Abstract
What is already known about this topic?
Severe fever with thrombocytopenia syndrome (SFTS) is a serious tick-borne disease in East Asia with high mortality, particularly affecting the elderly. Since its discovery in 2010, inconsistencies in small-scale studies and the lack of decade-long research on antibody levels in large population samples after natural infection, along with the absence of an effective vaccine, highlight the need for large-scale, long-term data in high-incidence regions of China.
What is added by this report?
This study of 1,410 serum samples from SFTS patients in high-incidence regions of China reveals that immunoglobulin M (IgM) levels peak at 8–14 days post-infection, declining to nearly undetectable levels by 180 days. Immunoglobulin G (IgG) and neutralizing antibodies (NAb) levels peak at 22–180 days, persisting up to 10 years. IgM levels correlate with viral load and various immune and coagulation parameters, with lower levels observed in fatal cases. During convalescence, elderly patients have lower IgG levels, whereas females exhibit higher IgG levels compared with males.
What are the implications for public health practice?
The study’s findings on long-term antibody dynamics in SFTS patients can significantly improve vaccine development, optimize therapy scheduling, inform public health policies, and enhance diagnostic tools, leading to better disease management and prevention in high-incidence areas.
Keywords: Severe fever with thrombocytopenia syndrome, Antibody dynamics, Longitudinal study, High-incidence regions
Severe fever with thrombocytopenia syndrome (SFTS) is a serious tick-borne disease in East Asia with high mortality, particularly among the elderly. Since its discovery in 2010, research on antibody levels in large populations following natural infection has been limited, and no effective vaccine is available, highlighting the need for comprehensive long-term data in high-incidence regions of China ( 1– 3). To address this public health challenge, we conducted a retrospective study on the dynamics of nucleoprotein (NP)-specific immunoglobulin M (IgM), immunoglobulin G (IgG), and neutralizing antibodies (NAb) using 1,410 serum samples from SFTS patients in high-incidence areas across Shandong, Anhui, Hubei, Liaoning, Henan, and Jiangsu provinces from 2010 to 2023. Our findings reveal that IgM levels peak at 8–14 days post-infection and decline to nearly undetectable levels by 180 days. In contrast, IgG and NAb levels peak at 22–180 days and persist for up to 10 years. Public health practitioners should prioritize long-term surveillance and vaccine research to combat SFTS effectively.
The study enrolled confirmed SFTS cases with laboratory evidence, including severe fever with thrombocytopenia syndrome virus (SFTSV) nucleic acid detection or a fourfold increase in IgG antibody titers during recovery ( 2). Participants aged 5 to 90 years were included, and general epidemiological data were collected, categorizing them by age (under 50, 50–70, over 70) and disease onset time (≤7 days, 8–14 days, 15–21 days, 22–180 days, 181–365 days, 1–2 years, 2–3 years, 3–4 years, 4–5 years, 5–6 years, 6–7 years, 7–8 years, 8–9 years, 9–10 years). Disease phases were classified as acute (within 2 weeks) and recovery (after 2 weeks). Serum samples were subjected to quantitative reverse transcription polymerase chain reaction (qRT-PCR) targeting the S segment of SFTSV for nucleic acid detection and viral load quantification, IgM-capture enzyme-linked immunosorbent assay (ELISA) for NP-specific IgM, indirect ELISA for NP-specific IgG, and microneutralization assays to assess NAb titers. Case questionnaires were used to collect data on clinical parameters, prognosis, clinical outcomes, and disease severity. Statistical analyses were performed using GraphPad Prism (version 10.1.2; La Jolla, CA). The Mann-Whitney U-test was used for group differences, the Wilcoxon signed-rank test for paired serum samples, the Kruskal-Wallis test for categorical variables, and Spearman’s rank correlation for correlation analysis. Significance levels were defined as follows: ns (not significant), P<0.05, P<0.01, P<0.001, P<0.0001. This study was approved by the ethics committee of the National Institute for Viral Disease Control and Prevention, China CDC (IVDC2022-011).
In high-incidence areas of China, 1,410 serum samples from 1,233 SFTSV-infected patients were collected, primarily between April and October, peaking in July with 541 samples (38.37%). Of these, 64.47% (909/1,410) were from the acute phase, and 35.53% (501/1,410) were from the convalescent phase. The samples comprised 53.45% (596/1,115) females and 46.55% (519/1,115) males. Regarding age distribution, 57.48% (638/1,110), 22.88% (254/1,110), and 19.64% (218/1,110) were collected from patients older than 70 years, 50–70 years, and younger than 50 years, respectively. Farmers constituted the largest occupational group. Samples were predominantly collected within 14 days post-onset, while convalescent-phase samples, especially those collected more than one year post-onset, were relatively few (Supplementary Figure S1A–D, available at https://weekly.chinacdc.cn/). IgM positivity was initially 51.22% (293/572) at 1–7 days post-onset, peaking at 64.89% (146/225) at 8–14 days. It then decreased slightly to 60.94% (39/64) at 15–21 days and declined to nearly 0% after six months. IgG positivity peaked at 76.83% (189/246) at 22–180 days and remained above 60% (6/10) after 1–10 years. NAb positivity peaked at 76.53% (163/213) at 22–180 days and remained above 50% (5/10) after 1–10 years. Initially 100% among the 108 PCR-positive serum samples out of 325 tested, PCR positivity declined significantly and became undetectable after 28 days ( Figures 1A–1C). IgM levels peaked at 8–14 days, declined at 15–21 days, and remained negligible after 180 days. IgG and NAb levels both rose significantly at 8–21 days, peaked at 22–180 days, declined slightly at 181–365 days, and remained relatively high after 1–10 years ( Figures 1D–1H).
Figure 1.
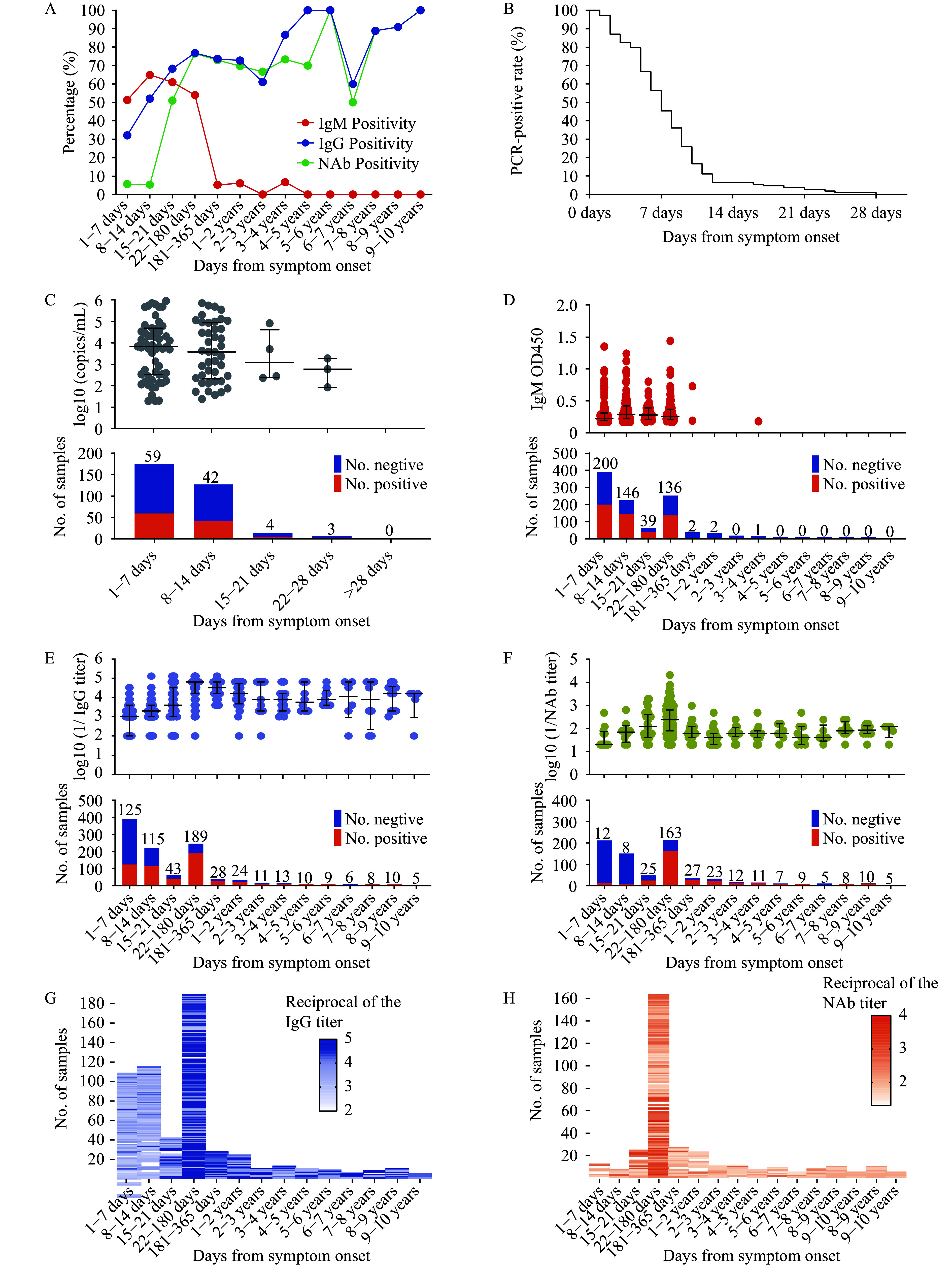
Temporal dynamics of antibody response and viral load post-symptom onset. (A) Percentage of patients testing positive for IgM, IgG, and NAb over time; (B) Decline in PCR positivity rates among the 108 patients who initially tested positive (out of 325 samples tested); (C) Changes in viral load over time post-symptom onset; (D) Temporal changes in IgM levels; (E) Temporal changes in IgG levels; (F) Temporal changes in NAb levels; (G) Temporal changes in IgG titers; (H) Temporal changes in NAb titers.
Note: For (C) to (F), the bar charts below each panel show the number of positive and negative samples for each interval, with positive sample counts noted above the bars. (G) and (H) heatmaps for IgG and NAb titers, respectively, displaying changes in titer concentrations over time post-symptom onset and offering a visual overview of antibody response dynamics.
Abbreviation: IgM=immunoglobulin M; IgG=immunoglobulin G; NAb=neutralizing antibody; PCR=polymerase chain reaction.
IgM levels positively correlated with viral load on days 1–7 ( r=0.2279, P<0.05) but not on days 8–14, while IgG levels showed no significant correlation with viral load. IgM levels positively correlated with IgG only on days 1–7 ( r=0.7178, P<0.05). IgM and NAb levels were positively correlated only during 22–180 days ( r=0.4254, P<0.0001), whereas IgG and NAb were not significantly correlated in the acute phase but showed a moderate positive correlation during the recovery phase ( r=0.4–0.6) ( Figure 2A–D). IgM levels were positively correlated with platelets (PLT) ( r=0.3551, P<0.01), white blood cells (WBC) ( r=0.2606, P<0.05), lymphocyte percentage (LY%) ( r=0.2791, P<0.05), monocyte percentage (MONO%) ( r=0.3389, P<0.01) and fibrinogen (FIB) ( r=0.4694, P<0.0001), and negatively correlated with activated partial thromboplastin time (APTT) ( r=−0.4706, P<0.0001), thrombin time (TT) ( r=−0.4531, P<0.001), prothrombin time (PT) ( r=0.4417, P<0.001), C-reactive protein (CRP) ( r=−0.2860, P<0.05), aspartate aminotransferase (AST) ( r=−0.2187, P<0.05), total protein (TP) ( r=−0.2921, P<0.05), blood urea nitrogen (BUN) ( r=−0.4838, P<0.0001), creatine kinase (CK) ( r=−0.2419, P<0.05), lactate dehydrogenase (LDH) ( r=−0.2468, P<0.05) and procalcitonin (PCT) (r=−0.4807, P<0.0001) (Supplementary Figure S2, available at https://weekly.chinacdc.cn/). Patients in the <50 age group had higher IgM levels during 8–14 days ( P<0.01) and 15–21 days ( P<0.05) compared to those in the 50–70 and >70 age groups. Similarly, patients in the >70 age group had lower IgG levels during 22–180 days ( P<0.05) and 181–365 days ( P<0.01) compared to those in the <50 and 50–70 age groups (Supplementary Figure S3A–B, available at https://weekly.chinacdc.cn/). Gender did not influence IgM and NAb levels, but females exhibited significantly higher IgG levels during the 22–180 day period ( P<0.05). IgM levels were significantly lower in fatal cases than in survivors ( P<0.05), with no significant differences between mild and severe cases or between non-fatal and severe survivors, while IgG levels showed no significant differences across all groups (Supplementary Figure S3C–G).
Figure 2.
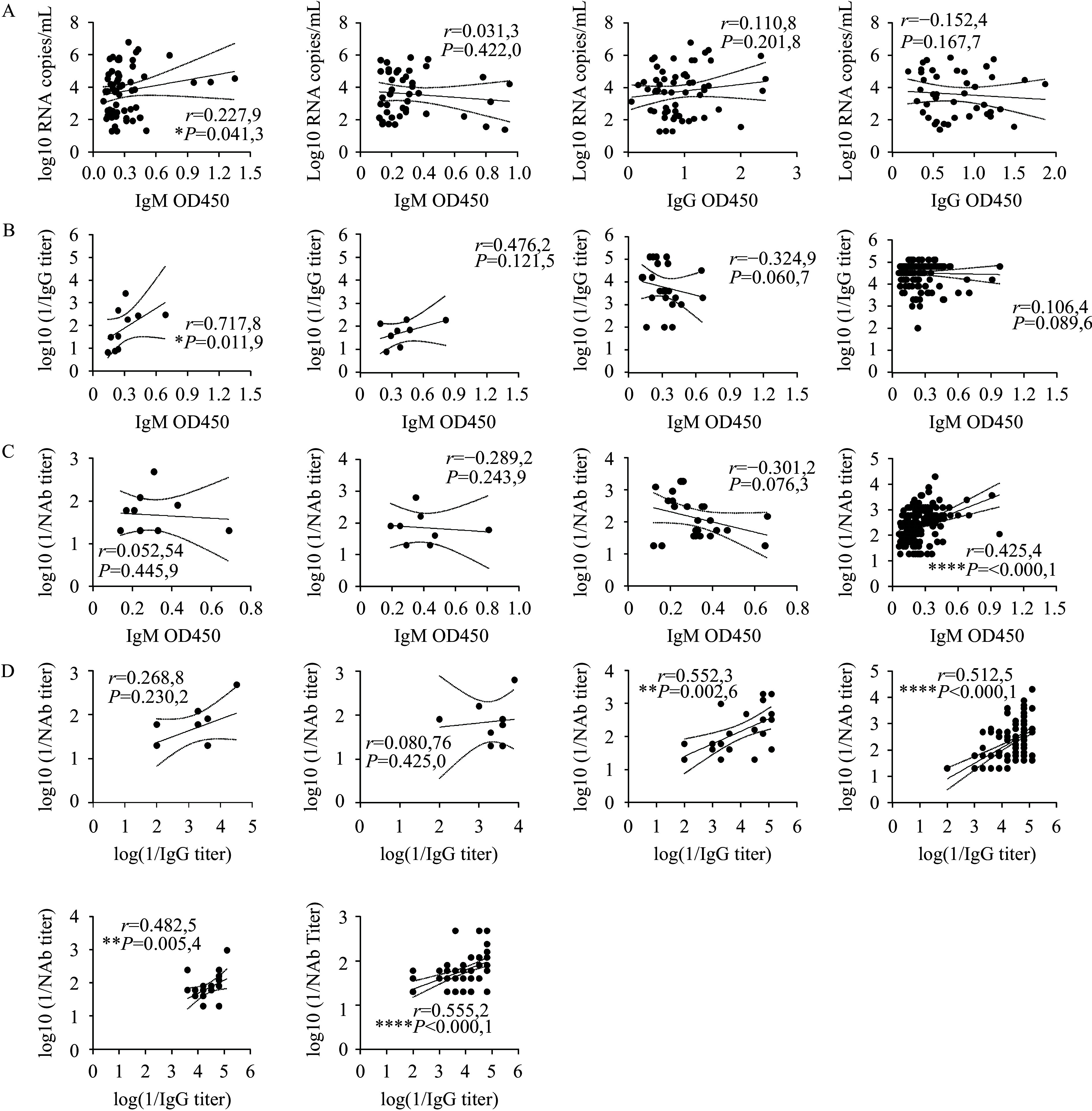
Correlations between IgM, IgG levels, and viral load, and among IgM, IgG, and NAb levels in SFTS patients. (A) Correlation of IgM and IgG levels with viral load during 1–7 and 8–14 days post-symptom onset; (B) Correlation between IgM and IgG levels at 1–7, 8–14, 15–21, and 22–180 days post-symptom onset; (C) Correlation of IgM with NAb levels at 1–7, 8–14, 15–21, and 22–180 days post-symptom onset; (D) Correlation of IgG with NAb levels at 1–7, 8–14, 15–21, 22–180, 181–365, and >365 days post-symptom onset.
Note: The correlation coefficient (r) denotes the strength of the correlation: very strong (0.8–1.0), strong (0.6–0.8), moderate (0.4–0.6), weak (0.2–0.4), or very weak/no correlation (0.0–0.2).
Abbreviation: IgM=immunoglobulin M; IgG=immunoglobulin G; NAb=neutralizing antibody; SFTS=severe fever with thrombocytopenia syndrome.
* P<0.05;
** P<0.01;
*** P<0.001;
**** P<0.0001.
This research also analyzed IgM, IgG, and NAb dynamics in 64 SFTS patients with paired acute and recovery phase serum samples. IgM levels increased significantly in 69.23% (9/13) and 55.56% (5/9) of patients at 15–21 and 22–30 days, respectively ( P<0.05). However, IgM levels decreased or stabilized in 50.00% (9/18) and 58.33% (7/12) of patients at 31–60 and 61–90 days, respectively (no significant difference), and decreased significantly in 66.67% (8/12) of patients after 90 days ( P<0.05) ( Figure 3A). IgG levels increased significantly in 84.62% (11/13) of patients at 15–21 days ( P<0.001), 100% (9/9) at 22–30 days ( P<0.01), 94.44% (17/18) at 31–60 days ( P<0.0001), and 75.00% (9/12) at 61–90 days ( P<0.01). Furthermore, 66.67% (8/12) of patients showed increases after 90 days, although this was not statistically significant. NAb levels increased significantly in 76.92% (10/13) of patients at 15–21 days ( P<0.001), 88.89% (8/9) at 22–30 days ( P<0.001), and 94.44% (17/18) at 31–60 days ( P<0.0001). Moreover, 58.33% (7/12) of patients showed increases at 61–90 days ( P<0.05), and 50.00% (6/12) after 90 days ( P<0.05) ( Figure 3B and 3C).
Figure 3.
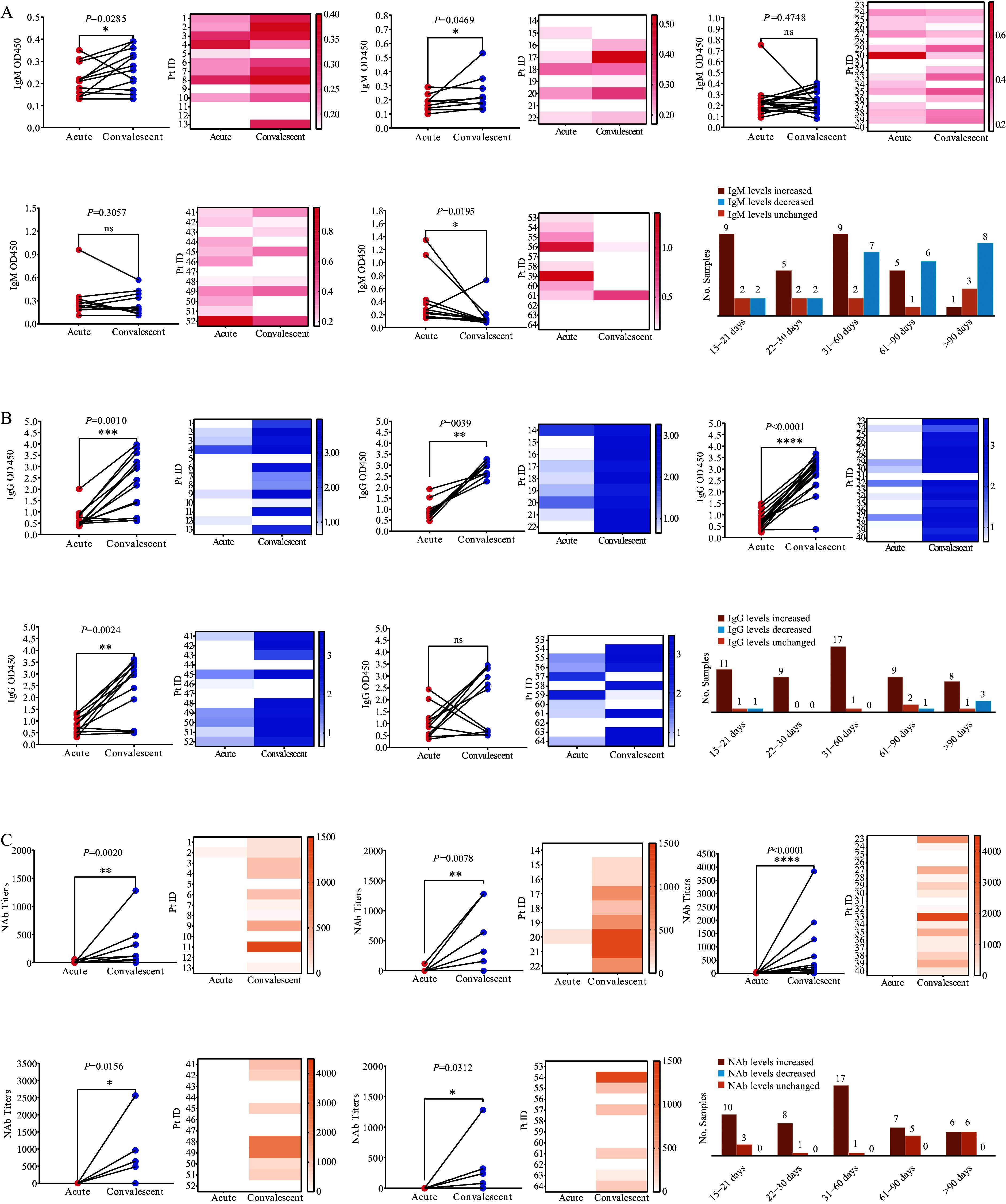
Comparative analysis of IgM, IgG, and NAb levels in paired serum sample of SFTS patients. (A), (B), and (C) show IgM, IgG, and NAb levels, respectively.
Note: This figure illustrates changes in IgM, IgG, and NAb levels in paired serum samples from 64 SFTSV-infected patients, comparing the acute phase with recovery stages at 15–21 days, 22–30 days, 31–60 days, 61–90 days, and beyond 90 days. The left sections depict individual patient changes, with red dots for the acute phase and blue dots for the recovery phase. The right sections present heatmaps displaying antibody level distributions at various stages, with darker shades indicating higher levels. Bar charts summarize antibody level changes: red bars for increases, orange bars for stable levels, and blue bars for decreases during recovery.
Abbreviation: IgM=immunoglobulin M; IgG=immunoglobulin G; NAb=neutralizing antibody; SFTS=severe fever with thrombocytopenia syndrome; SFTSV=severe fever with thrombocytopenia syndrome virus.
* P<0.05;
** P<0.01;
*** P<0.001;
**** P<0.0001.
DISCUSSION
This study presents essential longitudinal insights into the dynamics of SFTS antibodies in high-incidence areas of East Asia, particularly China, spanning a 10-year period. The temporal profile of IgM highlighted its value as an early diagnostic marker, with a rapid rise post-infection and becoming nearly undetectable after 180 days, reflecting its role in the acute immune response. In contrast, both IgG and NAb remained detectable for up to 10 years post-infection. Importantly, NAb retained functional neutralizing activity throughout this period, underscoring their critical role in long-term immune defense against SFTS. The comprehensive analysis of paired serum samples further reinforces these findings, providing a detailed trajectory of antibody responses over time. This study addresses the limitations of prior research, which often involved smaller cohorts and primarily focused on seroconversion without fully capturing the extended dynamics of antibody persistence and activity ( 3– 9).
Our analysis revealed that acute-phase IgM levels positively correlate with viral load and IgG levels, underscoring IgM’s pivotal role in early diagnosis and immune response. In the recovery phase, IgG and NAb levels exhibited a moderately positive correlation, suggesting that the IgG immune response corresponds with NAb production, thereby contributing to long-term immunity. IgM levels are significantly associated with both immune response and coagulation dysfunction, underscoring their value in identifying high-risk SFTS patients. The observed correlations with various clinical parameters reflect IgM’s role in pathophysiological processes, such as prolonged coagulation and potential liver impairment, reinforcing its utility in guiding diagnosis and therapeutic interventions. Additionally, elderly patients exhibited lower IgG levels during convalescence, likely attributable to age-related immune decline. In contrast, females showed higher IgG levels during days 22–180 compared to males, consistent with reports of higher antibody production in females ( 10). This highlighted age-related immune compromise and a more robust female immune response. Furthermore, lower IgM levels observed in fatal cases suggest a link with increased mortality, though whether this represents a causal relationship or a consequence of severe disease remains unclear.
However, limitations include insufficient long-term samples and uneven group sizes, highlighting the need for future research with diverse populations and standardized protocols to validate these findings and improve SFTS management and prevention.
Despite these limitations, this study examined a large cohort of naturally infected individuals over a 10-year period, offering key insights into SFTS immune response dynamics. The extensive sample analysis enhances our understanding of SFTS pathogenesis and provides a basis for refining diagnostic and therapeutic strategies.
Conflicts of interest
No conflicts of interest.
SUPPLEMENTARY DATA
Supplementary data to this article can be found online.
Funding Statement
Supported by the National Key R&D Program of China (ZDYF-2022YFC2303402), the Young Scientists Fund of China CDC (CCDC-2022A104), and the National Major Science and Technology Project of China (2018ZX10711001)
Contributor Information
Wei Wu, Email: wuwei@ivdc.chinacdc.cn.
Xinxian Dai, Email: daixinxian@sinopharm.com.
Shiwen Wang, Email: wangsw@ivdc.chinacdc.cn.
References
- 1.Yu XJ, Liang MF, Zhang SY, Liu Y, Li JD, Sun YL, et al Fever with thrombocytopenia associated with a novel bunyavirus in China. N Engl J Med. 2011;364(16):1523–32. doi: 10.1056/NEJMoa1010095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang XX, Ding SJ, Jiang XL, Pang B, Zhang QF, Li C, et al Detection of SFTS virus RNA and antibodies in severe fever with thrombocytopenia syndrome surveillance cases in endemic areas of China. BMC Infect Dis. 2019;19(1):476. doi: 10.1186/s12879-019-4068-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hu LF, Kong QX, Liu YY, Li JJ, Bian TT, Ma XJ, et al Time course of severe fever with thrombocytopenia syndrome virus and antibodies in patients by long-term follow-up study, China. Front Microbiol. 2021;12:744037. doi: 10.3389/fmicb.2021.744037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li JC, Ding H, Wang G, Zhang S, Yang X, Wu YX, et al Dynamics of neutralizing antibodies against severe fever with thrombocytopenia syndrome virus. Int J Infect Dis. 2023;134:95–8. doi: 10.1016/j.ijid.2023.05.018. [DOI] [PubMed] [Google Scholar]
- 5.Chung H, Kim E, Kwon B, Cho YG, Bae S, Jung J, et al Kinetics of glycoprotein-specific antibody response in patients with severe fever with thrombocytopenia syndrome. Viruses. 2022;14(2):256. doi: 10.3390/v14020256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang G, Chang HY, Jia B, Liu Y, Huang R, Wu WH, et al Nucleocapsid protein-specific IgM antibody responses in the disease progression of severe fever with thrombocytopenia syndrome. Ticks Tick Borne Dis. 2019;10(3):639–46. doi: 10.1016/j.ttbdis.2019.02.003. [DOI] [PubMed] [Google Scholar]
- 7.Song PX, Zheng N, Liu Y, Tian C, Wu XL, Ma XH, et al Deficient humoral responses and disrupted B-cell immunity are associated with fatal SFTSV infection. Nat Commun. 2018;9(1):3328. doi: 10.1038/s41467-018-05746-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li JC, Zhao J, Li H, Fang LQ, Liu W Epidemiology, clinical characteristics, and treatment of severe fever with thrombocytopenia syndrome. Infect Med. 2022;1:40–9. doi: 10.1016/j.imj.2021.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lu QB, Cui N, Hu JG, Chen WW, Xu W, Li H, et al Characterization of immunological responses in patients with severe fever with thrombocytopenia syndrome: a cohort study in China. Vaccine. 2015;33(10):1250–5. doi: 10.1016/j.vaccine.2015.01.051. [DOI] [PubMed] [Google Scholar]
- 10.Qi R, Huang YT, Yu XJ Persistence and gender differences in protection against severe fever with thrombocytopaenia syndrome virus with natural infection: a 4-year follow-up and mathematical prediction study. Epidemiol Infect. 2019;147:e78. doi: 10.1017/S1469440918003643. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data to this article can be found online.


