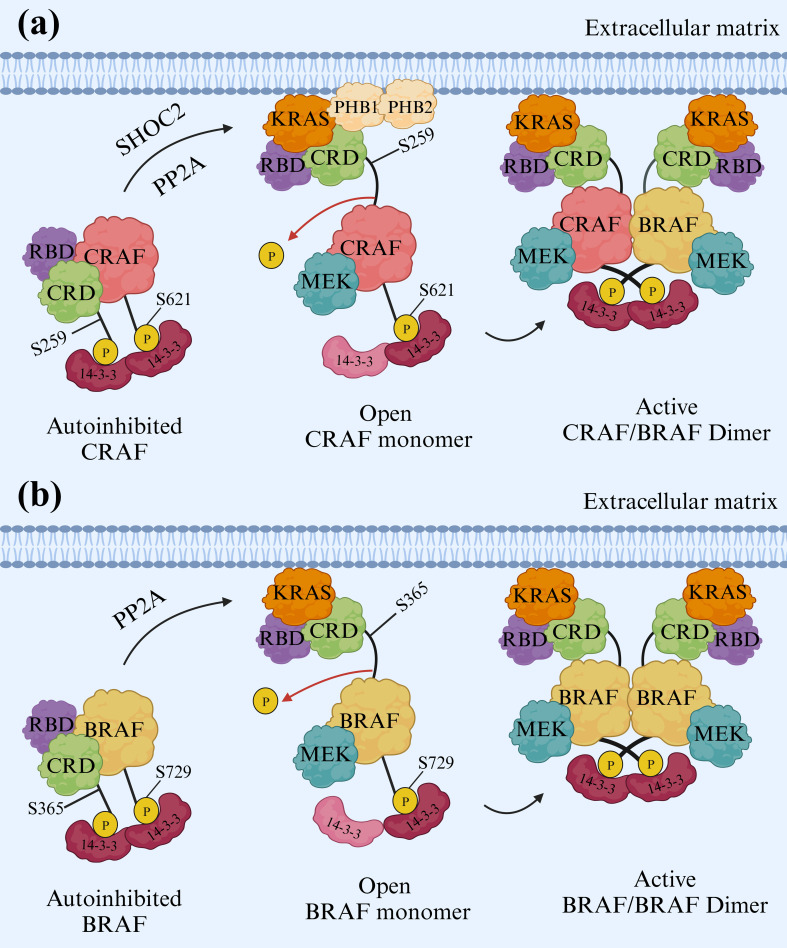
Figure 4.
Activation mechanisms of CRAF and BRAF. (A) CRAF Activation. To activate CRAF, the SHOC2 complex removes phosphate groups from S259 of CRAF. This dephosphorylation is vital, enabling CRAF to pair with other RAF kinases when stimulated by growth factors. The formation of these RAF heterodimers and removing the inhibitory “S259” site are crucial for CRAF activation. Prohibitin 1 (PHB1) also plays a key role by directly interacting with CRAF and stimulating ERK1/2. (B) BRAF Activation. BRAF activation resembles a lock-and-key mechanism. BRAF forms a complex with its partner, MEK, and a 14-3-3 dimer in its inactive state. The 14-3-3 dimer acts like a lock, encircling specific sites (pS365 and pS729) on both sides of the BRAF kinase domain. This interaction effectively keeps the BRAF inactive by preventing dimerization, a crucial step for activation. Two types of 14-3-3 proteins, single and mixed types, actively participate in RAF activation. In this arrangement, the cysteine-rich domain (CRD) is centrally shielded from interactions with the cell membrane and RAS, while the RAS-binding domain (RBD) of BRAF is exposed and ready to interact with RAS.