To the Editor:
Young persons with youth-onset type 2 diabetes (T2D), particularly males, are disproportionately affected by hyperuricemia compared with peers without diabetes and those with type 1 diabetes (T1D).1 Research on the effects of reductions in SUA levels on kidney and cardiovascular outcomes in youth-onset T2D is limited. Existing trials have primarily focused on xanthine oxidase inhibitors, achieving SUA level reductions of 1-4 mg/dL, which may not significantly affect cardio-renal outcomes.2, 3, 4
Uricases like rasburicase and pegloticase rapidly break down uric acid into the water-soluble allantoin.5 This pilot trial aimed to determine the impact of rapid and substantial lowering of SUA levels with pegloticase on markers of kidney and cardiovascular health in young adult men with T2D.
Methods
We conducted an open-label, non-placebo-controlled pilot trial, recruiting young adult men from diabetes clinics at the University of Colorado Hospital and Children’s Hospital Colorado. The trial (registration number NCT03899883) was approved by the Colorado Multiple Institutional Review Board. All participants provided written informed consent.
Participants
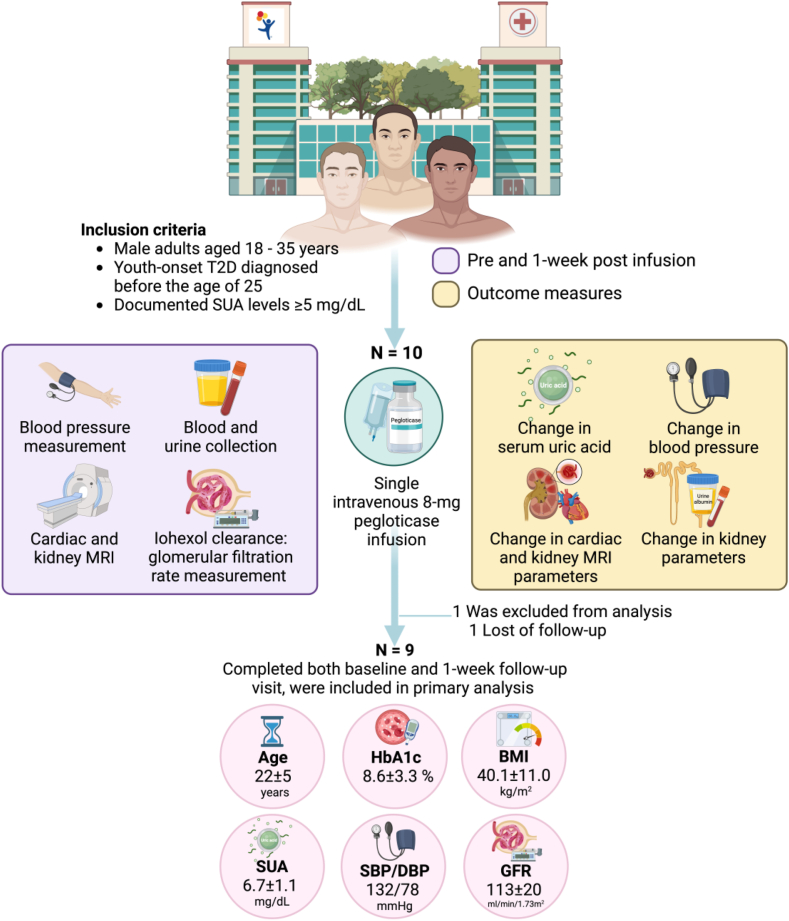
Enrollment was limited to male adults aged 18 to 35 years with T2D diagnosed before age 25 and SUA levels ≥ 5 mg/dL. Participants using other uric acid-lowering medications, with glucose-6-phosphate dehydrogenase deficiency, recent diabetic ketoacidosis, or hyperglycemic hyperosmolar episodes were excluded. The full inclusion and exclusion criteria are listed in Table S1.
Trial Procedures
Participants followed a 3-day controlled diet and refrained from strenuous activity before admission. Baseline measurements included fasting urine and blood collections, blood pressure, iohexol clearance for glomerular filtration rate (GFR) and cardiac and kidney magnetic resonance imaging (MRI). A single-dose of pegloticase (8 mg) was infused intravenously. Baseline measures were repeated 1 week later under the same conditions. Full detail of trial procedures are described in Item S1.
Results
Nine participants completed both baseline and follow-up visits (Fig 1 and Table S2). One week post-pegloticase, median (p25, p75) SUA levels decreased from 6.4 (5.6, 7.6) to 0.5 (0.5, 4.6) mg/dL (P = 0.01), and urinary uric acid levels decreased from 52.0 (34.0, 73.9) to 5.5 (2.8, 19.4) mg/dL (P = 0.004). GFR increased in all participants, irrespective of their baseline GFR (Fig S1), increasing from a mean (± standard deviation [SD]) of 154 ± 22 to 172 ± 32 mL/min (P = 0.005) and from 113 ± 20 to 122 ± 19 mL/min per 1.73 m2 (P = 0.004). Diastolic blood pressure increased from 77 ± 9 to 85 ± 11 mm Hg (P = 0.04), and end-diastolic volume increased from 136 ± 31 to 145 ± 25 mL (P = 0.02). Peak longitudinal cardiac strain (Fig S2) and mean wall shear stress decreased from 15.6 ± 2.0% to 13.4 ± 2.2% (P = 0.02), and 1.5 ± 0.2 to 1.4 ± 0.1 Pa (P = 0.05), respectively. Serum bilirubin increased from 0.6 ± 0.2 to 0.9 ± 0.3 mg/dL (P = 0.005). Comparison between baseline and 1-week variables is shown in Table 1.
Figure 1.
Study design.
Table 1.
Comparison of Baseline versus 1-Week Variables of Interest Between All Participants and Pegloticase Responders
| Measurement | All Participants |
P valueb | Pegloticase Responders |
P valueb | ||
|---|---|---|---|---|---|---|
| Baseline, N = 9a | 1 week, N = 9a | Baseline, N = 7a | 1 week, N = 7a | |||
| Weight (kg) | 123 ± 35 | 122 ± 34 | 0.44 | 113 ± 28 | 113 ± 28 | 0.79 |
| Metabolic measures | ||||||
| Serum uric acid (mg/dL) | 6.4 (5.6, 7.6) | 0.5 (0.5, 4.6) | 0.01c | 6.4 (5.5, 7.8) | 0.5 (0.5, 0.5) | 0.02c |
| Fasting blood glucose (mg/dL) | 157 ± 71 | 144 ± 46f | 0.30 | 139 ± 44 | 152 ± 51d | >0.99 |
| Serum bilirubin (mg/dL) | 0.6 ± 0.2 | 0.9 ± 0.3 | 0.005 | 0.6 ± 0.2 | 1.0 ± 0.3 | 0.02 |
| Renal measures | ||||||
| Serum urea nitrogen | 13.2 ± 3.4 | 13.4 ± 4.9 | 0.86 | 12.7 ± 2.3 | 13.0 ± 3.2 | 0.85 |
| Serum creatinine (mg/dL) | 0.8 ± 0.2 | 0.7 ± 0.3 | 0.14 | 0.8 ± 0.2 | 0.7 ± 0.2 | 0.06 |
| Serum cystatin C (mg/L) | 0.9 ± 0.2g | 0.9 ± 0.1g | 0.79 | 0.8 ± 0.2 | 0.9 ± 0.1 | 0.73 |
| Urine uric acid (mg/dL) | 52.0 (34.0, 73.9) | 5.5 (2.8, 19.4) | 0.004c | 52.0 (37.8, 69.6) | 2.8 (2.8, 8.2) | 0.02c |
| Urine creatinine (mg/dL) | 135.8 ± 95.0 | 176.0 ± 157.7 | 0.17 | 144.0 ± 106.7 | 202.1 ± 170.2 | 0.12 |
| Urine microalbumin (μg) | 30.4 (7.7, 312.0) | 33.2 (13.5, 156.5)g | 0.11c | 21.9 (6.1, 171.2) | 22.1 (11.8, 90.0)e | 0.16c |
| Urine albumin-creatinine ratio (UACR) | 18 (9, 578) | 21 (6, 211)g | 0.84c | 15 (8, 298) | 10 (6, 38)e | 0.44c |
| Iohexol glomerular filtration rate (GFR) measures | ||||||
| Absolute GFR (mL/min) | 154 ± 22g | 172 ± 32 | 0.005 | 159 ± 18 | 169 ± 17 | 0.01 |
| GFR standardized body surface area (mL/min/1.73 m2) | 113 ± 20g | 122 ± 19 | 0.004 | 117 ± 17 | 125 ± 16 | 0.009 |
| 2D PC-MRI | ||||||
| Renal artery (mL/cycle) | 18.4 ± 5.0g | 18.2 ± 5.3g | 0.81 | 19.9 ± 4.9e | 20.1 ± 4.5e | 0.84 |
| Renal vein (mL/cycle)∗ | 18.3 ± 4.9g | 16.8 ± 3.6g | 0.32 | 19.5 ± 4.2e | 17.6 ± 3.0e | 0.31 |
| Cardiovascular measures | ||||||
| Systolic blood pressure (SBP) | 133 ± 10 | 132 ± 9 | 0.38 | 133 ± 12 | 130 ± 10 | 0.29 |
| Diastolic blood pressure (DBP) | 77 ± 9 | 85 ± 11 | 0.04 | 74 ± 7 | 82 ± 10 | 0.13 |
| Mean arterial pressure (MAP) | 96 ± 7 | 101 ± 10 | 0.06 | 94 ± 6 | 98 ± 9 | 0.19 |
| Cardiac MRI | ||||||
| End-diastolic volume (mL) | 136.2 ± 31.5f | 144.6 ± 24.6g | 0.02 | 145.1 ± 22.8e | 154.5 ± 16.9e | 0.06 |
| End systolic volume (mL) | 57.3 ± 12.8f | 60.8 ± 18.5g | 0.50 | 60.8 ± 9.7e | 66.1 ± 17.2e | 0.47 |
| Stroke volume (mL) | 79.0 ± 25.5f | 83.8 ± 14.6g | 0.45 | 84.4 ± 23.1e | 88.4 ± 13.7e | 0.67 |
| Ejection fraction | 57.3 ± 7.6f | 58.5 ± 7.7g | 0.75 | 57.5 ± 8.3e | 57.5 ± 8.3e | 0.99 |
| Cardiac output (L/min) | 6.4 ± 1.8f | 7.0 ± 0.9g | 0.41 | 6.7 ± 1.7e | 7.0 ± 1.1e | 0.64 |
| Peak radial strain (%) | 28.5 ± 3.1f | 27.3 ± 6.4f | 0.69 | 28.7 ± 3.3e | 26.1 ± 6.1e | 0.39 |
| Peak circumferential strain (%) | 17.2 ± 1.3f | 16.6 ± 2.5f | 0.58 | 17.3 ± 1.4e | 16.2 ± 2.5e | 0.35 |
| Peak longitudinal strain (%) | 15.6 ± 2.0f | 13.4 ± 2.2f | 0.02 | 15.4 ± 2.2e | 13.1 ± 2.2e | 0.03 |
| Aortic 4D flow MRI | ||||||
| Pulse wave velocity (m/s) | 4.8 ± 1.4f | 4.3 ± 0.8f | 0.31 | 4.4 ± 0.9e | 4.1 ± 0.5e | 0.56 |
| Mean wall shear stress (Pa) | 1.5 ± 0.2f | 1.4 ± 0.1f | 0.046 | 1.5 ± 0.2e | 1.4 ± 0.1e | 0.05 |
| Maximum wall shear stress (Pa) | 2.6 ± 0.3f | 2.5 ± 0.3f | 0.32 | 2.6 ± 0.4e | 2.6 ± 0.4e | 0.53 |
Note: In instances where sample sizes differ between baseline and 1 week, the reported P values are based on the smaller sample size, as incomplete pairs of data were excluded from the analyses. Bold values were considered statistically significant (P < 0.05).
Descriptive statistics are reported as mean ± SD or median (p25, p75).
Paired t-test.
Wilcoxon signed rank test with continuity correction; Wilcoxon signed rank exact test.
Data were collected from 5 participants.
Data were collected from 6 participants.
Data were collected from 7 participants.
Data were collected from 8 participants.
Kidney MRI data focusing on renal blood flow (Fig S3) found a positive correlation between increase in GFR and renal artery blood flow (r = 0.60, P = 0.24) as well as venous blood flow (r = 0.89, P = 0.03) in participants with a reduction in SUA levels of at least 3 mg/dL after pegloticase infusion (Fig S4A-B). Additionally, the reduction in SUA levels correlated with increased renal artery (r = –0.31, P = 0.56) and venous blood flow (r = –0.99, P = 0.003) and the increase in GFR (r = –0.79, P = 0.05) (Fig S4C-E). The correlation between the reduction in SUA levels and the increase in GFR was similar across all participants (r = –0.69, P = 0.07).
No serious adverse events occurred in this study (Table S3).
Discussion
In young men with T2D and elevated SUA levels, pegloticase infusion rapidly reduced SUA levels and consistently increased GFR across all participants, irrespective of their baseline kidney function, likely ascribed to improved renal blood flow. Cardiac MRI showed worsening of cardiac function, evidenced by reduced peak longitudinal strain, with an increase in diastolic blood pressure observed 1 week following pegloticase administration.
The FEATHER, PERL, and CKD-FIX studies showed no effects of SUA reduction on GFR with xanthine oxidase inhibitors in adults with T1D and T2D.2, 3, 4 Similarly, the SAPPHIRE study found no nephroprotective effects from SUA reduction in diverse chronic kidney disease populations.6 These discrepancies may stem from differences in study populations given that prior trials involved later-stage chronic kidney disease, whereas our study involved individuals with normal-to-elevated GFR. Additionally, our study used pegloticase, an infused uricase, leading to greater reductions in SUA levels compared with xanthine oxidase inhibitors or uricosuric agents. This decrease was associated with improved renal blood flow and GFR. Given that uric acid is thought to cause arteriolar vasoconstriction, lowering SUA levels with pegloticase may relieve this vasoconstriction, as indicated by reduced wall shear stress. These changes may enhance renal blood flow and GFR, supporting its renoprotective effects on renal hemodynamics.7
Cardiac MRI data suggest a potential adverse impact of pegloticase infusion on cardiac function, possibly due to the rapid reduction in the levels of SUA, a major plasma antioxidant, causing an imbalance between oxidative stress and antioxidant.8,9 Mild adverse events, such as muscle pain and respiratory distress, occurred during infusion and resolved afterward. A significant increase in serum bilirubin suggested subclinical hemolysis.
Strengths of our study include effective urate lowering and comprehensive cardiac and kidney evaluations. However, the small sample size, specific participant group, and lack of control participants limit the generalizability of our findings. Larger randomized controlled trials are needed to validate pegloticase’s long-term safety and benefits on kidney and cardiovascular health in T2D patients.
Article Information
Authors’ Contributions
PN, SP, AJB, and PB designed the study. PN, SP, CRJ, AJB, and PB analyzed the data. AJB and PB researched the data. Each author contributed important intellectual content during article drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Support
This work was supported by Horizon Pharma and Colorado Clinical and Translational Sciences Institute microgrant.
Financial Disclosure
Author Bjornstad reports serving or having served as a consultant for AstraZeneca, Bayer, Bristol-Myers Squibb, Boehringer Ingelheim, Eli-Lilly, LG Chemistry, Sanofi, Novo Nordisk, and Horizon Pharma. Author Bjornstad also serves or has served on the advisory boards and/or steering committees of AstraZeneca, Bayer, Boehringer Ingelheim, Novo Nordisk, and XORTX. Author Nadeau has received device and drug donation from Abbott and Novo Nordisk. Author Antenor reports serving or having served as a consultant for Horizon Pharma. All other authors declare that they have no relevant financial interests.
Peer Review
Received June 10, 2024. Evaluated by 2 external peer reviewers, with direct editorial input from the Statistical Editor and the Editor-in-Chief. Accepted in revised form September 11, 2024.
Footnotes
Figure S1: Pre- and post-treatment changes in absolute GFR (A) and BSA-standardized GFR (B).
Figure S2: Schematic of cardiac MRI analyses. (A) Left ventricle (LV) endo- and epi-cardial contours and right ventricle (RV) endo-cardial contour were semi-automatically drawn, and 3D volumetric information was reconstructed. Global function pre- and post-treatment were subsequently calculated from the results of end-systole and end-diastole. (B) The magnitude of peak strain was also obtained using time-resolved strain curves. Color shadows indicate the minimum and maximum values of each strain within the overall group.
Figure S3: Schematic of kidney MRI analyses. (A) Contours were manually drawn based on the time-resolved phase and magnitude images. (B) Total flow volumes were extracted from the left renal vein and artery, and the right renal vein and artery. (C) An example of the time-resolved flow rate from the left renal vein.
Figure S4: Correlation between (A) changes in renal artery blood flow obtained from 2D PC-MRI flow measurement and changes in absolute GFR, (B) changes in renal vein blood flow obtained from 2D PC-MRI flow measurement and changes in absolute GFR, (C) changes in renal artery blood flow obtained from 2D PC-MRI flow measurement and changes in SUA, (D) changes in renal vein blood flow obtained from 2D PC-MRI flow measurement and changes in SUA levels, (E) changes in SUA and changes in absolute GFR in participants with a reduction in SUA levels of at least 3 mg/dL after pegloticase infusion. Note: One post-infusion 2D PC kidney MRI was excluded from analysis due to the presence of image artifact affecting the reliability of the measurement, resulting in fewer than 7 scatter points being displayed in panels A-D.
Item S1: Methods.
Table S1: Inclusion and Exclusion Criteria.
Table S2: Baseline Characteristics at Screening Visit.
Table S3: Safety Outcomes of Special Interest.
Supplementary Material
Figures S1-S4; Item S1; Tables S1-S3.
References
- 1.Bjornstad P., Laffel L., Lynch J., et al. Elevated serum uric acid is associated with greater risk for hypertension and diabetic kidney diseases in obese adolescents with type 2 diabetes: an observational analysis from the Treatment Options for Type 2 Diabetes in Adolescents and Youth (TODAY) Study. Diabetes Care. 2019;42:1120–1128. doi: 10.2337/dc18-2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maahs D.M., Caramori L., Cherney D.Z., et al. Uric acid lowering to prevent kidney function loss in diabetes: the preventing early renal function loss (PERL) allopurinol study. Curr Diab Rep. 2013;13:550–559. doi: 10.1007/s11892-013-0381-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kimura K., Hosoya T., Uchida S., et al. Febuxostat therapy for patients with stage 3 CKD and asymptomatic hyperuricemia: a randomized trial. Am J Kidney Dis. 2018;72:798–810. doi: 10.1053/j.ajkd.2018.06.028. [DOI] [PubMed] [Google Scholar]
- 4.Badve S.V., Pascoe E.M., Tiku A., et al. Effects of allopurinol on the progression of chronic kidney disease. N Engl J Med. 2020;382:2504–2513. doi: 10.1056/NEJMoa1915833. [DOI] [PubMed] [Google Scholar]
- 5.Biggers K., Scheinfeld N. Pegloticase, a polyethylene glycol conjugate of uricase for the potential intravenous treatment of gout. Curr Opin Investig Drugs. 2008;9:422–429. [PubMed] [Google Scholar]
- 6.Heerspink H.J.L., Stack A.G., Terkeltaub R., et al. Combination treatment with verinurad and allopurinol in CKD: a randomized placebo and active controlled trial. J Am Soc Nephrol. 2024;35(5):594–606. doi: 10.1681/ASN.0000000000000326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Uedono H., Tsuda A., Ishimura E., et al. Relationship between serum uric acid levels and intrarenal hemodynamic parameters. Kidney Blood Press Res. 2015;40:315–322. doi: 10.1159/000368507. [DOI] [PubMed] [Google Scholar]
- 8.Fabbrini E., Serafini M., Colic Baric I., Hazen S.L., Klein S. Effect of plasma uric acid on antioxidant capacity, oxidative stress, and insulin sensitivity in obese subjects. Diabetes. 2014;63:976–981. doi: 10.2337/db13-1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sautin Y.Y., Johnson R.J. Uric acid: the oxidant-antioxidant paradox. Nucleosides Nucleotides Nucleic Acids. 2008;27:608–619. doi: 10.1080/15257770802138558. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figures S1-S4; Item S1; Tables S1-S3.