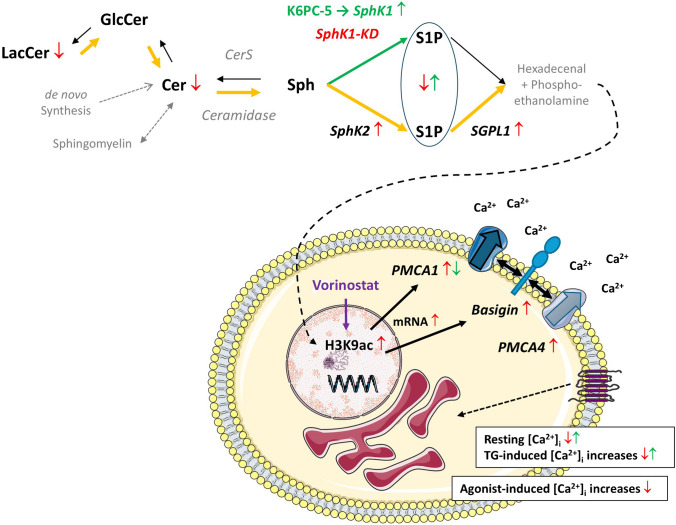
Fig. 11.
Graphical summary of the alterations in sphingolipid concentrations and Ca2+ homeostasis in EA.hy926 cells with stable knockdown of SphK1. Enzymes and lipids written in gray letters have not been addressed in the present study. The lipid analysis did not differentiate between S1P derived from SphK1 or SphK2. In SphK1-KD cells (red arrows), the concentrations of S1P, ceramides (Cer), and lactosylceramides (LacCer) were decreased. SphK2 and SGPL1 were upregulated, suggesting that there was enhanced flux via the sphingolipid degradation pathway (orange arrows). Conversely, the SphK1 activator, K6PC-5 (green arrows), caused an upregulation of SphK1 and increased S1P concentrations. SphK1-KD cells had reduced resting [Ca2+]i and diminished [Ca2+]i increases in response to thapsigargin (TG) and diverse GPCR agonists. In agreement, PMCA1 and its auxiliary subunit, basigin, were upregulated on mRNA and protein levels. Basigin is known to stabilize PMCA complexes and probably contributed to the increase in PMCA4 protein. Transcriptional regulation of PMCA1 and basigin was associated with enhanced histone acetylation (specifically, H3K9ac) and mimicked by the HDAC inhibitor, vorinostat. Conversely, K6PC-5 caused a decrease in PMCA1 expression and elevated both resting [Ca2+]i and thapsigargin-induced [Ca2+]i increases. Recently published data [11] show that hexadecenal, the product of SGPL1, induced HDAC inhibition, and we propose that this was the mechanism of enhanced histone acetylation in SphK1-KD cells