Abstract
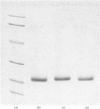
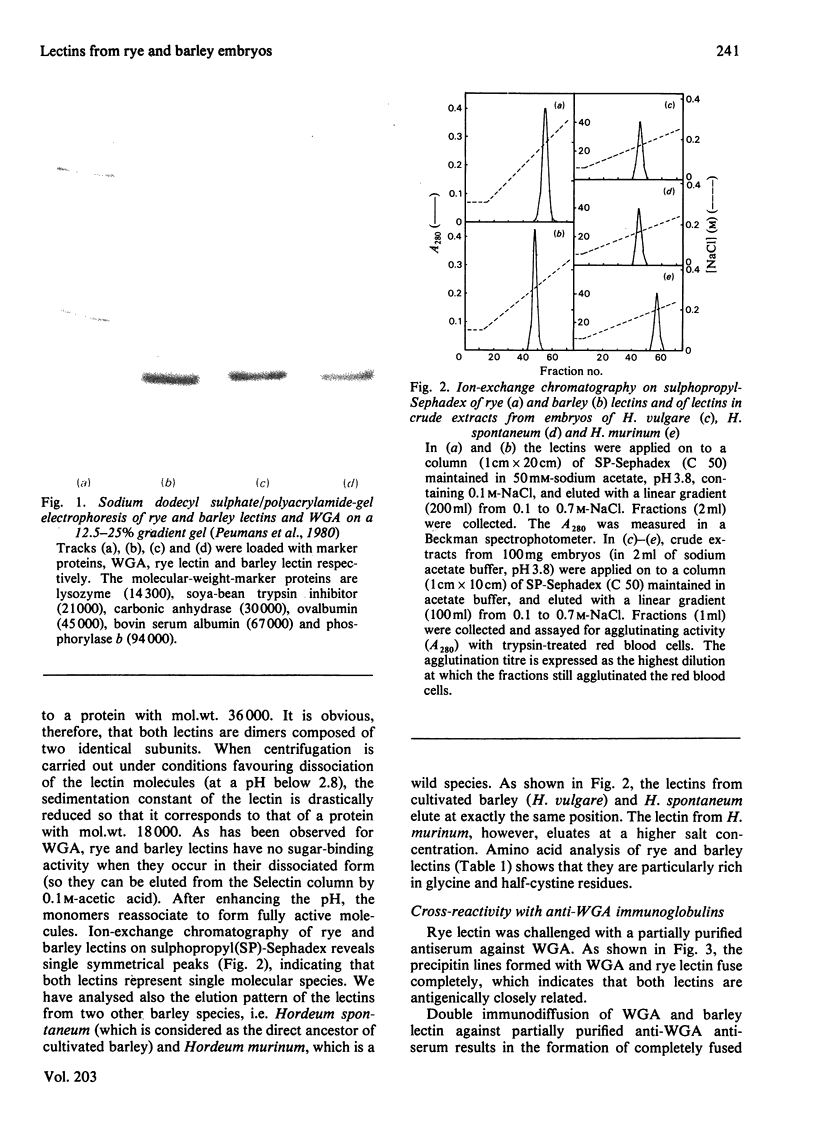
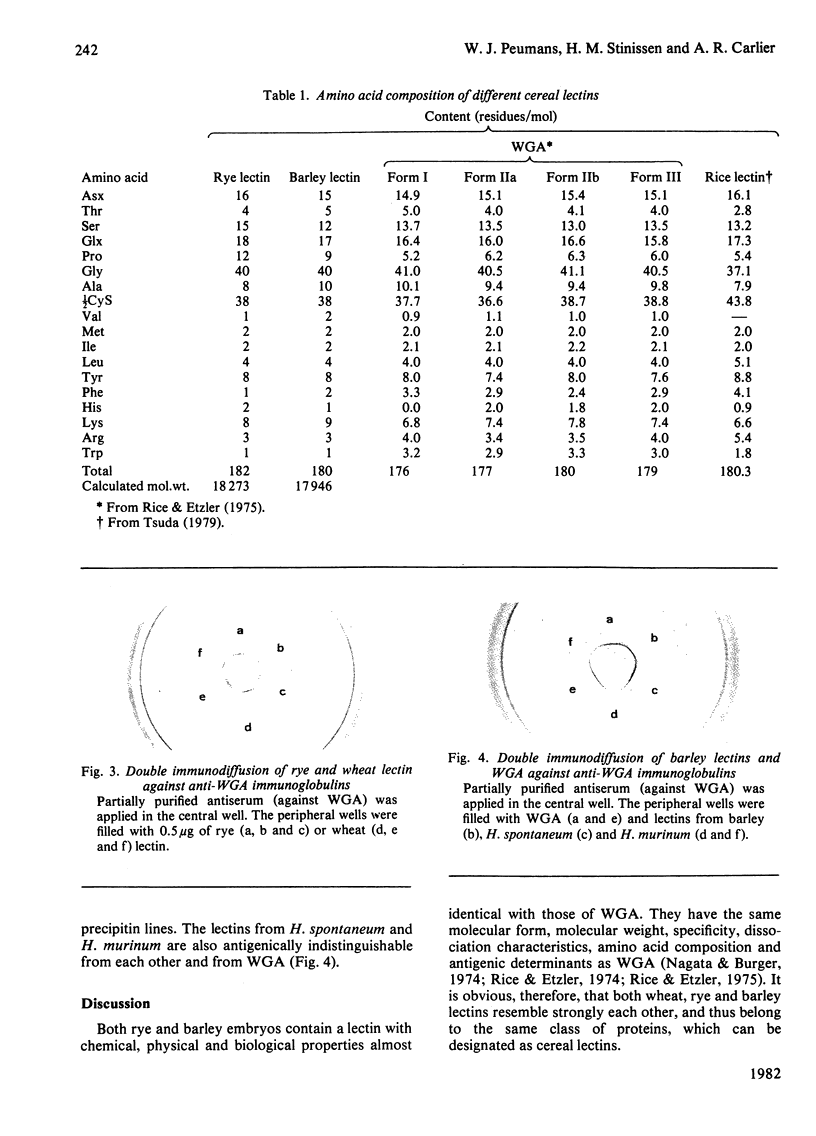
Lectins have been isolated from embryos of Secale cereale (rye) and Hordeum vulgare (barley) by affinity chromatography on immobilized N-acetylglucosamine. Both lectins are dimeric proteins of two identical subunits of mol.wt. 18000. They resemble strongly wheat-germ agglutinin with respect to their chemical, physical, biological and immunological properties.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen A. K., Neuberger A., Sharon N. The purification, composition and specificity of wheat-germ agglutinin. Biochem J. 1973 Jan;131(1):155–162. doi: 10.1042/bj1310155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlier A. R., Peumans W. J. The rye embryo system as an alternative to the wheat system for protein synthesis in vitro. Biochim Biophys Acta. 1976 Nov 1;447(4):436–444. doi: 10.1016/0005-2787(76)90081-2. [DOI] [PubMed] [Google Scholar]
- Foriers A., De Neve R., Kanarek L. Proceedings: Specificity and partial purification of the barley-germ agglutinin. Arch Int Physiol Biochim. 1975 May;83(2):362–362. [PubMed] [Google Scholar]
- Foriers A., De Neve R., Kanarek L. Purification of barley-germ agglutinin. Arch Int Physiol Biochim. 1976;84(3):617–618. [PubMed] [Google Scholar]
- Goldstein I. J., Hayes C. E. The lectins: carbohydrate-binding proteins of plants and animals. Adv Carbohydr Chem Biochem. 1978;35:127–340. doi: 10.1016/s0065-2318(08)60220-6. [DOI] [PubMed] [Google Scholar]
- Mirelman D., Galun E., Sharon N., Lotan R. Inhibition of fungal growth by wheat germ agglutinin. Nature. 1975 Jul 31;256(5516):414–416. doi: 10.1038/256414a0. [DOI] [PubMed] [Google Scholar]
- Nagata Y., Burger M. M. Wheat germ agglutinin. Molecular characteristics and specificity for sugar binding. J Biol Chem. 1974 May 25;249(10):3116–3122. [PubMed] [Google Scholar]
- Partridge J., Shannon L., Gumpf D. A barley lectin that binds free amino sugars. I. Purification and characterization. Biochim Biophys Acta. 1976 Dec 21;451(2):470–483. doi: 10.1016/0304-4165(76)90142-2. [DOI] [PubMed] [Google Scholar]
- Privat J. P., Delmotte F., Monsigny M. Protein-sugar interactions. Association of beta-(1 leads to 4) linked N-acetyl-D-glucosamine oligomer derivatives with wheat germ agglutinin (lectin). FEBS Lett. 1974 Sep 15;46(1):224–228. doi: 10.1016/0014-5793(74)80373-x. [DOI] [PubMed] [Google Scholar]
- Rice R. H., Etzler M. E. Subunit structure of wheat germ agglutinin. Biochem Biophys Res Commun. 1974 Jul 10;59(1):414–419. doi: 10.1016/s0006-291x(74)80222-6. [DOI] [PubMed] [Google Scholar]
- Rice R. H. Wheat germ agglutinin. Evidence for a genetic basis of multiple forms. Biochim Biophys Acta. 1976 Aug 24;444(1):175–180. doi: 10.1016/0304-4165(76)90234-8. [DOI] [PubMed] [Google Scholar]
- Wright C. S. Crystallographic elucidation of the saccharide binding mode in wheat germ agglutinin and its biological significance. J Mol Biol. 1980 Aug 15;141(3):267–291. doi: 10.1016/0022-2836(80)90181-3. [DOI] [PubMed] [Google Scholar]