curricula. Ensure that home dialysis is part of the training |
|
| Dialysis provider level | Does not provide PD | Centres must be held accountable professionally to ensure that, where feasible, all kidney replacement therapy modalities, including home dialysis and transplantation are available |
| PD program very small (<20 patients) or lack of staff | Develop networks with other local providers to develop critical mass, experience (follow the example of paediatric units) | |
| No structured pre-dialysis education program | Centres must provide this alongside provision of the modality | |
| No PD-responsive services | Non-surgical routes to PD catheter insertion, urgent start PD | |
| No assisted PD | Develop an assisted PD program | |
| Poor organisation culture that does not empower patients | Multiple: Support patient empowerment and presumption of eligibility if this is what the patient wants, address unconscious bias, use decision aids, provide peer support and engage in quality improvement | |
| Patient level | Out-of-pocket expenses | Patient/family contribution to their care should be equitable across the treatment modalities, preferably zero or adjusted to ability to pay |
| Ethnic minority or more socio-economically disadvantaged | The principle of equity should be applied—more disadvantages groups will require more support. These groups may need additional support to develop trust, e.g. through employment of ethnically diverse staff or collaboration with community groups |
Figure 2:
Regional variation in the proportion of people on home dialysis as a percentage of all treatments including transplantation (data from the UK Renal Registry 25th Annual Report).
Country-level inequity of access—the example of England
The use of PD to treat kidney failure in UK dialysis centres varies several-fold, which strongly suggests inequity of access [53]. Embedded within this, the chance of getting home dialysis is affected by ethnicity and socio-economic status, being less likely in ethnic minorities and those living in more socially deprived areas. This was the impetus for the Inter-CEPt study, funded by the National Institute of Health and Care Research (NIHR), which seeks to devise interventions to redress this imbalance [54]. The research included an ethnographic study of dialysis centres in which access to home dialysis was good, a national survey of home dialysis practice that was informed by ethnography and the linking of these survey findings to a patient-level analysis of UK Renal Registry data that interrogated how patient- and unit-level characteristics and practices associated with home dialysis use. The latter modelled causal relationships using a sequence of regression analyses and multistate modelling to underpin a patient-level cost–utility analysis.
The findings of the ethnographic and national surveys were complimentary [55]. They strongly suggested that it is not how services are organised that seem to matter, but rather it is the organisational culture that is important. This led to practices that empowered patients to consider home dialysis due to a presumption of their eligibility. Patients and their families likened choosing to have their dialysis at home as an ‘act of faith’, and their ability to place their trust in the PD team as key in them making this decision. Centres with strong home dialysis leadership that engaged in service development and quality improvement were much more likely to see their patients use home dialysis. Equally, lack of staff capacity, such that the centre could not engage in these activities, was associated with less PD use. In contrast to the relatively recent National Institute of Clinical Excellence guidance, the Inter-CEPt cost–utility analysis found that PD is more cost effective than in-centre HD (even if the costs were the same) and that it would be worth spending more resources to develop leadership roles and enable engagement in quality improvement. Inter-CEPt did not set out specific targets for home dialysis use, but rather aimed to identify ways to enable services to optimise its use in different contexts, recognising this may increase overall uptake. However, a goal for home dialysis use—at least 20% of prevalent dialysis patients in each unit—has been set by the national Renal Services Transformation Project. It will be interesting to see how effective this is in the coming few years.
Inequity of access to PD in Europe
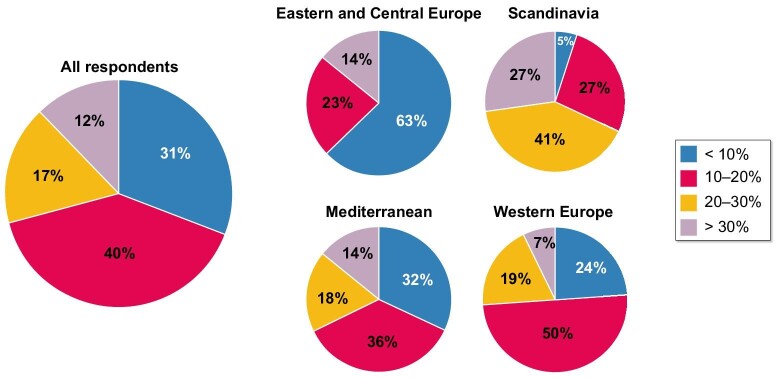
The use of home dialysis in Europe, which is dominated by PD, varies dramatically. By 3 months of kidney replacement treatment, 13% of patients are on PD, but in parts of Scandinavia this can average >30%, whereas in countries such as Belarus and the Czech Republic it is <3%, (Table 2). In 2019, at the Ljubljana meeting, EuroPD set up a group of future leaders in PD from across Europe who subsequently undertook a survey-based project to better understand the reasons for this large discrepancy in PD use. Most striking was the decreasing gradient across Europe in the proportion of prevalent patients on PD, from Scandinavia to the Mediterranean to western Europe to Eastern and Central Europe (Fig. 1). To tease out the barriers, 628 respondents from 288 centres were asked to focus on a clinical vignette of an unplanned urgent starter and to indicate the chance of their being commenced on PD [56]. The chances were greater if centres had 50–200 dialysis patients, if they were non-academic but not privately run, if catheters could be placed by a nephrologist, if assisted PD was available and if a high-quality, structured pre-dialysis education program was in place. Not surprisingly, if PD was less profitable than HD then it was less likely to be used. The data on assisted PD was especially informative, as having a program was five times more likely in Scandinavian and Western centres, twice as common in non-academic centres and almost three times as common in centres with a dedicated team delivering pre-dialysis education and was associated with double the incidence and prevalence of patients on home dialysis [57].
Table 2:
Percentage of dialysis patients starting on PD in a selection of European countries reporting aggregated or patient-level data (taken from the ERA Registry Report for 2021).
| Data type | Country | Percentage |
|---|---|---|
| Countries returning aggregated data | Albania | 2.6 |
| Belarus | 2.7 | |
| Croatia | 5.9 | |
| Czech Republic | 3.0 | |
| Italy (7 of 20 regions) | 15.6 | |
| Latvia | 21.3 | |
| Lithuania | 8.4 | |
| Poland | 5.4 | |
| Portugal | 10.2 | |
| Spain (country average) | 16.8 | |
| Turkey | 10.0 | |
| Ukraine | 6.0 | |
| Countries or regions returning individual-level data | Norway | 29.2 |
| Spain (Rioja) | 33.3 | |
| Spain (Extremadura) | 8.1 | |
| Romania | 1 | |
| Austria | 8.8 | |
| Denmark | 31.7 | |
| UK (England) | 22 | |
| Greece | 3.9 |
Figure 1:
Results of a survey of European centres (n = 288) showing the proportion of prevalent patients treated with PD by region across Europe [56, 57].
To follow this through, EuroPD held a policy forum to address the inequity of access to home dialysis in Europe during its last meeting in Brugge, Belgium in November 2024. This meeting deliberately involved all the key stakeholders, including the European Kidney Health Alliance and European Kidney Patients’ Federation, the International Society of Peritoneal Dialysis, the European Dialysis and Transplantation Nurses Association and industry leaders Baxter Healthcare and Fresenius Medical Care. Engaging with the European Renal Association was less easy. In depth discussions were held covering three main topic areas: dialysis provider motivation, empowering the patient and education of professionals and workforce capacity. Proposals to tackle the issues raised will be published in a forthcoming position paper, but to summarise briefly here, these will build on a number of key points. These include the economic drivers of home dialysis use (or lack of), emphasising that for all stakeholders, home dialysis must not result in financial disadvantage, but at the same time recognising that this is a necessary but not sufficient condition. It is clear that there are professional issues related to a lack of inertia and a failure to deliver patient-centred modality choice that border on the unethical. Patients and their families should be empowered to get the care they want both individually and collectively, recognising that they are the best advocates for home dialysis. The workforce crisis in Europe, especially in nursing, often preferentially depletes staffing in home therapies teams while paradoxically it is a potential solution to the problem. The training of doctors in home therapies in parts of Europe is little short of a disgrace.
Global inequity of access to PD
The barriers to being treated with PD worldwide undoubtedly include all of the above, but there are additional problems that lead to a complex picture. Globally there is a serious lack of dialysis provision, especially in low- and low- and middle-income countries (L/LMICs) [58, 59], and on the face of it, PD should be a very attractive option in these parts of the world. It does away with the construction of expensive infrastructure, does not require access to large volumes of high-quality water and should be especially attractive in rural communities that contribute larger proportions of the population in L/LMICs. For a country wishing to develop a universal healthcare system that supports dialysis treatment (and there is no country in the world that makes dialysis available to all its citizens without a universal coverage scheme), then PD, provided it can be delivered less expensively than HD, is an attractive option [60]. This has led to the development of both ‘PD-first’ and ‘PD-preferred’ options [61], of which there are notable successes, especially the Hong Kong model [62]. Key to the development of these approaches is strong government engagement and a willingness to evolve over time. Thailand developed such a model when extending its healthcare coverage for dialysis from those with civil service insurance to the whole population, and this led to a remarkable increase in the availability of dialysis that was developed by healthcare professionals in a very impressive way [12, 63–65]. Subsequently there has been some dismantling of this model, in part because by definition it is choice restricted, but also because of perceptions that PD, which was being used in the poorer sections of society, is a second-class treatment. This is reflected in higher mortality rates, which can be attributed to socio-economic factors, but points to the main limitation in employing PD-first or PD-preferred policies—they need to apply to the whole population rather than creating a two-tiered system based on wealth or privilege [66].
The other very significant barrier to growth of PD in L/LMICs is the high cost of PD fluid, and when combined with the relatively low cost of labour, this makes the treatment more expensive overall in many countries [67, 68]. In many parts of Africa and Latin America, PD is simply not competitive, and where universal healthcare coverage is not available it is far more profitable for private nephrologists to set up HD units in which dialysis quality may be poorly regulated and there is a risk of catastrophic healthcare expenditures by patients and their families [69]. PD fluid is expensive if it is manufactured in a high-income environment and then transferred for use in low-income settings. There is also the cost of transport, added to which are the official and unofficial taxes incurred when crossing country borders, which may be multiple. While this is currently an issue for L/LMICs, it is important to recognize that this is a truly global problem for PD in the future with respect to climate change. Dialysis is one of the worst long-term treatments with respect to global warming. Recent data from high-income settings suggest that overall PD is less bad for the planet than HD, with typical estimates from Australia ranging from 1500 to 2700 kg of yearly carbon dioxide equivalent emissions (CO2e) for continuous ambulatory PD and 2350 to 4500 kg CO2e for automated PD [70], compared with estimates for HD ranging from 3800 to 10 200 kg CO2e [71–73]. Nevertheless, it makes no sense to transport large quantities of bags of salty water around the planet when in principle, at least, the overall use of water by PD should be considerably less than for HD [74].
Is local PD solution production the solution?
It can be argued that this is the greatest technological challenge facing the future of PD and the one most likely to transform the therapy. In addition to addressing the economic and environmental costs of manufacturing and transporting large quantities of salty water, there would be benefits to patients in terms of dialysis fluid storage, the biocompatibility of dialysis solutions (no formation of glucose degradation products) and the potential to individualise dialysis fluid composition (e.g. sodium and glucose levels). However, the challenges involved are not trivial—mainly because of the need to produce fluid that is both sterile and free of endotoxin. The additional challenge worldwide is that the device producing the PD fluid at the point of care needs to be low cost and robust. In 2016, the winner of the Affordable Dialysis Prize, presented by the International Society of Nephrology and the George Institute for Global Health, was announced. Won by Vincent Garvey, he proposed a portable, lightweight distiller producing medical-quality distilled water that could be reconstituted with salts. A device that demonstrates proof of principle has been developed and used by patients in their homes, but there is still some way to go in progressing this to being clinically available [75]. Existing dialysis companies have indicated that they are working on point-of-care dialysis fluid production and, recently, early promising results from the Baxter APD Solution Generation System have been published [76].
CONCLUSIONS
PD is established as the leading home treatment for kidney failure, and when considering a 5-year time horizon, patient outcomes and cost–utility analyses compare favourably with centre-based HD in high-income settings. Further improvements in transfer rates to HD are needed, but the requisites for achieving future improvements through quality improvement initiatives are clear. There are a number of promising new technologies and dialysis solutions aimed at reducing the harmful effects of PD associated with peritonitis and exposure of the peritoneal membrane to glucose. Further improvements in survival in the future can be envisaged, but large effects are likely to be across the dialysis population as a whole, such as slowing the loss of residual kidney function or supressing inflammation, rather than being PD specific. Further maximising small solute clearance, which has dominated dialysis for too long, is unlikely to have a transformational effect and is limited by physical constraints. The main challenge facing PD use is the inequity of access, which is observed at every level—dialysis centre, country, continent and worldwide. Europe demonstrates this inequity particularly starkly and there is a need for country and European professional organisations to take this seriously. Ultimately this is about empowering people living with kidney failure. All dialysis modalities represent a threat to the environment, which presents technical challenges for the industry, but a focus on developing point-of-care dialysis fluid production should be a priority.
ACKNOWLEDGEMENTS
I am grateful to my colleagues in EuroPD and for the many who contributed to the policy forum addressing inequity of access to home dialysis held at the conference in Brugge, Belgium in November 2024. The Inter-CEPt study is funded by the NIHR Health Services and Delivery Research Programme (NIHR award identifier 128364). The views expressed are those of the author and not necessarily those of the NIHR or the Department of Health and Social Care.
FUNDING
This paper is part of a Supplement that was financially supported by the ERA. The topic of this paper was also presented at the 61st ERA Congress, Stockholm & Virtual, May 23-26, 2024.
AUTHOR'S CONTRIBUTIONS
Simon Davies is the sole author and the article is based on the invited lecture at the ERA meeting held in Stockholm, 2024.
DATA AVAILABILITY STATEMENT
No new data were generated or analysed in support of this research. The data for Figure 2 was derived from the UK Renal Registry 25th Annual Report, Available from https://ukkidney.org/audit-research/annual-report
CONFLICT OF INTEREST STATEMENT
The author has in the past received research funding and lecture fees from Baxter HealthCare and Fresenius Medical Care. He was previously on the advisory board for Ellen Medical. He is lead UK investigator for the POSIBIL-6 ESKD Study, CSL300,2301
REFERENCES
- 1. Davies SJ. Peritoneal dialysis—current status and future challenges. Nat Rev Nephrol 2013;9:399–408. 10.1038/nrneph.2013.100 [DOI] [PubMed] [Google Scholar]
- 2. Mehrotra R, Devuyst O, Davies SJ et al. The current state of peritoneal dialysis. J Am Soc Nephrol 2016;27:3238–52. http://www.ncbi.nlm.nih.gov/pubmed/27339663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Davies S. Individual, providers, countries and continents: the challenge ahead for peritoneal dialysis. 17th Congress of the International Society for Peritoneal Dialysis: Dimitrios G. Oreopoulos Lecture. 2018. https://ispd.org/wp-content/uploads/3.-Simon-Davies-Oreopoulos-Lecture-ISPD2018-Individuals-providers-countries-and-continents-the-challenges-ahead.pdf
- 4. Vonesh EF, Snyder JJ, Foley RN et al. The differential impact of risk factors on mortality in hemodialysis and peritoneal dialysis. Kidney Int 2004;66:2389–401. 10.1111/j.1523-1755.2004.66028.x [DOI] [PubMed] [Google Scholar]
- 5. Stack AG, Murthy BVR, Molony DA. Survival differences between peritoneal dialysis and hemodialysis among “large” ESRD patients in the United States. Kidney Int 2004;65:2398–408. 10.1111/j.1523-1755.2004.00654.x [DOI] [PubMed] [Google Scholar]
- 6. Bloembergen WE, Port FK, Mauger EA et al. A comparison of mortality between patients treated with hemodialysis and peritoneal dialysis. J Am Soc Nephrol 1995;6:177–83. https://journals.lww.com/jasn/fulltext/1995/08000/a_comparison_of_mortality_between_patients_treated.6.aspx [DOI] [PubMed] [Google Scholar]
- 7. van de Luijtgaarden MWM, Jager KJ, Segelmark M et al. Trends in dialysis modality choice and related patient survival in the ERA-EDTA Registry over a 20-year period. Nephrol Dial Transplant 2016;31:120–8. 10.1093/ndt/gfv295 [DOI] [PubMed] [Google Scholar]
- 8. Marshall MR, Polkinghorne KR, Boudville N et al. Home versus facility dialysis and mortality in Australia and New Zealand. Am J Kidney Dis 2021;78:826–36.e1. 10.1053/j.ajkd.2021.03.018 [DOI] [PubMed] [Google Scholar]
- 9. United States Renal Data System . 2020 Annual Data Report. https://usrds-adr.niddk.nih.gov/2020
- 10. Wang V, Coffman CJ, Sanders LL et al. Comparing mortality of peritoneal and hemodialysis patients in an era of Medicare payment reform. Med Care 2021;59:155–62. 10.1097/MLR.0000000000001457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lambie M, Davies S. An update on absolute and relative indications for dialysis treatment modalities. Clin Kidney J 2023;16:i39–47. 10.1093/ckj/sfad062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lambie M, Zhao J, McCullough K et al. Variation in peritoneal dialysis time on therapy by country results from the Peritoneal Dialysis Outcomes and Practice Patterns Study. Clin J Am Soc Nephrol 2022;17:861–71. 10.2215/CJN.16341221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cho Y, Htay H, Johnson DW. Centre effects and peritoneal dialysis-related peritonitis. Nephrol Dial Transplant 2017;32:913–5. https://academic.oup.com/ndt/article-lookup/doi/10.1093/ndt/gfx054 [DOI] [PubMed] [Google Scholar]
- 14. Htay H, Cho Y, Pascoe EM et al. Multicenter registry analysis of center characteristics associated with technique failure in patients on incident peritoneal dialysis. Clin J Am Soc Nephrol 2017;12:1090–9. http://cjasn.asnjournals.org/lookup/doi/10.2215/CJN.12321216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Al Sahlawi M, Zhao J, McCullough K et al. Variation in peritoneal dialysis-related peritonitis outcomes in the Peritoneal Dialysis Outcomes and Practice Patterns Study (PDOPPS). Am J Kidney Dis 2022;79:45–55.e1. 10.1053/j.ajkd.2021.03.022 [DOI] [PubMed] [Google Scholar]
- 16. Jose MD, Johnson DW, Mudge DW et al. Peritoneal dialysis practice in Australia and New Zealand: a call to action. Nephrology 2011;16:19–29. http://www.ncbi.nlm.nih.gov/pubmed/21175973 [DOI] [PubMed] [Google Scholar]
- 17. Neu AM, Richardson T, De Souza HG et al. Continued reduction in peritonitis rates in pediatric dialysis centers: results of the Standardizing Care to Improve Outcomes in Pediatric End Stage Renal Disease (SCOPE) Collaborative. Pediatr Nephrol 2021;36:2383–91. 10.1007/s00467-021-04924-0 [DOI] [PubMed] [Google Scholar]
- 18. Brown EA, Bargman J, van Biesen W et al. Length of time on peritoneal dialysis and encapsulating peritoneal sclerosis—position paper for ISPD: 2017 update. Perit Dial Int 2017;37:362–74. http://www.pdiconnect.com/lookup/doi/10.3747/pdi.2017.00018 [DOI] [PubMed] [Google Scholar]
- 19. Morelle J, Stachowska-Pietka J, Öberg C et al. ISPD recommendations for the evaluation of peritoneal membrane dysfunction in adults: classification, measurement, interpretation and rationale for intervention. Perit Dial Int 2021;41:352–72. 10.1177/0896860820982218 [DOI] [PubMed] [Google Scholar]
- 20. Lambie M, Teece L, Johnson DW et al. Estimating risk of encapsulating peritoneal sclerosis accounting for the competing risk of death. Nephrol Dial Transplant 2019;34:1585–91. 10.1093/ndt/gfz034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nadeau-Fredette A-C, Chan CT, Cho Y et al. Outcomes of integrated home dialysis care: a multi-centre, multi-national registry study. Nephrol Dial Transplant 2015;30:1897–904. http://www.ncbi.nlm.nih.gov/pubmed/26044832 [DOI] [PubMed] [Google Scholar]
- 22. Chan CT, Blankestijn PJ, Dember LM et al. Dialysis initiation, modality choice, access, and prescription: conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int 2019;96:37–47. 10.1016/j.kint.2019.01.017 [DOI] [PubMed] [Google Scholar]
- 23. Brown EA, Blake PG, Boudville N et al. International Society for Peritoneal Dialysis practice recommendations: prescribing high-quality goal-directed peritoneal dialysis. Perit Dial Int 2020;40:244–53. 10.1177/0896860819895364 [DOI] [PubMed] [Google Scholar]
- 24. Mehrotra R, Davison SN, Farrington K et al. Managing the symptom burden associated with maintenance dialysis: conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int 2023;104:441–54. 10.1016/j.kint.2023.05.019 [DOI] [PubMed] [Google Scholar]
- 25. Blake PG, Dong J, Davies SJ. Incremental peritoneal dialysis. Perit Dial Int 2020;40:320–6. 10.1177/0896860819895362 [DOI] [PubMed] [Google Scholar]
- 26. Manera KE, Johnson DW, Craig JC et al. Establishing a core outcome set for peritoneal dialysis: report of the SONG-PD (Standardized Outcomes in Nephrology–Peritoneal Dialysis) Consensus Workshop. Am J Kidney Dis 2020;75:404–12. https://www.sciencedirect.com/science/article/pii/S0272638619311163 [DOI] [PubMed] [Google Scholar]
- 27. Lawrence C, Sharma S, Da Silva-Gane M et al. Exploring the views of patients not on the transplant waiting list: a qualitative study. J Ren Care 2013;39:118–24. http://www.ncbi.nlm.nih.gov/pubmed/23683305 [DOI] [PubMed] [Google Scholar]
- 28. Fletcher BR, Damery S, Aiyegbusi OL et al. Symptom burden and health-related quality of life in chronic kidney disease: a global systematic review and meta-analysis. PLoS Med 2022;19:e1003954. 10.1371/journal.pmed.1003954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Goodlad C, George S, Sandoval S et al. Measurement of innate immune response biomarkers in peritoneal dialysis effluent using a rapid diagnostic point-of-care device as a diagnostic indicator of peritonitis. Kidney Int 2020;97:1253–9. 10.1016/j.kint.2020.01.044 [DOI] [PubMed] [Google Scholar]
- 30. Mehrotra R, Williamson DE, Betts CR et al. A Prospective Clinical Study to EvaluAte the AbiliTy of the CloudCath System to Detect Peritonitis During In-Home Peritoneal Dialysis (CATCH). Kidney Int Rep 2024;9:929–40. 10.1016/j.ekir.2024.01.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Govindji-Bhatt N, Kennedy SM, Barker MG et al. Novel colorimetric and light scatter methods to identify and manage peritoneal dialysis-associated peritonitis at the point-of-care. Kidney Int Rep 2024;9:589–600. 10.1016/j.ekir.2023.12.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Heimbürger O, Hegbrant J, Martus G et al. Effects of steady concentration peritoneal dialysis on ultrafiltration volume and sodium removal: a pilot crossover trial. Clin J Am Soc Nephrol 2023;19:224–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Masola V, Bonomini M, Onisto M et al. Biological effects of xylocore, a glucose sparing pd solution, on mesothelial cells: focus on mesothelial-mesenchymal transition, inflammation and angiogenesis. Nutrients 2021;13:2282. 10.3390/nu13072282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Vychytil A, Herzog R, Probst P et al. A randomized controlled trial of alanyl-glutamine supplementation in peritoneal dialysis fluid to assess impact on biomarkers of peritoneal health. Kidney Int 2018;94:1227–37. 10.1016/j.kint.2018.08.031 [DOI] [PubMed] [Google Scholar]
- 35. Bonomini M, Pandolfi A, Di Liberato L et al. L-carnitine is an osmotic agent suitable for peritoneal dialysis. Kidney Int 2011;80:645–54. http://www.ncbi.nlm.nih.gov/pubmed/21525850 [DOI] [PubMed] [Google Scholar]
- 36. Lambie M, Bonomini M, Davies SJ et al. Insulin resistance in cardiovascular disease, uremia, and peritoneal dialysis. Trends Endocrinol Metab 2021;32:721–30. 10.1016/j.tem.2021.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jansen MAM, Hart AAM, Korevaar JC et al. Predictors of the rate of decline of residual renal function in incident dialysis patients. Kidney Int 2002;62:1046–53. http://www.ncbi.nlm.nih.gov/pubmed/12164889 [DOI] [PubMed] [Google Scholar]
- 38. Medcalf JF, Harris KP, Walls J. Role of diuretics in the preservation of residual renal function in patients on continuous ambulatory peritoneal dialysis. Kidney Int 2001;59:1128–33. http://www.ncbi.nlm.nih.gov/pubmed/11231370 [DOI] [PubMed] [Google Scholar]
- 39. Htay H, Johnson DW, Wiggins KJ et al. Biocompatible dialysis fluids for peritoneal dialysis. Cochrane Database Sys Rev 2018;10:CD007554. http://www.ncbi.nlm.nih.gov/pubmed/30362116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Davies SJ, Coyle D, Lindley EJ et al. Bio-impedance spectroscopy added to a fluid management protocol does not improve preservation of residual kidney function in incident hemodialysis patients in a randomized controlled trial. Kidney Int 2023;104:587–98. 10.1016/j.kint.2023.05.016 [DOI] [PubMed] [Google Scholar]
- 41. Johnson DW, Brown FG, Clarke M et al. Effects of biocompatible versus standard fluid on peritoneal dialysis outcomes. J Am Soc Nephrol 2012;23:1097–107. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3358767&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bargman JM, Thorpe KE, Churchill DN. Relative contribution of residual renal function and peritoneal clearance to adequacy of dialysis: a reanalysis of the CANUSA study. J Am Soc Nephrol 2001;12:2158–62. http://www.ncbi.nlm.nih.gov/pubmed/11562415 [DOI] [PubMed] [Google Scholar]
- 43. Obi Y, Rhee CM, Mathew AT et al. Residual kidney function decline and mortality in incident hemodialysis patients. J Am Soc Nephrol 2016;27:3758–68. http://www.jasn.org/cgi/doi/10.1681/ASN.2015101142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mayne KJ, Staplin N, Keane DF et al. Effects of empagliflozin on fluid overload, weight and blood pressure in chronic kidney disease. J Am Soc Nephrol 2024;35:202–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Istanbuly O, Belcher J, Tabinor M et al. Estimating the association between systemic Interleukin-6 and mortality in the dialysis population. Re-analysis of the global fluid study, systematic review and meta-analysis. BMC Nephrol 2023;24:312. 10.1186/s12882-023-03370-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Cesari M, Leeuwenburgh C, Lauretani F et al. Frailty syndrome and skeletal muscle: results from the Invecchiare in Chianti study. Am J Clin Nutr 2006;83:1142–8. https://pubmed.ncbi.nlm.nih.gov/16685058/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lambie M, Chess J, Donovan KL et al. Independent effects of systemic and peritoneal inflammation on peritoneal dialysis survival. J Am Soc Nephrol 2013;24:2071–80. https://journals.lww.com/jasn/fulltext/2013/12000/independent_effects_of_systemic_and_peritoneal.19.aspx [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yu Z, Lambie M, Chess J et al. Peritoneal protein clearance is a function of local inflammation and membrane area whereas systemic inflammation and comorbidity predict survival of incident peritoneal dialysis patients. Front Physiol 2019;10:105. 10.3389/fphys.2019.00105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Yu Z, Lambie M, Davies SJ. Longitudinal study of small solute transport and peritoneal protein clearance in peritoneal dialysis patients. Clin J Am Soc Nephrol 2014;9:326–34. http://www.ncbi.nlm.nih.gov/pubmed/24262505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Treharne C, Liu FX, Arici M et al. Peritoneal dialysis and in-centre haemodialysis: a cost-utility analysis from a UK payer perspective. Appl Health Econ Health Policy 2014;12:409–20. http://link.springer.com/10.1007/s40258-014-0108-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Busink E, Kendzia D, Kircelli F et al. A systematic review of the cost-effectiveness of renal replacement therapies, and consequences for decision-making in the end-stage renal disease treatment pathway. Eur J Health Econ 2023;24:377–92. 10.1007/s10198-022-01478-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Perl J, Brown EA, Chan CT et al. Home dialysis: conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int 2023;103:842–58. [DOI] [PubMed] [Google Scholar]
- 53. Tabinor M, Casula A, Wilkie M et al. UK Renal Registry 19th Annual Report: Chapter 13 Home therapies in 2015: national and centre-specific analyses. Nephron 2017;137(Suppl 1):297–325. [DOI] [PubMed] [Google Scholar]
- 54. Tshimologo M, Allen K, Coyle D et al. Intervening to eliminate the centre-effect variation in home dialysis use: protocol for Inter-CEPt-a sequential mixed-methods study designing an intervention bundle. BMJ Open 2022;12:e060922. 10.1136/bmjopen-2022-060922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Damery S, Lambie M, Williams I et al. Centre variation in home dialysis uptake: a survey of kidney centre practice in relation to home dialysis organisation and delivery in England. Perit Dial Int 2024;44:265–74. 10.1177/08968608241232200 [DOI] [PubMed] [Google Scholar]
- 56. Hahn Lundström U, Abrahams AC, Allen J et al. Barriers and opportunities to increase PD incidence and prevalence: lessons from a European survey. Perit Dial Int 2021;41:542–51. 10.1177/08968608211034988 [DOI] [PubMed] [Google Scholar]
- 57. van Eck van der Sluijs A, van Jaarsveld BC, Allen J et al. Assisted peritoneal dialysis across Europe: practice variation and factors associated with availability. Perit Dial Int 2021;41:533–41. 10.1177/08968608211049882 [DOI] [PubMed] [Google Scholar]
- 58. Liyanage T, Ninomiya T, Jha V et al. Worldwide access to treatment for end-stage kidney disease: a systematic review. Lancet 2015;385:1975–82. 10.1016/S0140-6736(14)61601-9 [DOI] [PubMed] [Google Scholar]
- 59. Luyckx VA, Al-Aly Z, Bello AK et al. Sustainable Development Goals relevant to kidney health: an update on progress. Nat Rev Nephrol 2021;17:15–32. 10.1038/s41581-020-00363-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Harris DCH, Davies SJ, Finkelstein FO et al. Increasing access to integrated ESKD care as part of universal health coverage. Kidney Int 2019;95(4S):S1–33. http://www.ncbi.nlm.nih.gov/pubmed/30904051 [DOI] [PubMed] [Google Scholar]
- 61. Liu FX, Gao X, Inglese G et al. A global overview of the impact of peritoneal dialysis first or favored policies: an opinion. Perit Dial Int 2015;35:406–20. 10.3747/pdi.2013.00204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Li PKT, Lu W, Mak SK et al. Peritoneal dialysis first policy in Hong Kong for 35 years: global impact. Nephrology 2022;27:787–94. 10.1111/nep.14042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Teerawattananon Y, Mugford M, Tangcharoensathien V. Economic evaluation of palliative management versus peritoneal dialysis and hemodialysis for end-stage renal disease: evidence for coverage decisions in Thailand. Value Health 2007;10:61–72. [DOI] [PubMed] [Google Scholar]
- 64. Assanatham M, Pattanaprateep O, Chuasuwan A et al. Economic evaluation of peritoneal dialysis and hemodialysis in Thai population with end-stage kidney disease. BMC Health Serv Res 2022;22:1384. 10.1186/s12913-022-08827-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Kanjanabuch T, Takkavatakarn K. Global dialysis perspective: Thailand. Kidney360 2020;1:671–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Changsirikulchai S, Sriprach S, Thokanit NS et al. Survival analysis and associated factors in Thai patients on peritoneal dialysis under the PD-First policy. Perit Dial Int 2018;38:172–8. http://www.ncbi.nlm.nih.gov/pubmed/29437140 [DOI] [PubMed] [Google Scholar]
- 67. Caskey FJ, Kramer A, Elliott RF et al. Global variation in renal replacement therapy for end-stage renal disease. Nephrol Dial Transplant 2011;26:2604–10. http://www.ncbi.nlm.nih.gov/pubmed/21245131 [DOI] [PubMed] [Google Scholar]
- 68. Klarenbach SW, Tonelli M, Chui B et al. Economic evaluation of dialysis therapies. Nat Rev Nephrol 2014;10:644–52. 10.1038/nrneph.2014.145 [DOI] [PubMed] [Google Scholar]
- 69. Jha V, Martin DE, Bargman JM et al. Ethical issues in dialysis therapy. Lancet 2017;389:1851–6. 10.1016/S0140-6736(16)32408-4 [DOI] [PubMed] [Google Scholar]
- 70. McAlister S, Talbot B, Knight J et al. The carbon footprint of peritoneal dialysis in Australia. J Am Soc Nephrol 2024;35:1095–103. 10.1681/ASN.0000000000000361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Connor A, Lillywhite R, Cooke MW. The carbon footprints of home and in-center maintenance hemodialysis in the United Kingdom. Hemodial Int 2011;15:39–51. 10.1111/j.1542-4758.2010.00523.x [DOI] [PubMed] [Google Scholar]
- 72. Lim AEK, Perkins A, Agar JWM. The carbon footprint of an Australian satellite haemodialysis unit. Aus. Health Rev 2013;37:369–74. 10.1071/AH13022 [DOI] [PubMed] [Google Scholar]
- 73. Sehgal AR, Slutzman JE, Huml AM. Sources of variation in the carbon footprint of hemodialysis treatment. J Am Soc Nephrol 2022;33:1790–5. 10.1681/ASN.2022010086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Luyckx VA, Alasfar S, Bajpai D et al. Providing environmentally sustainable nephrology care: focus in low- and middle-income countries. Kidney Int 2024;105:259–68. 10.1016/j.kint.2023.09.034 [DOI] [PubMed] [Google Scholar]
- 75. Talbot B, Davies S, Burman J et al. The Point-of-Care Peritoneal Dialysis System Early Evaluation Study (POC-PDEE): a pilot proof-of-principal study of the Ellen Medical Devices Point-of-Care affordable peritoneal dialysis system. Perit Dial Int 2024:8968608231209850. 10.1177/08968608231209850 [DOI] [PubMed] [Google Scholar]
- 76. Sharma S, Shamy OE, Wilmington A et al. Performance evaluation of an automated peritoneal dialysis solution generation system in patients using automated peritoneal dialysis. Kidney Int Rep 2024;9:1752–7. 10.1016/j.ekir.2024.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were generated or analysed in support of this research. The data for Figure 2 was derived from the UK Renal Registry 25th Annual Report, Available from https://ukkidney.org/audit-research/annual-report