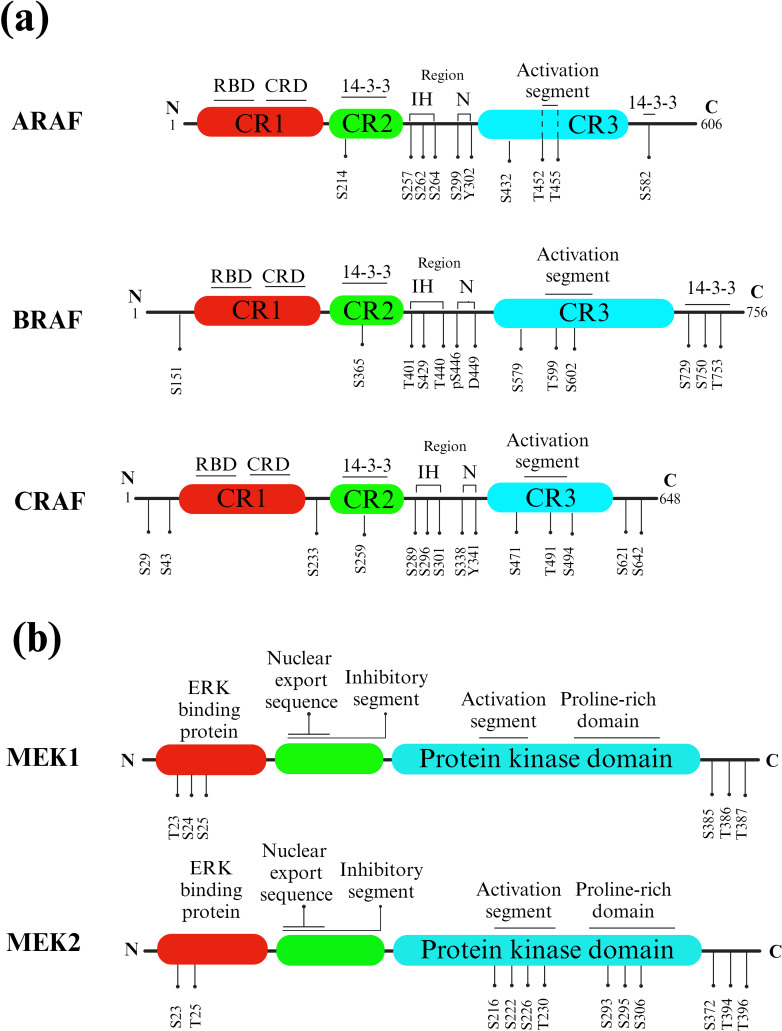
Figure 3.
RAF and MEK isoforms and phosphorylation sites. (A) RAFs. Key structural features of different RAF isotypes. The three highly conserved regions (CR1, CR2, and CR3) are indicated, with CR1 containing the RAS binding domain (RBD) and cysteine-rich domain (CRD), CR2 comprising serine and threonine regulatory phosphorylation sites, and CR3 housing the P-loop or glycine-rich loop and the kinase domain, including the activation segment. Phosphorylated CR2 (pS365) and the C-terminal region (pS729) of BRAF, as well as phosphorylated CR2 (pS259) and the C-terminal region (pS621) of CRAF, act as binding sites for 14-3-3 proteins. Instead, KRAS is linked to the RAS-binding domain (RBD). (B) MEKs. Activated RAF phosphorylates and activates MEK, which is a dual-specificity kinase. MEK has two kinase domains, and it phosphorylates a specific tyrosine and threonine residue on ERK proteins.