Abstract
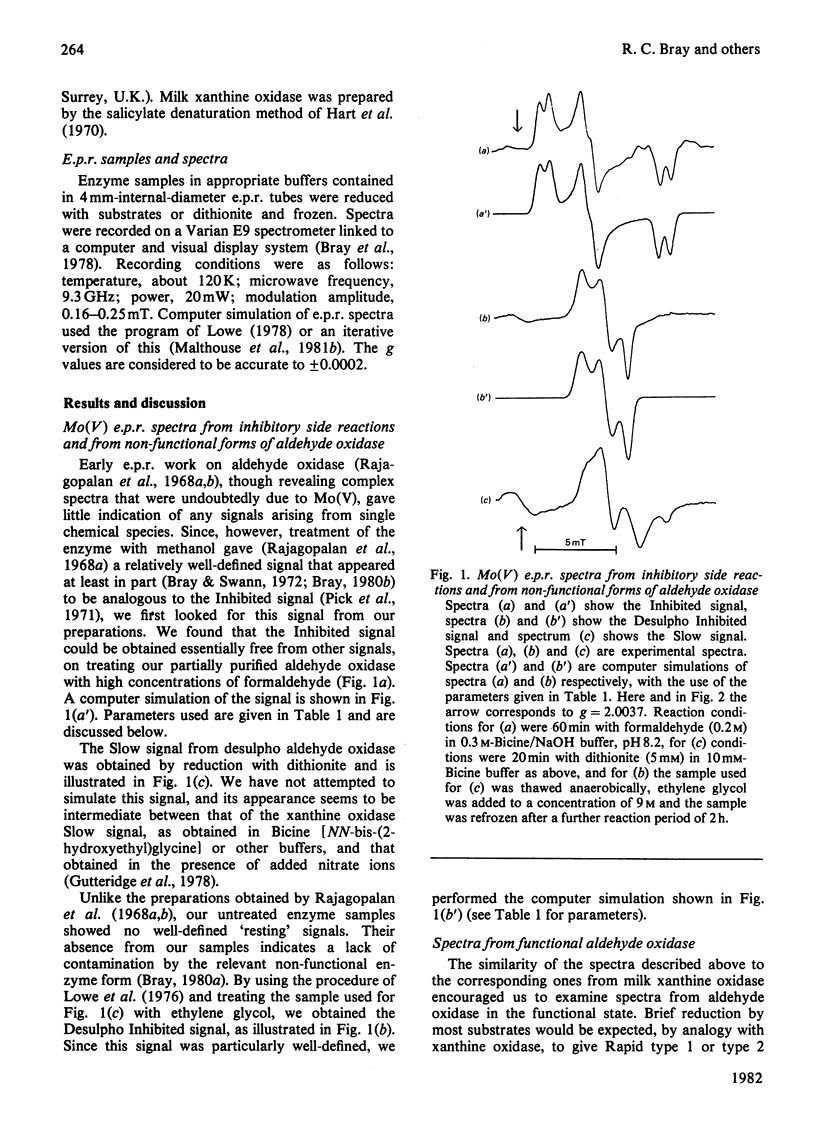
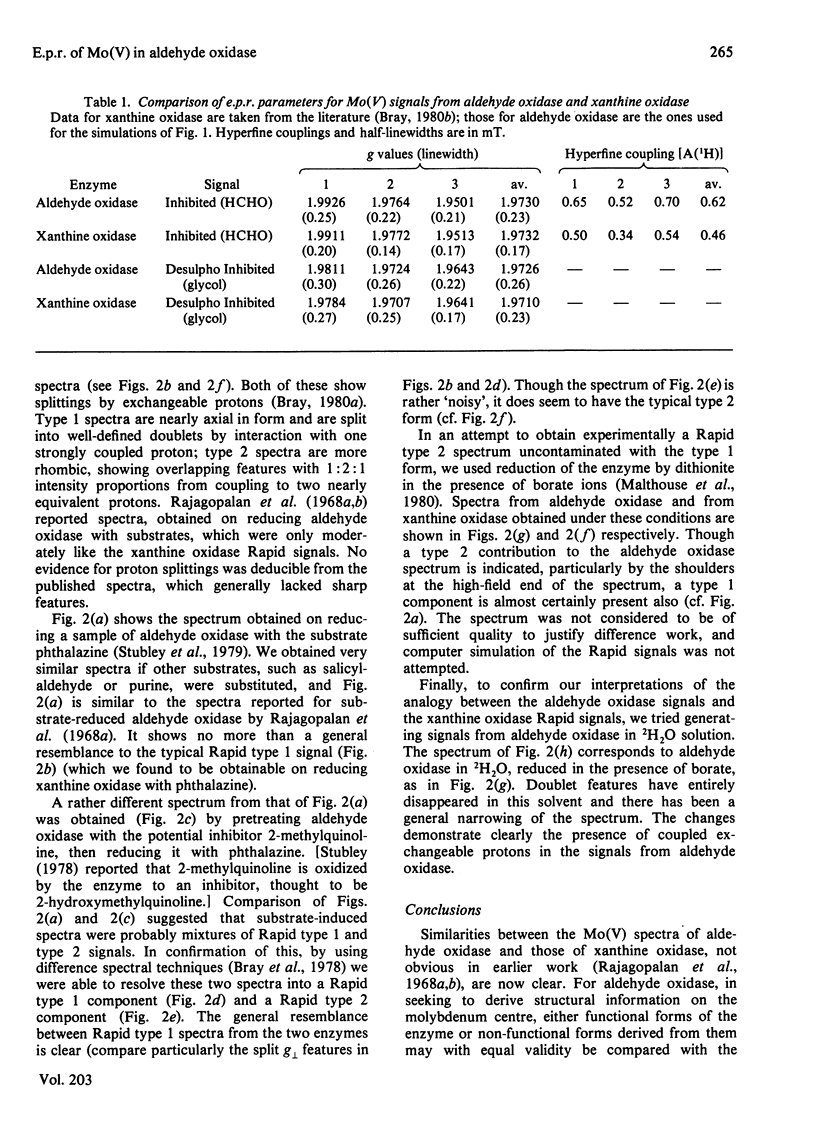
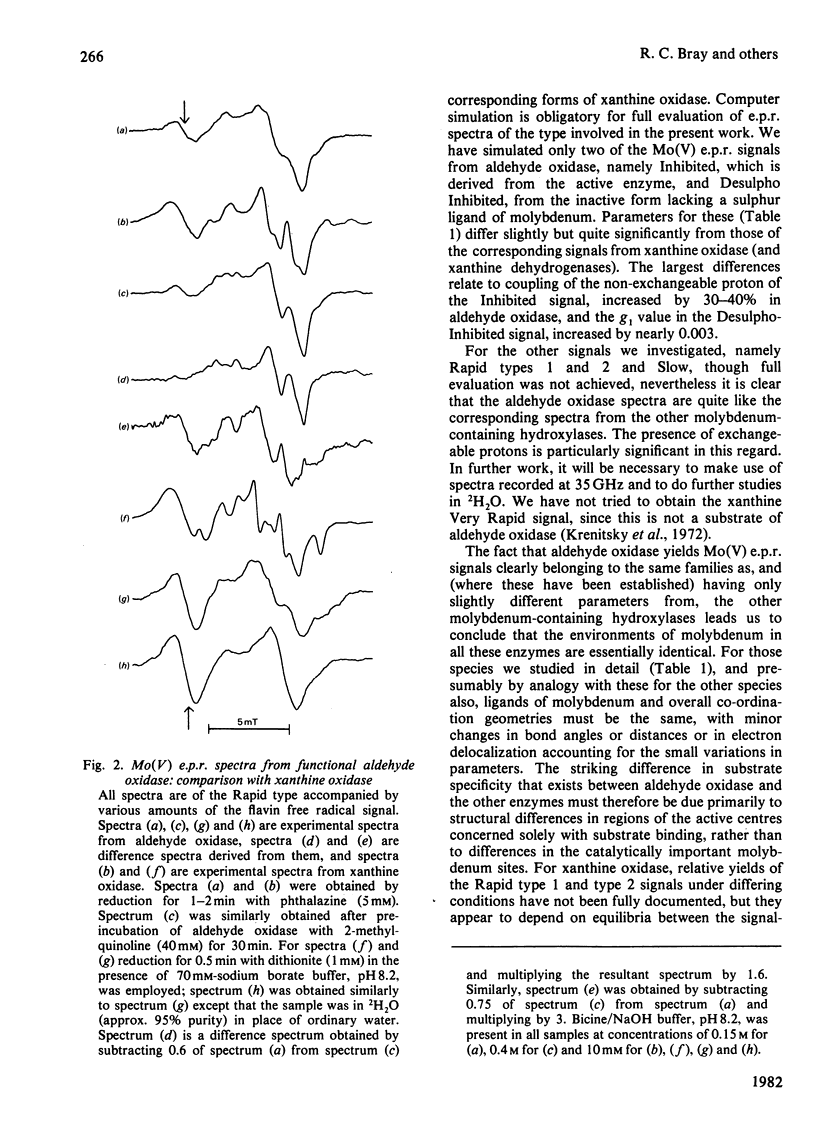
Molybdenum(V) e.p.r. spectra from reduced forms of aldehyde oxidase were obtained and compared with those from xanthine oxidase. Inhibited and Desulpho Inhibited signals from aldehyde oxidase were fully characterized, and parameters were obtained with the help of computer simulations. These differ slightly but significantly from the corresponding parameters for the xanthine oxidase signals. Rapid type 1 and type 2 and Slow signals were obtained from aldehyde oxidase, but were not fully characterized. From the general similarities of the signals from the two enzymes, it is concluded that the ligands of molybdenum must be identical and that the overall co-ordination geometries must be closely similar in the enzymes. The striking differences in substrate specificity must relate primarily to structural differences in a part of the active centre concerned with substrate binding and not involving the catalytically important molybdenum site.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barber M. J., Bray R. C., Lowe D. J., Coughlan M. P. Studies by electron-paramagnetic-resonance spectroscopy and stopped-flow spectrophotometry on the mechanism of action of turkey liver xanthine dehydrogenase. Biochem J. 1976 Feb 1;153(2):297–307. doi: 10.1042/bj1530297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branzoli U., Massey V. Evidence for an active site persulfide residue in rabbit liver aldehyde oxidase. J Biol Chem. 1974 Jul 25;249(14):4346–4349. [PubMed] [Google Scholar]
- Branzoli U., Massey V. Preparation of aldehyde oxidase in its native and deflavo forms. Comparison of spectroscopic and catalytic properties. J Biol Chem. 1974 Jul 25;249(14):4399–4345. [PubMed] [Google Scholar]
- Bray R. C., Barber M. J., Lowe D. J. Electron-paramagnetic-resonance spectroscopy of complexes of xanthine oxidase with xanthine and uric acid. Biochem J. 1978 Jun 1;171(3):653–658. doi: 10.1042/bj1710653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray R. C. The reactions and the structures of molybdenum centers in enzymes. Adv Enzymol Relat Areas Mol Biol. 1980;51:107–165. doi: 10.1002/9780470122969.ch3. [DOI] [PubMed] [Google Scholar]
- Dalton H., Lowe D. J., Pawlik T., Bray R. C. Studies by electron-paramagnetic-resonance spectroscopy on the mechanism of action of xanthine dehydrogenase from Veillonella alcalescens. Biochem J. 1976 Feb 1;153(2):287–295. doi: 10.1042/bj1530287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutteridge S., Tanner S. J., Bray R. C. Comparison of the molybdenum centres of native and desulpho xanthine oxidase. The nature of the cyanide-labile sulphur atom and the nature of the proton-accepting group. Biochem J. 1978 Dec 1;175(3):887–897. doi: 10.1042/bj1750887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart L. I., McGartoll M. A., Chapman H. R., Bray R. C. The composition of milk xanthine oxidase. Biochem J. 1970 Mar;116(5):851–864. doi: 10.1042/bj1160851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krenitsky T. A., Neil S. M., Elion G. B., Hitchings G. H. A comparison of the specificities of xanthine oxidase and aldehyde oxidase. Arch Biochem Biophys. 1972 Jun;150(2):585–599. doi: 10.1016/0003-9861(72)90078-1. [DOI] [PubMed] [Google Scholar]
- Krenitsky T. A., Tuttle J. V., Cattau E. L., Jr, Wang P. A comparison of the distribution and electron acceptor specificities of xanthine oxidase and aldehyde oxidase. Comp Biochem Physiol B. 1974 Dec 15;49(4):687–703. doi: 10.1016/0305-0491(74)90256-9. [DOI] [PubMed] [Google Scholar]
- Lowe D. J., Barber M. J., Pawlik R. T., Bray R. C. A new non-functional form of milk xanthine oxidase containing stable quinquivalent molybdenum. Biochem J. 1976 Apr 1;155(1):81–85. doi: 10.1042/bj1550081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe D. J. Electron paramagnetic resonance in biochemistry. Computer simulation of spectra from frozen aqueous samples. Biochem J. 1978 Jun 1;171(3):649–651. doi: 10.1042/bj1710649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malthouse J. P., Gutteridge S., Bray R. C. Rapid type 2 molybdenum(V) electron-paramagnetic resonance signals from xanthine oxidase and the structure of the active centre of the enzyme. Biochem J. 1980 Mar 1;185(3):767–770. doi: 10.1042/bj1850767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pick F. M., McGartoll M. A., Bray R. C. Reaction of formaldehyde and of methanol with xanthine oxidase. Eur J Biochem. 1971 Jan 1;18(1):65–72. doi: 10.1111/j.1432-1033.1971.tb01215.x. [DOI] [PubMed] [Google Scholar]
- Rajagopalan K. V., Handler P., Palmer G., Beinert H. Studies of aldehyde oxidase by electron paramagnetic resonance spectroscopy. I. Spectra at equilibrium states. J Biol Chem. 1968 Jul 25;243(14):3784–3796. [PubMed] [Google Scholar]
- Rajagopalan K. V., Handler P., Palmer G., Beinert H. Studies of aldehyde oxidase by electron paramagnetic resonance spectroscopy. II. Kinetic studies by rapid freezing. J Biol Chem. 1968 Jul 25;243(14):3797–3806. [PubMed] [Google Scholar]
- Stubley C., Stell J. G., Mathieson D. W. The oxidation of azaheterocycles with mammalian liver aldehyde oxidase. Xenobiotica. 1979 Aug;9(8):475–484. doi: 10.3109/00498257909087261. [DOI] [PubMed] [Google Scholar]


