Abstract
Background
Human papillomavirus (HPV) self-sampling is a new method for collecting cervical isolated cells, but research been carried out in multi-ethnic and multi-regional areas of China is scarce.
Objectives
We aimed to evaluate the accuracy and acceptability of HPV self-sampling and analyze the characteristics of HPV infection.
Study design
Women aged 25–65 years were recruited from 8 provinces in China. Women underwent clinician-sampling and self-sampling and were asked to complete a 65-question questionnaire on their acceptance of HPV self-sampling. The paired samples were analyzed for 23 genotypes of HPV by polymerase chain reaction.
Results
5551 women were recruited, of which 5417 were eligible for analysis. 3163 women have completed and submitted the questionnaire. The top five infection genotypes were HPV 52, 58, 16, 39, and 68. The highest infection rate was in the 25–30 years group. The crude agreement between self-sampling and clinician-sampling was 93.06 %. 43.79 % of women preferred self-sampling over clinician-sampling, and 67.59 % preferred doing self-sampling at the hospital.
Conclusion
HPV self-sampling could be an effective supplement to traditional cervical screening in China. Clinicians’ advocacy, timely reminders and guidance for women with abnormal self-sampling results are needed. In addition, new vaccination and cervical screening recommendations might be adjusted to fit populations with different characteristics.
Keywords: Human papilloma virus, Self-sampling, Agreement, Acceptance, cervical screening
1. Background
Cervical cancer is the fourth most frequently diagnosed cancer and the fourth leading cause of cancer death in women worldwide, causing approximately 662,301 new cases and 348,874 deaths in 2022, which is increasing year by year [1]. More than 90 % of cervical cancers are caused by human papillomavirus (HPV) infection [2]. Incidence and mortality of cervical cancer have declined in several countries due to effective HPV vaccination and cervical cancer screening [3]. However, cervical cancer remains the fifth life-threatening and most common cancer in Chinese women [4]. 18 % of all cervical cancer cases and 17 % of deaths from cervical cancer occurred in China [5]. According to the report of the National Cancer Center and International Agency for Research on Cancer in 2024, the age-standardized incidence rates and age-standardized mortality rates of female cervical cancer in China were 13.83 % and 4.54 %, respectively, both increased significantly from 2000 to 2022 [4,6,7].
In 2020, the World Health Organization (WHO) proposed an intermediate target of eliminating cervical cancer, which is to have 90 % of adolescent girls vaccinated, 70 % of women screened regularly in the age range of 35–45 years, and 90 % of women with precancerous lesion or cervical cancer received appropriate treatment [8]. While HPV vaccination has not been included in the Chinese national immunization program yet, cervical screening remains the principal method to prevent and eliminate cervical cancer [9,10]. Traditional cervical screening methods include visual inspection with acetic acid, pap smear test, liquid-based cytology test, and clinician-collected HPV testing [11]. These screening methods require professional medical personnel and equipment, which are relatively scarce in low- and middle-income countries and may not meet the requirement of increasing the participation rate of women. China is a vast country with 432.9 million females aged 15–59 years who are at risk of developing cervical cancer [12]. China has launched a free screening program called “Two-Cancer Screening” for women, which contains cervical and breast cancer screening [13]. Cervical cancer screening methods differ across China, with clinician-collected HPV test and Thinprep cytology test (TCT) being the mainstream methods in most areas. In 2015, the cervical screening coverage rate in China was only 37 %, far lower than the 70 % level set by the WHO [12].
HPV DNA testing could detect high-risk HPV (HR-HPV), which causes the vast majority of cervical cancers [14]. Unlike tests that rely on visual inspection, the HPV DNA test is an objective diagnostic method, leaving no space to interpret results. HPV self-sampling is a method for women to take a sample by inserting a brush or swab into their vagina, instead of being sampled by a gynecologist at the hospital. In some countries, HPV self-sampling has been promoted for national cervical cancer screening programs [15]. Few multi-center and population-based studies on HPV self-sampling have been conducted in China, and no self-sampling-based cervical screening program has been implemented.
2. Objectives
The aim of this study is to evaluate the accuracy and feasibility of HPV self-sampling in different regions and ethnic groups of China.
2.1. Study design
The Medical Ethics Committee of Beijing Obstetrics and Gynecology Hospital (BJOGH) approved this study on May 18, 2022. Eligible women were 25–65 years and had a need for cervical cancer screening. Women who were pregnant, had a history of vaginal bleeding, female genital tract tumors, previous hysterectomy and cervical surgery, had used topical drugs in the vagina within the past 3 days, or were unable to complete self-sampling were excluded.
2.2. Study populations
5551 eligible women were recruited for the study in Beijing, Shandong, Hubei, Yunnan, Qinghai, Inner Mongolia, Xinjiang and Tibet. All eligible women were invited to participate, and virtually everyone agreed and was accepted until our target number was reached. At gynecological clinics in each region, the trial was explained by a professional gynecologist and informed consent was obtained from all participants. Women included in the pooled analysis all received HPV DNA testing for self-collected and clinician-obtained samples.
2.3. Screening tests
2.3.1. HPV clinician-sampling
A physician obtained specimens for clinician-collected HPV tests from the endocervix during internal examination using a female sample collection kit (Hybribio, Chaozhou, China). After routine smears, the brush head was placed into a vial (Hybribio, Chaozhou, China) containing 3.5 ml of fixative solution for direct HPV detection. 48 h after clinician sampling, patients performed HPV self-sampling.
2.3.2. HPV self-sampling
All participants received both video and verbal instructions on how to perform self-sampling. The HPV self-tests were provided with a disposable brush, cap and column (Female self-sampling kit, Hybribio). Patients were instructed to clean and disinfect their hands before sampling, remove the cap, and insert the swab with the column into the vagina until the stopping plate reached the vaginal entrance. They were then to gently twist the swab in one direction for 5 rotations, noting a "tick" feeling for each rotation to count easily. They took out the swab and column with care, twisted them back into the cover tube, held the cover tube, and twisted the cap tightly to secure the swab. The kit was then placed back into the plastic packaging, sealed with the provided zip bag, and the name and date were written on the information card provided before sending the kit within 7 days. The self-sampling kit was transported dry to the laboratory and then suspended in 3.5 mL of Hybribio cell preservation solution.
2.3.3. HPV testing
The 23 HPV Genotyping Real-time PCR Kit is designed for in vitro detection of 23 HPV types in cervical cell specimens, including HPV16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, 68, 6, 11, 42, 43, 44, 53, 81, 73 and 82. Cellular internal control is included for each sample to monitor the whole testing process starting from DNA extraction to signal detection [16].
All samples were tested according to the manufacturer's instructions: 0.5–1 mL of the cervical or vaginal sample was pipetted into a 1.5 mL microcentrifuge or screw-cap tube. The sample was then centrifuged, the supernatant was discarded, and the pellet was lysed. After boiling the sample in a hot water bath, 2.0 μL of the DNA template was pipetted from the center of the volume for the PCR reaction. Through real-time fluorescence PCR, one-step nucleic acid amplification and detection are achieved. One sample is divided into six reaction tubes by using four fluorescence channels to detect 23 types of HPV. The control, β-globin DNA, is used to evaluate sample quality and PCR inhibition results. If the Ct value of a sample is undetected, the sample is negative. If the Ct value of a sample is ≤ 40, the sample is positive. The cutoff Ct values of self- and clinician-collected samples were the same. All sample processing and laboratory testing were performed at BJOGH in Beijing, China. Tests for both sampling methods are free of charge.
2.4. Questionnaire
A 65-question questionnaire was used to investigate women's demographic data, awareness of cervical cancer screening, acceptance and feelings about HPV self-sampling.
2.5. Data analysis
Two researchers collected all the data and entered it into a Microsoft Excel sheet. Data analysis was performed using SPSS software version 22.0. Statistical significance was set at p ≤ 0.05.
The sociodemographic characteristics of participants were analyzed using descriptive statistics. The accuracy of HPV self-sampling was examined by the concordance in PCR test results between paired self- and clinician-collected samples. The concordance was assessed by calculating the overall agreement rate and Cohen's kappa statistics with the following interpretation: poor or slight agreement (≤0.2), fair agreement (0.2–0.4), moderate agreement (0.4–0.6), good agreement (0.6–0.8), and very good agreement (0.8–1.0). Factors influencing women's acceptability of HPV self-sampling were analyzed using both univariate and multivariate logistic regression.
3. Results
-
1.
Patient characteristics
A total of 5551 women were recruited, of which 5417 (97.59 %) were eligible for analysis. 134 women were excluded from the analysis because samples contained too few cells to perform PCR tests or leaked during transportation. The mean age of 5417 women was 43.82 ± 9.42 years. 3163 women have completed and submitted the questionnaire. Table 1 presents the sociodemographic characteristics of the study participants who completed questionnaires. The mean age was 44.25 ± 9.32 years old. The majority of women were married (92.16 %) and employed (72.15 %), had 1 or 2 children (84.57 %), and graduated from high school or higher education (69.23 %). Women who had never participated in cervical screening and women whose last screening interval was more than 3 years accounted for 44.96 %.
-
2.
Characteristics of HPV infection
Table 1.
Sociodemographic characteristics of the study participants.
| Characteristics | No.(%) |
|---|---|
| Total | 5417 |
| Completed the questionnaire | 3163 |
| Age(year) | |
| Mean ± standard deviation | 44.25 ± 9.32 |
| 25–30 | 190(6.01) |
| 31–40 | 1005(31.77) |
| 41–50 | 1093(34.56) |
| 51–65 | 875(27.66) |
| Marital status | |
| Unmarried | 112(3.54) |
| Married | 2915(92.16) |
| Divorced | 103(3.26) |
| Widowed | 33(1.04) |
| Parity | |
| 0 | 107(3.64) |
| 1 | 1600(54.38) |
| 2 | 1075(36.54) |
| 3 | 131(4.45) |
| 4 | 20(0.68) |
| ≥5 | 9(0.31) |
| Menstruation | |
| Not-Menopausal | 2304 (72.84) |
| Menopausal | 859(27.16) |
| Highest level of school attended | |
| Primary school | 274(8.66) |
| Junior high school | 699(22.1) |
| Senior high school | 625(19.76) |
| College or higher | 1565(49.48) |
| Occupation | |
| Worker | 294(9.29) |
| Farmer | 288(9.11) |
| Individual business | 289(9.14) |
| Server | 428(13.53) |
| Housewife | 300(9.48) |
| Clerk | 630(19.92) |
| Retired | 294(9.29) |
| Others | 640(20.23) |
| Income per month(CNY) | |
| <3000 | 1227(38.79) |
| 3001–5000 | 979(30.95) |
| 5001–8000 | 586(18.53) |
| >8000 | 371(11.73) |
| Medical insurance | |
| Insured | 3020(95.48) |
| Not-insured | 143(4.52) |
| Time of last cervical screening | |
| Within 1 year | 581(18.37) |
| 1–3 years | 950(30.03) |
| 3–5 years | 281(8.88) |
| Above 5 years | 141(4.46) |
| Never been screened | 1000(31.62) |
| Forgotten | 210(6.64) |
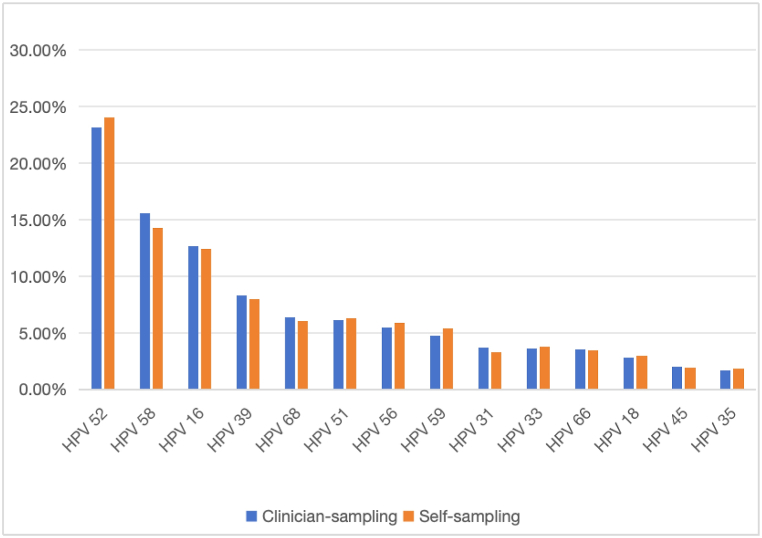
The top five infection genotypes were HPV 52, 58, 16, 39, and 68, and the infection rates were 23.17 %, 15.56 %, 12.73 %, 8.31 %, and 6.37 %, respectively. Fig. 1 shows the 14 HR-HPV genotypes detected in clinician-collected samples.
Fig. 1.
14 genotypes of HR-HPV detected in clinician-collected samples
Abbreviations: HPV, human papilloma virus; HR-HPV, high-risk HPV.
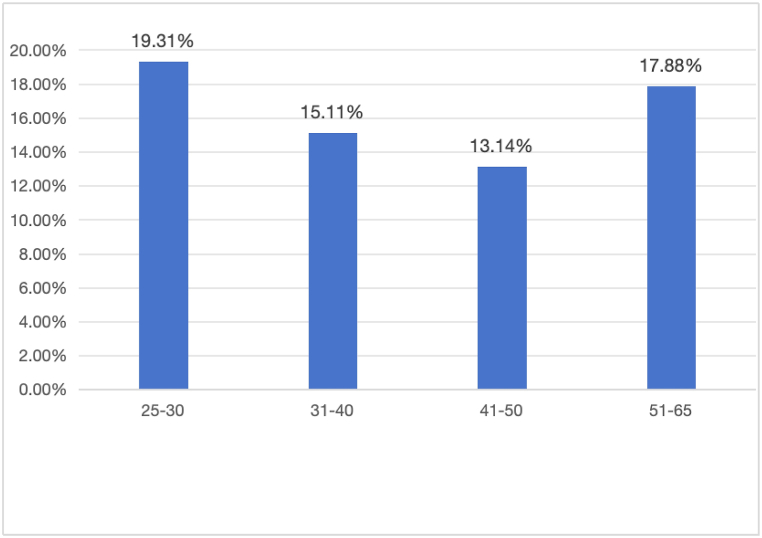
Fig. 2 shows infection rates of HR-HPV in different age groups. The highest infection rate was shown in the group of age 25–30 years (19.31 %), followed by 51–65 years (17.88 %), 31–40 years (15.11 %) and 41–50 years(13.14 %). Table 2 shows infection rates of each HR-HPV type in different age groups. In women aged 25–30, the top five infective types were HPV 16, 58, 52, 68, and 56. In ages 31–40 years, the top six infective types were HPV 52, 58, 16, 39, 59, and 68, in which the infection rates of HPV 59 and 68 were equal. In age 41–50 and 51–65, the top five were HPV 52, 58, 16, 39, and 51.
-
3.
Accuracy of HPV self-sampling
Fig. 2.
Infection rates of HR-HPV in different age groups
Abbreviations: HR-HPV, high-risk human cytomegalovirus.
Table 2.
Infection rates of each HR-HPV type in different age groups.
| Age(yr, no) | 25-30, 404 | 31-40, 1774 | 41-50, 1751 | 51-65, 1488 |
|---|---|---|---|---|
| HR-HPV type | No.(%) | No.(%) | No.(%) | No.(%) |
| 16 | 18(4.46) | 31(1.75) | 25(1.43) | 34(1.08) |
| 18 | 2(0.50) | 10(0.56) | 7(0.40) | 8(0.54) |
| 31 | 0(0) | 10(0.56) | 14(0.80) | 14(0.94) |
| 33 | 3(0.74) | 12(0.68) | 8(0.46) | 10(0.67) |
| 35 | 2(0.50) | 5(0.28) | 5(0.29) | 5(0.34) |
| 39 | 5(1.24) | 23(1.30) | 21(1.20) | 25(1.68) |
| 45 | 2(0.50) | 6(0.34) | 4(0.23) | 8(0.54) |
| 51 | 4(0.99) | 13(0.73) | 18(1.03) | 20(1.34) |
| 52 | 9(2.22) | 69(3.89) | 60(3.43) | 74(4.97) |
| 56 | 6(1.49) | 9(0.51) | 5(0.29) | 24(1.61) |
| 58 | 15(3.71) | 41(2.31) | 34(1.94) | 48(3.23) |
| 59 | 5(1.24) | 17(0.96) | 12(0.69) | 12(0.81) |
| 66 | 5(1.24) | 10(0.56) | 4(0.23) | 14(0.94) |
| 68 | 7(1.73) | 17(0.96) | 16(0.91) | 19(1.28) |
| Total | 83 | 273 | 233 | 315 |
Abbreviations: HR-HPV, high-risk human papilloma virus.
Table 3 summarizes the test results obtained using the two sampling methods. When testing for 23 HPV types, 4036 women were tested negative for HPV in both samples. Of women with HPV detected in both samples, 795(14.68 %) had a complete HPV type match, and 205 (3.78 %) were incompletely matched. 5 (0.09 %) were completely mismatched. In 92 women (1.70 %), one or more HPV types identified in clinician-collected samples were missed in self-collected samples, while in 284 women (5.24 %), HPV was identified in self-collected samples but missed in clinician-collected samples. The crude overall agreement was 93.06 % (95 % confidence interval [CI] = 0.924–0.937). When the results with incomplete matches were excluded, the overall agreement was 89.18 % (95 % CI = 0.883–0.900), kappa value was 0.798 (95 % CI = 0.778–0.994).
Table 3.
Test results obtained using the two sampling methods.
| Characteristics | Self-sampling | Clinician-sampling | No. (%) |
|---|---|---|---|
| Negative in both samples | Negative | Negative | 4036(74.51) |
| HPV detected in clinician-samples missed in self-samples | Positive | Negative | 92(1.70) |
| HPV detected in self-sampling missed in clinician-sampling | Negative | Positive | 284(5.24) |
| Complete match of HPV types in both samples | Positive | Positive | 795(14.68) |
| HPV detected in both samples but incomplete matched | Positive | Positive | 205(3.78) |
| HPV detected in both samples but completely mismatched | Positive | Positive | 5(0.09) |
Abbreviations: HPV, human papilloma virus.
When testing for HR-HPV, 4354 women were tested negative. 842 were tested positive in clinician-sampling, while 996 were tested positive in self-sampling. 669 women had completely matched genotypes in both sampling methods. 67 were clinician-sampling positive and self-sampling negative. 221 were positive for self-sampling and negative for clinician-sampling. 104 were incompletely matched and 2 were utterly mismatched (Table 4).
Table 4.
Test results of HR-HPV obtained using the two sampling methods.
| Characteristics | Self-sampling | Clinician-sampling | No. (%) |
|---|---|---|---|
| Negative in both samples | Negative | Negative | 4354(80.38) |
| HPV detected in clinician-samples missed in self-samples | Positive | Negative | 67(1.24) |
| HPV detected in self-sampling missed in clinician-sampling | Negative | Positive | 221(4.08) |
| Complete match of HPV types in both samples | Positive | Positive | 669(12.35) |
| HPV detected in both samples but incomplete matched | Positive | Positive | 104(1.92) |
| HPV detected in both samples but completely mismatched | Positive | Positive | 2(0.03) |
Abbreviations: HR-HPV, high-risk human papilloma virus.
Samples from women who were 25–30 years old showed the best concordance, the agreement was 95.79 % (95 % CI = 93.4–97.4), kappa value = 0.872 (95 % CI = 81.3–93.1). The agreement between self-sampling and physician-sampling in all age groups was more than 90 %, and the kappa value was higher than 0.8(Table 5).
-
4.
Acceptability of self-sampling
Table 5.
Agreement between self-sampling and physician-sampling in each age group.
| Characteristics |
Clinician-sampling |
Self-sampling |
Both positive | Both negative | Clinician-sampling positive and self-sampling negative | Clinician-sampling negative and self-sampling positive | Agreement(%) | 95%CI | Kappa value | p- value | 95%CI | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age(yr) | Positive | Negative | Positive | Negative | |||||||||
| 25–30 | 78 | 326 | 89 | 315 | 75 | 312 | 3 | 14 | 95.79 | (0.934,0.974) | 0.872 | 0.000 | (0.813,0.931) |
| 31–40 | 268 | 1506 | 323 | 1451 | 247 | 1430 | 21 | 76 | 94.53 | (0.934,0.955) | 0.803 | 0.000 | (0.766,0.840) |
| 41–50 | 230 | 1521 | 275 | 1476 | 211 | 1457 | 19 | 64 | 95.26 | (0.942,0.962) | 0.808 | 0.000 | (0.769,0.847) |
| 51–65 | 266 | 1222 | 309 | 1179 | 242 | 1155 | 24 | 67 | 93.88 | (0.926,0.950) | 0.804 | 0.000 | (0.765,0.843) |
| Total | 842 | 4575 | 996 | 4421 | 775 | 4354 | 67 | 221 | 94.68 | (0.941,0.953) | 0.812 | 0.000 | (0.790,0.834) |
Abbreviations: CI, confidence interval.
Table 6 presents the results of the understanding and acceptance questionnaires. Among 3163 participants, 52.89 % reported that they weren't aware of HPV self-sampling at all. 56.94 % reported that the instructions for self-sampling were easy or very easy to understand, and 53.52 % felt comfortable collecting the sample by themselves. However, 44.19 % of women were concerned about the accuracy of the self-sampling test results. Regarding participant preferences, 43.79 % preferred self-sampling. However, 67.59 % preferred doing self-sampling at a hospital, and only 23.08 % preferred it at home. A total of 2682 women indicated that they could accept HPV self-sampling as a method for cervical cancer screening. Age and monthly income were identified as the independent factors influencing women's acceptability (Table 7).
Table 6.
Understanding and acceptability of self-sampling versus clinician-sampling.
| Characteristics | No.(%) |
|---|---|
| Total | 3163 |
| Awareness of self-sampling | |
| Not aware at all | 1673(52.89) |
| Not aware much | 292(9.23) |
| General aware | 391(12.36) |
| Well aware | 164(5.18) |
| Full aware | 643(20.33) |
| Understanding of self-sampling instructions | |
| Very easy | 1250(39.52) |
| Easy | 551(17.42) |
| Moderate | 646(20.42) |
| Difficult | 168(5.31) |
| Very difficult | 548(17.33) |
| Comfort of self-sampling | |
| Uncomfortable at all | 462(14.61) |
| Not so well | 230(7.27) |
| Moderate | 778(24.60) |
| Comfortable | 563(17.80) |
| Very comfortable | 1130(35.72) |
| Pain | |
| Very painful | 371(11.73) |
| Painful | 167(5.28) |
| Moderate | 804(25.42) |
| Not so painful | 611(19.32) |
| No pain at all | 1210(38.25) |
| Privacy protection | |
| Not private at all | 226(7.15) |
| Not so private | 120(3.79) |
| Moderate | 598(18.91) |
| Private | 532(16.82) |
| Very private | 1687(53.34) |
| Embarrassment | |
| Very embarrassed | 357(11.29) |
| Embarrassed | 175(5.53) |
| Moderate | 675(21.34) |
| Not so embarrassed | 481(15.21) |
| Not embarrassed at all | 1475(46.53) |
| Concern about the accuracy of the result | |
| Not concern at all | 1005(24.15) |
| Not so concerned | 393(10.59) |
| Moderate | 666(21.06) |
| Concerned | 335(12.42) |
| Very concerned | 764(31.77) |
| Preference of self-sampling | |
| Not prefer at all | 760(24.03) |
| Not so preferred | 306(9.67) |
| Moderate | 712(22.51) |
| Prefer | 395(12.49) |
| Very prefer | 990(31.30) |
| Preferred sites for self-sampling HPV screening | |
| Hospital | 2138(67.59) |
| Clinics near home | 68(2.15) |
| Home | 730(23.08) |
| Regular physical examination center | 92(2.91) |
| Others | 135(4.27) |
Abbreviations: HPV, human papilloma virus.
Table 7.
Univariate and multivariate logistic regression analysis for acceptability of HPV self-sampling.
| Characteristics |
Acceptance |
Univariate analysis |
Multivariate analysis |
|||||
|---|---|---|---|---|---|---|---|---|
| Yes | No | p-value | OR | OR (95 % CI) | p-value | OR | OR (95 % CI) | |
| Age | <0.001 | 0.048 | ||||||
| 25–30 | 175 | 14 | Reference | |||||
| 31–40 | 889 | 117 | 0.091 | 0.608 | (0.341, 1.083) | 0.155 | 0.634 | (0.339, 1.187) |
| 41–50 | 913 | 180 | 0.002 | 0.406 | (0.230, 0.716) | 0.023 | 0.480 | (0.254, 0.905) |
| 51–65 | 705 | 170 | <0.001 | 0.332 | (0.188, 0.586) | 0.036 | 0.478 | (0.239, 0.953) |
| Education level | <0.001 | 0.446 | ||||||
| Primary school | 227 | 47 | Reference | Reference | ||||
| Junior high school | 564 | 135 | 0.437 | 0.865 | (0.600. 1.247) | 0.120 | 0.744 | (0.512, 1.08) |
| Senior high school | 516 | 109 | 0.917 | 0.980 | (0.673. 1.427) | 0.173 | 0.762 | (0.516, 1.126) |
| College or above | 1375 | 190 | 0.023 | 1.498 | (1.057, 2.124) | 0.318 | 0.812 | (0.539, 1.222) |
| Monthly income (Chinese Yuan)* | <0.001 | 0.003 | ||||||
| <3000 | 985 | 242 | Reference | Reference | ||||
| 3001–5000 | 840 | 139 | <0.001 | 1.485 | (1.182, 1.865) | 0.040 | 1.308 | (1.012, 1.690) |
| 5001–8000 | 528 | 58 | <0.001 | 2.237 | (1.648, 3.305) | <0.001 | 1.938 | (1.354, 2.773) |
| >8000 | 329 | 42 | <0.001 | 1.925 | (1.355, 2.733) | 0.018 | 1.644 | (1.088, 2.483) |
| Marital status | 0.004 | 0.147 | ||||||
| Unmarried | 103 | 9 | Reference | Reference | ||||
| Married | 2474 | 441 | 0.042 | 0.490 | (0.246, 0.976) | 0.799 | 0.905 | (0.420, 1.951) |
| Divorce | 83 | 20 | 0.018 | 0.363 | (0.157, 0.838) | 0.341 | 0.643 | (0.260, 1.594) |
| Widowed | 22 | 11 | <0.001 | 0.175 | (0.065, 0.472) | 0.131 | 0.438 | (0.150, 1.278) |
| Medical insurance | 0.650 | 0.893 | (0.547, 1.458) | 0.970 | 0.990 | (0.601, 1.633) | ||
| Yes | 2582 | 461 | ||||||
| No | 100 | 20 | ||||||
| Menstrual status | <0.001 | 0.626 | (0.509, 0.768) | 0.209 | 0.817 | (0.596, 1.120) | ||
| Not in menopause | 1994 | 310 | ||||||
| Menopause | 688 | 171 | ||||||
Abbreviations: HPV, human papilloma virus.
4. Discussion
Despite several studies on HPV self-sampling have been implemented, this study is the first to cover all regions in China simultaneously [[17], [18], [19], [20]]. China's vast size, varied natural environments, and diverse economic and medical development levels make finding an effective and convenient cervical cancer screening method crucial for advancing the WHO's strategy to eliminate cervical cancer. Beijing and Inner Mongolia represent northern China, Shandong Eastern China, Qinghai, Xinjiang and Tibet Western China, Hubei Central China, and Yunnan Southern China. Moreover, the above areas are different in economic development levels, medical resources, natural environment, and ethnic distribution. Ethnic minority women such as Mongolian, Tibetan, and Uyghur comprised more than 20 % of all women in this study. Both self- and clinician-collected samples from different parts of China remained valid when mailed to the laboratory in Beijing, which indicates that the sampler we used could overcome problems caused by high altitude, long-distance, and long-time transportation.
According to this analysis, the positive rates of self-sampling and clinician-sampling are 18.47 % and 14.66 %, respectively. HPV self-sampling has detected more positive results than clinician-sampling, which may associated with the process of sampling. Gynecologists used a speculum to open the vagina and brush directly on the surface of the cervix to get isolated cells, whereas, in the process of self-sampling, women put the swab into their vagina without a speculum. The swab might collect isolated cells not only from the cervix but also from the vulva and vagina, suggesting that self-sampling swabs capture a broader range and greater quantity of cells compared to clinician-sampling brushes. Incidences of vulva and vagina cancer are increasing [21,22], but there is no routine screening method for these cancers. HPV self-sampling may help to find HPV infection and lesions of vulva and vagina.
Our data showed that the top five frequent high-risk genotypes were HPV 52, 58, 16, 39, and 68, which was different from the results of several published studies previously [17,23]. HPV types 16 and 18 were considered the most pathogenic and were included in both the two-valent and four-valent HPV vaccines [[24], [25], [26], [27]]. Given the different characteristics of the Chinese population and the expansion of HPV vaccination, the predominant infection types of HPV may change, and screening guidelines may be adjusted. Women older than 50 years had a higher HPV infection rate than women aged 31–50 years. The recommendations for vaccination may be changed in the future as well.
Most women enrolled in this study expressed a high acceptance and preference for HPV self-sampling, and they could understand how to collect samples by themselves. This suggests that HPV self-sampling has a broad prospect for application in China, especially for remote, ethnic minority and less developed areas. For women who are HPV-positive without cervical precancerous lesions but require follow-up, HPV self-sampling offers a convenient follow-up method for monitoring the persistence of the same HPV genotype. Additionally, the HPV assay used in this study could perform HPV genotyping, allowing for the triage of positive women and saving costs and medical resources. With the increase in HPV vaccination and cervical screening rates [21], a greater focus on screening the unscreened/hard-to-reach populations may improve the risk/benefit ratio of cervical cancer screening programs.
Despite the high level of acceptance of HPV self-sampling among women, more than 60 % had never heard of what self-sampling was. The lack of awareness of HPV self-sampling is also one of the critical reasons for it's limited application. More than half of the women enrolled in this study felt self-sampling comfortable, private and not embarrassing, and showed their preference for self-sampling. Younger women and those with higher incomes demonstrated greater acceptance of self-sampling, similar to He et al.'s study [28]. Those women were more likely to know about HPV and related healthcare knowledge. A study involving a predominantly Hispanic population living along the United States-Mexico border found that once participants were educated about the test, they showed a high level of acceptance for self-sampling [29]. Clinicians and social health workers are needed to carry out propaganda on HPV self-sampling to promote and improve the participation of cervical screening. Women also expressed their preference for doing self-sampling in hospitals and concerns about the accuracy of self-sampling results, indicating the importance of clinicians' instruction. Furthermore, the follow-up management of positive screening results is a matter of great account. When women take self-sampling at home and mail the sample to the testing institution, special personnel to inform them of abnormal results and treatment methods are needed to avoid missed diagnoses of cervical cancer and precancerous lesions. Recently, new HPV testing methods, such as urine and menstrual blood sampling, have been developed for cervical cancer screening and have demonstrated high accuracy. However, these studies' had small sample sizes and needed further validation in larger populations. These new approaches hold promise for increasing participation rates in cervical cancer screening among women from diverse regions, religious beliefs, and cultures [30,31].
The strength of this study is that it covers women from all regions of China as well as different ethnic groups. However, there are also several limitations of this study. One weakness is that the outcome of cervical HPV infection remains unknown. However, we have built a platform from which we can follow and manage women's data over the long term. Moreover, our study enrolled women who went to the hospital only, which may weaken its representation given that some women attended cervical screening at community health centers in China.
In conclusion, this study shows the high consistency between HPV self- and clinician-sampling. HPV self-sampling was widely accepted by women in different regions of China and could be an effective alternative to traditional cervical screening methods. Professional gynecologists and social health workers are needed to publicize self-sampling and inform women with abnormal results. The HPV infection data represented by this analysis exhibited some unique features. New vaccination and cervical screening recommendations should be tailored to different population characteristics, which may have higher socioeconomic value.
CRediT authorship contribution statement
Xuechao Ji: Writing – original draft, Formal analysis, Data curation, Conceptualization. Menglin Hao: Formal analysis, Data curation. Yixiao Wang: Methodology, Funding acquisition. Zangyu Pan: Methodology, Formal analysis. Ruiye Yang: Methodology, Formal analysis. Xinbo Wang: Data curation. Hui Wang: Data curation. Chunlian Zhang: Data curation. Yiqun Zhang: Data curation. Xumei Zhang: Data curation. Ge Yang: Data curation. Sarendalai: Data curation. Tunala: Data curation. Jinwei Miao: Writing – review & editing, Funding acquisition, Conceptualization.
Data availability
The data supporting this study's findings are available from the corresponding authors upon reasonable request.
Ethics statements
The Medical Ethics Committee of Beijing Obstetrics and Gynecology Hospital approved this study (No.2022-KY043-01).
Ethics approval statement
The Human Subjects Review Boards of Beijing Obstetrics and Gynecology Hospital approved this study (No.2022-KY043-01).
Patient consent statement
All participants provided written informed consent.
Funding
Beijing Municipal Health Commission, China Capital's Funds for Health Improvement and Research (Grant number: 2022-1G-2112); Beijing Hospitals Authority's, China Ascent Plan (Grant number: DFL20221201); Demonstration Construction Project of Clinical Research Ward of Beijing Obstetrics and Gynecology Hospital, Capital Medical University, China (Grant number: BCRW202109); Laboratory for Clinical Medicine, Capital Medical University.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We thank Chaozhou Hybribio Biochemistry Ltd for assistance in offering 23 HPV Genotyping Real-time PCR Kits.
References
- 1.Bray F., Laversanne M., Sung H., Ferlay J., Siegel R.L., Soerjomataram I., Jemal A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Ca - Cancer J. Clin. 2024;74:229–263. doi: 10.3322/caac.21834. [DOI] [PubMed] [Google Scholar]
- 2.Mix J.M., Van Dyne E.A., Saraiya M., Hallowell B.D., Thomas C.C. Assessing impact of HPV vaccination on cervical cancer incidence among women aged 15-29 Years in the United States, 1999-2017: an ecologic study. Cancer Epidemiol Biomark Prev Publ Am Assoc Cancer Res Cosponsored Am Soc Prev Oncol. 2021;30:30–37. doi: 10.1158/1055-9965.EPI-20-0846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Singh D., Vignat J., Lorenzoni V., Eslahi M., Ginsburg O., Lauby-Secretan B., et al. Global estimates of incidence and mortality of cervical cancer in 2020: a baseline analysis of the WHO Global Cervical Cancer Elimination Initiative. Lancet Glob Health. 2023;11:e197–e206. doi: 10.1016/S2214-109X(22)00501-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Han B., Zheng R., Zeng H., Wang S., Sun K., Chen R., et al. J Natl Cancer Cent; 2022. Cancer Incidence and Mortality in China. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arbyn M., Weiderpass E., Bruni L., de Sanjosé S., Saraiya M., Ferlay J., et al. Estimates of incidence and mortality of cervical cancer in 2018: a worldwide analysis. Lancet Glob Health. 2020;8:e191–e203. doi: 10.1016/S2214-109X(19)30482-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sun K., Zheng R., Lei L., Zhang S., Zeng H., Wang S., et al. Trends in incidence rates, mortality rates, and age-period-cohort effects of cervical cancer — China, 2003–2017. China CDC Wkly. 2022;4:1070–1076. doi: 10.46234/ccdcw2022.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen W., Zheng R., Baade P.D., Zhang S., Zeng H., Bray F., et al. Cancer statistics in China, 2015. Ca - Cancer J. Clin. 2016;66:115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 8.Global strategy to accelerate the elimination of cervical cancer as a public health problem. https://www.who.int/publications-detail-redirect/9789240014107
- 9.Jiang M., Chen S., Yan X., Ying X., Tang S. The coverage and challenges of increasing uptake of non-National Immunization Program vaccines in China: a scoping review. Infect Dis Poverty. 2023;12:114. doi: 10.1186/s40249-023-01150-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Song D., Liu P., Wu D., Zhao F., Wang Y., Zhang Y. Knowledge and attitudes towards human papillomavirus vaccination (HPV) among healthcare providers involved in the governmental free HPV vaccination program in shenzhen, southern China. Vaccines. 2023;11:997. doi: 10.3390/vaccines11050997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crum C.P., Abbott D.W., Quade B.J. Cervical cancer screening: from the Papanicolaou smear to the vaccine era. J Clin Oncol Off J Am Soc Clin Oncol. 2003;21:224s. doi: 10.1200/JCO.2003.01.116. 30s. [DOI] [PubMed] [Google Scholar]
- 12.National Bureau of Statistics of China 2021 China statistical yearbook. 2021 May 11. https://www.stats.gov.cn/sj/pcsj/rkpc/d7c/202111
- 13.Progress of the "two cancer" screening project for rural women. http://www.nhc.gov.cn/jkfpwlz/gzdt1ur/201902/6a19776dd4374223a07dfe9f76ed5157.shtml
- 14.Schiffman M.H., Bauer H.M., Hoover R.N., Glass A.G., Cadell D.M., Rush B.B., et al. Epidemiologic evidence showing that human papillomavirus infection causes most cervical intraepithelial neoplasia. J Natl Cancer Inst. 1993;85:958–964. doi: 10.1093/jnci/85.12.958. [DOI] [PubMed] [Google Scholar]
- 15.Serrano B., Ibáñez R., Robles C., Peremiquel-Trillas P., de Sanjosé S., Bruni L. Worldwide use of HPV self-sampling for cervical cancer screening. Prev. Med. 2022;154 doi: 10.1016/j.ypmed.2021.106900. [DOI] [PubMed] [Google Scholar]
- 16.Yin J., Cheng S., Liu D., Tian Y., Hu F., Zhang Z., Zhu T., Su Z., Liu Y., Wang S., Liu Y., Peng S., Li L., Xu S., Zhang C., Qiao Y., Chen W. Head-to-head comparison of 7 high-sensitive human papillomavirus nucleic acid detection technologies with the SPF10 LiPA-25 system. J Natl Cancer Cent. 2022;2:148–154. doi: 10.1016/j.jncc.2022.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Du H., Luo H., Wang C., Qu X., Belinson J.L., Wu R. The prevalence of HR-HPV infection based on self-sampling among women in China exhibited some unique epidemiologic features. J. Clin. Epidemiol. 2021;139:319–329. doi: 10.1016/j.jclinepi.2021.06.009. [DOI] [PubMed] [Google Scholar]
- 18.Li J., Wu R., Qu X., Huang X., Li L., Lin Z., et al. Effectiveness and feasibility of self-sampling for human papillomavirus testing for internet-based cervical cancer screening. Front. Public Health. 2022;10 doi: 10.3389/fpubh.2022.938272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goldstein A., Goldstein L.S., Lipson R., Bedell S., Wang J., Stamper S.A., et al. Assessing the feasibility of a rapid, high-volume cervical cancer screening programme using HPV self-sampling and digital colposcopy in rural regions of Yunnan, China. BMJ Open. 2020;10 doi: 10.1136/bmjopen-2019-035153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goldstein A., Lei Y., Goldstein L., Goldstein A., Bai Q.X., Felix J., et al. A rapid, high-volume cervical screening project using self-sampling and isothermal PCR HPV testing. Infect Agent Cancer. 2020;15:64. doi: 10.1186/s13027-020-00329-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu S., Xu X., Zhang Y., Liu Y., Yang C., Wang Y., et al. A nationwide post-marketing survey of knowledge, attitude and practice toward human papillomavirus vaccine in general population: implications for vaccine roll-out in mainland China. Vaccine. 2021;39:35–44. doi: 10.1016/j.vaccine.2020.11.029. [DOI] [PubMed] [Google Scholar]
- 22.Li X., Xie H., Fu Y., Zhang X., Dong X., Ji Y., et al. Epidemiology characteristics and potential clinical value of vulvar human papillomavirus in Chinese women: a multicenter cross-sectional study. Arch. Pathol. Lab Med. 2023 doi: 10.5858/arpa.2023-0255-OA. [DOI] [PubMed] [Google Scholar]
- 23.Bray F., Laversanne M., Weiderpass E., Arbyn M. Geographic and temporal variations in the incidence of vulvar and vaginal cancers. Int. J. Cancer. 2020;147:2764–2771. doi: 10.1002/ijc.33055. [DOI] [PubMed] [Google Scholar]
- 24.Zhu B., Liu Y., Zuo T., Cui X., Li M., Zhang J., et al. The prevalence, trends, and geographical distribution of human papillomavirus infection in China: the pooled analysis of 1.7 million women. Cancer Med. 2019;8:5373–5385. doi: 10.1002/cam4.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Muñoz N., Bosch F.X., de Sanjosé S., Herrero R., Castellsagué X., Shah K.V., et al. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N. Engl. J. Med. 2003;348:518–527. doi: 10.1056/NEJMoa021641. [DOI] [PubMed] [Google Scholar]
- 26.Clifford G.M., Smith J.S., Aguado T., Franceschi S. Comparison of HPV type distribution in high-grade cervical lesions and cervical cancer: a meta-analysis. Br. J. Cancer. 2003;89:101–105. doi: 10.1038/sj.bjc.6601024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Castellsagué X., Díaz M., de Sanjosé S., Muñoz N., Herrero R., Franceschi S., et al. Worldwide human papillomavirus etiology of cervical adenocarcinoma and its cofactors: implications for screening and prevention. J Natl Cancer Inst. 2006;98:303–315. doi: 10.1093/jnci/djj067. [DOI] [PubMed] [Google Scholar]
- 28.He L., He J. Attitudes towards HPV self-sampling among women in Chengdu, China: a cross-sectional survey. J. Med. Screen. 2020;27(4):201–206. doi: 10.1177/0969141319895543. [DOI] [PubMed] [Google Scholar]
- 29.Penaranda E., Molokwu J., Flores S., Byrd T., Brown L., Shokar N. Women's attitudes toward cervicovaginal self-sampling for high-risk HPV infection on the US-Mexico border. J. Low. Genit. Tract Dis. 2015;19(4):323–328. doi: 10.1097/LGT.0000000000000134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Daponte A., Michail G., Daponte A.I., et al. Urine HPV in the context of genital and cervical cancer screening-an update of current literature. Cancers. 2021;13(7) doi: 10.3390/cancers13071640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martinelli M., Giubbi C., Sechi I., et al. Evaluation of bd onclarity HPV assay on self-collected vaginal and first-void urine samples as compared to clinician-collected cervical samples: a pilot study. Diagnostics. 2022;12(12) doi: 10.3390/diagnostics12123075. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data supporting this study's findings are available from the corresponding authors upon reasonable request.