Abstract
Rationale & Objective
Cilastatin is an inhibitor of drug metabolism in the proximal tubule that demonstrates nephroprotective effects in animals. It has been used in humans in combination with the antibiotic imipenem to block imipenem’s renal metabolism. This systematic review and meta-analysis evaluated the nephroprotective effects of cilastatin in humans.
Study Design
Systematic review and meta-analysis of observational (comparative effectiveness) studies or randomized clinical trials (RCTs).
Setting & Study Populations
People of any age at risk of acute kidney injury (AKI).
Selection Criteria for Studies
We systematically searched MEDLINE, Embase, Web of Science, and the Cochrane Controlled Trials registry from database inception to November 2023 for observational studies or RCTs that compared kidney outcomes among groups treated with cilastatin, either alone or as combination imipenem-cilastatin, versus an inactive or active control group not treated with cilastatin.
Data Extraction
Two reviewers independently evaluated studies for inclusion and risk of bias.
Analytical Approach
Treatment effects were estimated using random-effects models, and heterogeneity was quantified using the I2 statistic.
Results
We identified 10 studies (5 RCTs, n = 531 patients; 5 observational studies, n = 6,321 participants) that met the inclusion criteria, including 4 studies with comparisons to inactive controls and 6 studies with comparisons to alternate antibiotics. Based on pooled results from 7 studies, the risk of AKI was lower with imipenem-cilastatin (risk ratio [RR], 0.52; 95% confidence intervals [CI], 0.40-0.67; I2 = 26.5%), with consistent results observed in RCTs (3 RCTs, RR, 0.26; 95% CI, 0.09-0.77; I2 = 44.4%) and observational studies (4 studies, RR, 0.54; 95% CI, 0.41-0.72; I2 = 44.4%). Based on results from 6 studies, serum creatinine concentration was lower following treatment with imipenem-cilastatin than comparators (weighted mean difference in serum creatinine −0.14 mg/dL (95% CI, −0.21 to −0.07; I2 = 0%). The overall certainty of the evidence was low due to heterogeneity of the results, high risk of bias, and indirectness among the identified studies.
Limitations
Clinical and statistical heterogeneity could not be fully explained due to a limited number of studies.
Conclusions
Patients treated with imipenem-cilastatin developed AKI less frequently and had lower serum creatinine concentration following treatment than control groups or those who had received comparator antibiotics. Larger clinical trials with less risk of detection bias due to lack of allocation concealment and blinding are needed to establish the efficacy of cilastatin for AKI prevention.
Index Words: Kidney failure, nephroprotective drugs, nephrotoxicity, renal disease
Plain-Language Summary
Cilastatin, used with the antibiotic imipenem, has shown kidney-protective effects in animals and preclinical studies of acute kidney injury (AKI). This systematic review and meta-analysis identified 10 studies (5 randomized controlled trials and 5 observational studies) of imipenem-cilastatin involving people at risk of AKI. Pooled estimates of treatment effects indicated that patients who received imipenem-cilastatin had a lower incidence of AKI and lower serum creatinine concentrations following treatment compared to comparator groups. Despite these promising findings, the overall certainty of the evidence was low due to heterogeneity among studies, high risk of bias, and indirectness of the data. Although cilastatin appears to be a promising medication for preventing AKI, larger, well-designed trials are needed to establish its effectiveness.
Acute kidney injury (AKI) refers to a reduction in kidney function based on serum creatinine changes observed within 48 hours to 7 days or reduced urine output for ≥6 hours.1 AKI is associated with increased mortality and the development of chronic conditions such as hypertension, cardiovascular disease, and chronic kidney disease (CKD). Complications from AKI result in higher health care costs and can also lead to reduced quality of life for affected individuals.2, 3, 4, 5, 6
Several types of medications have the potential to induce kidney tubular injury, thereby increasing the risks of acute and CKD.7,8 Nephrotoxic AKI is most often caused by chemotherapeutic agents, antibiotics, calcineurin inhibitors, and radiocontrast dyes.7,9,10 Hospitalized patients often receive these medications in the setting of acute illness, such as infection or at the time of procedures such as surgery or vascular procedures.11,12 This places them at further risk of AKI, occurring in up to 25% of exposed patients, particularly among those with other health conditions. Effective strategies for preventing AKI are needed to improve patient outcomes, reduce health care costs, and enhance quality of life.
Cilastatin was initially developed in the 1980s to reduce the renal metabolism of imipenem, a broad-spectrum antibiotic prescribed for severe systemic infections.13, 14, 15 Many animal studies have demonstrated the nephroprotective effects of cilastatin, particularly following exposure to nephrotoxic drugs.16, 17, 18 Specifically, cilastatin has been shown to reduce the risk of kidney injury in rats following treatment with cyclosporin, imipenem, cisplatin, vancomycin, gentamycin, and radiocontrast dye.18, 19, 20, 21 Studies have also shown that cilastatin lowers the risk of kidney injury in rats undergoing kidney transplantation and in those receiving chemotherapeutic agents, without reducing the potency of the anticancer effect of these drugs.18,19,21
The approval of imipenem-cilastatin for clinical use has enabled several human studies that suggest cilastatin may protect against drug-induced nephrotoxicity. Studies of imipenem-cilastatin have been completed in various patient groups, including those undergoing solid organ transplantation,22, 23, 24 bone marrow transplantation,25 cancer therapy,26 treatment of nosocomial pneumonia,27,28 and childhood bacterial infections.29 A previous meta-analysis of studies testing imipenem-cilastatin among organ transplant recipients receiving cyclosporin reported better kidney function and lower incidence of acute kidney injury among patients who received imipenem-cilastatin when compared with a control group.30 However, in the 16 years since that review, additional trials and comparative effectiveness studies have been published, suggesting that an updated systematic review and meta-analysis is needed to synthesize the current evidence base.31 In this systematic review and meta-analysis, we examined the effects of cilastatin on AKI, kidney function, and subsequent clinical outcomes among people at risk of kidney injury.
Methods
We followed a prespecified study protocol that was registered in the International Prospective Register of Systematic Reviews (PROSPERO) database (ID: CRD42023488809)32 and adhered to the Preferred Reporting for Systematic Review and Meta-analysis (PRISMA) guidelines.33
Search Strategy
We conducted a comprehensive search using 4 electronic bibliographic databases: MEDLINE via OVID (from January 1946 to November 21, 2023, and updated on May 28, 2024), Embase via OVID (from January 1974 to November 2023, and updated on May 28, 2024), Web of Science (from January 1976 to November 22, 2023, and updated on May 28, 2024), and the Cochrane Controlled Trials Registry (from January 1996 to November 21, 2023, and updated on May 28, 2024) (Table S1).
We developed the search strategy with the guidance of a health sciences librarian proficient in systematic search methodology. We used the following search terms as Medical Subject Heading terms and keywords combined with Boolean operators for the bibliographic database search; Medical Subject Heading terms “Acute kidney injury,” OR “chronic kidney failure,”; Key heading word/Text word: “Acute kidney injury” OR “kidney injury” OR “renal injury” OR “renal insufficiency” OR “ kidney insufficiency” OR “chronic kidney injury” OR “end-stage kidney disease” OR “end-stage renal disease” OR “renal failure” or “kidney failure” OR “end-stage kidney failure” OR “end-stage renal failure” OR “kidney dysfunction” OR “nephroprotection” OR “nephrotoxicity” OR “all-cause mortality” OR “kidney function” OR “Creatinine” or “Cystatin C” or “glomerular filtration rate,” “urine output,” “allograft function” OR “proteinuria” or “albuminuria” or “kidney biomarkers” AND “Cilastatin” OR “cilastatin, imipenem drug combination.” We limited the search to studies in humans and included randomized controlled trials (RCTs) as well as comparative effectiveness observational study designs. There were no restrictions imposed on age or language of publications. We excluded publications that were not primary research studies (eg, editorials, narrative reviews, opinion pieces, letters, and research protocols, etc). Citations and reference lists from the included studies were also searched to identify other potentially relevant studies. The detailed literature search strategy for each electronic database is provided in the supplement (Table S1).
Study Selection
Studies were eligible for inclusion if the population included human participants of any age at risk of AKI, acute kidney disease, or CKD arising from acute illness (eg, infection, malignancy), medical or surgical procedures (eg, transplant), or nephrotoxic exposures. Eligible studies were those including treatment with cilastatin either alone or in combination with imipenem and included a comparator group not treated with cilastatin; this could be an inactive control group with or without a placebo or ≥1 active comparator groups not receiving cilastatin. Studies were included if they reported ≥1 outcomes of interest related to nephrotoxicity including a measure of kidney function (eg, urine output, serum creatinine, cystatin C, measured or estimated glomerular filtration rate using any technique), kidney structure (eg, albuminuria/proteinuria, abnormal urine sediment, kidney injury biomarkers including markers of tubular damage such as neutrophil gelatinase-associated lipocalin, kidney injury molecule-1, interleukin-18, kidney imaging, or kidney biopsy features), or AKI based on serum creatinine changes or urine output criteria aligned with the Kidney Disease: Improving Global Outcomes (KDIGO), Acute Kidney Injury Network (AKIN), or risk, injury, failure, loss, end-stage kidney disease (RIFLE) criteria,34 or as defined by the study authors. Additional outcomes of interest included downstream clinical outcomes of AKI, including all-cause mortality, development or progression of CKD, kidney failure, and cardiovascular events.
Screening
We conducted a 2-staged screening process to assess each article’s suitability for inclusion in our review. During the first stage of screening, each article’s title and abstract were independently reviewed by 2 authors (DA and FG). If there was uncertainty regarding inclusion based on the title and abstract alone by either reviewer, the article was retained for full-text review. Subsequently, a full-text review of all articles identified from the first stage was undertaken independently by the same 2 authors. In case of any disagreements arising among the reviewers at each screening stage, consensus was sought, and remaining disagreements were resolved by a third reviewer (MJ). To effectively organize the identified literature, we used Endnote 21 reference management software (Clarivate Analytics).35
Data Extraction
A data extraction template was developed to systematically compile information from each eligible study. The data extraction process was distinct based on study design: RCTs and comparative observational studies. Two authors (DA and FG) completed the data extraction from all studies. The specific data elements acquired included the primary author names, year of publication, geographical origin, study design, sample size, nature of the study population, participant age, sex distribution, and the documented study outcomes and their definitions. We sought to preferentially use definitions of AKI that aligned with the KDIGO, AKIN, and RIFLE criteria34 whenever possible, but used the definition provided by the study authors if the former were not reported. For studies in which measures of kidney function were taken at multiple time points, we used the results from the last time point reported up to 90 days to define short-term changes and measurements after 90 days to identify long-term kidney function from each study.
Risk of Bias Assessment
We assessed the risk of bias of each study using the Cochrane risk of bias tool for randomized trials (RoB tool version 2)36 and the JBI critical appraisal tool for observational studies.37 Each study underwent evaluation and was categorized into 1 of 3 levels of risk of bias: low, unclear, or high.
Statistical Analyses
We quantified the agreement on article eligibility between reviewers in the first and second stages of article selection using the kappa (κ) statistic. The decision to perform meta-analysis was contingent on the availability of ≥2 studies that met our predefined study inclusion criteria for each outcome and that were considered clinically similar enough to justify pooling results.
Given expected clinical and statistical heterogeneity between studies, we estimated pooled dichotomous outcomes using random-effects models according to the DerSimonian and Laird method,38 with treatment effects estimated as risk ratios (RRs) with 95% confidence intervals (CIs). Continuous outcomes were also pooled using random-effects models incorporating restricted maximum likelihood weighting to estimate weighted mean differences with 95% CIs.39 Between-study heterogeneity was assessed using the I2 statistic. We conducted prespecified subgroup analyses and meta-regression for each outcome according to study design (RCT or observational study). We also performed a subgroup analysis and meta-regression according to whether studies used an active comparator antibiotic or used comparison to a control or placebo group. Publication bias was investigated using funnel plots and Egger’s test.40,41 The statistical significance threshold for all tests was set at P < 0.05. Analyses were conducted using Stata statistical software, version 17 (Stata Corporation), using the ‘metan’ package.42
Assessment of Certainty of the Evidence
The certainty of evidence was evaluated by 2 authors (DA and MJ) using the Grading of Recommendations, Assessment, Development and Evaluations (GRADE) approach to determine whether the overall certainty of the evidence for the nephroprotective effects of cilastatin in humans was very low, low, moderate, or high.43
Results
Selection of Studies
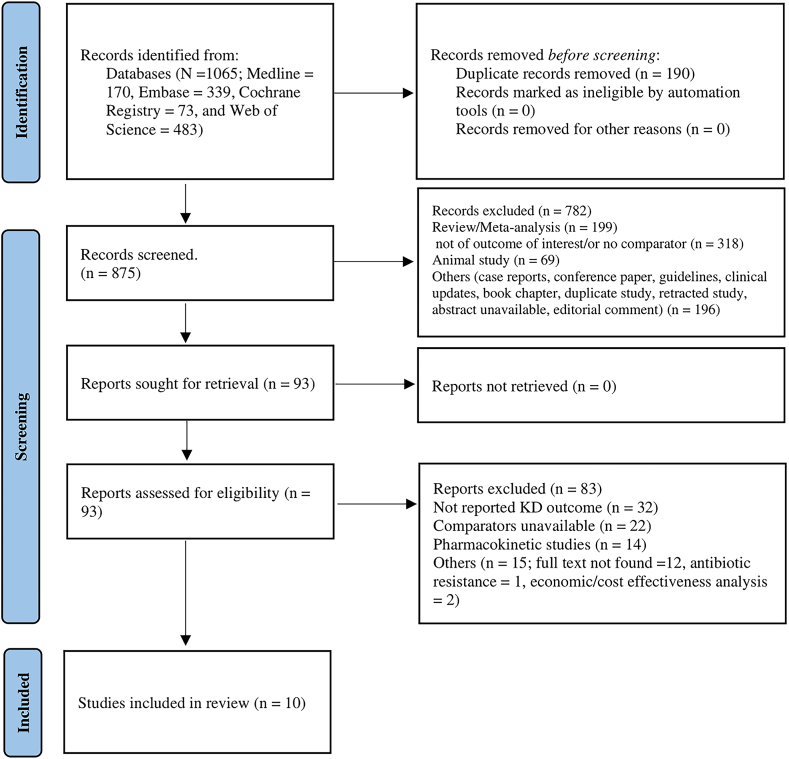
The electronic database search yielded 1,065 citations (1,015 citations retrieved in November 2023 and 50 additional citations retrieved on May 28, 2024). Among these, 190 citations were identified as duplicates and removed. In the first stage of screening, 782 articles were excluded based on their titles and abstracts, resulting in 93 articles selected for full-text review. Of these, 10 studies met the inclusion criteria. There was a high level of agreement between reviewers in the selection of articles for inclusion (κ = 0.61). The study selection process is represented in further detail in the PRISMA flowchart (Fig 1).44
Figure 1.
PRISMA flow diagram. KD, kidney disease.
Study Characteristics
The characteristics of the included studies are reported in Table 1.22, 23, 24, 25, 26, 27, 28, 29,45,46 Of the 10 included studies, 5 were RCTs23,24,27,28,45 (n = 531 patients), and 5 were observational comparative effectiveness studies22,25,26,29,46 (n = 6,321 participants). Publication dates ranged from 1994 to 2021, and the number of participants per study varied from 20 to 5,566. Study populations included kidney transplant recipients (2 studies),23,24 heart and lung transplant recipients (1 study),22 bone marrow transplant recipients (1 study),25 patients treated for nosocomial infections (2 studies),27,28 heart transplant recipients (1 study),45 infants with severe bacterial infection (1 study),29 and patients receiving cisplatin chemotherapy for peritoneal carcinomatosis (1 study).26 All studies tested imipenem-cilastatin as the intervention. The comparison groups varied across studies, with an inactive control used in 4 studies (with 1 describing use of a placebo control)22, 23, 24, 25, 26,45 and an active comparator used in the other 6 studies, including ceftazidime in 2 studies,23,24 meropenem in 2 studies,29,46 piperacillin/tazobactam in 1 study,27 and cefepime in 1 study.28 The volume of intravenous fluids administered in each treatment arm was not reported by any of the studies.
Table 1.
Characteristics of the Included Studies
| First Author (Year), Country | Study Design | Comparison | Daily Drug Doses (IC Group)/(Comparator Group) | Study Population | Total No. of Participants (IC Group/Comparator Group) | Sex M:F, (IC Group)/(Comparator Group) | Age, IC Group/Comparator Group, Mean (SD), y | Follow-up Duration, d | Outcomes Reported |
|---|---|---|---|---|---|---|---|---|---|
| Randomized controlled trials | |||||||||
| Carmellini et al23 (1997), Italy | RCT | IC vs ceftazidime | (500 mg/500 mg every 8 h)/(1 g every 12 h) before surgery, for 2 d | Kidney transplant recipients receiving cyclosporin | 69 (33/36) | (15:18)/(15:11) | 44.2 (9.7)/43.1 (9.8) | 30 | Scra and mortality |
| Carmellini et al24 (1998), Italy | RCT | IC vs ceftazidime | (500 mg/500 mg every 8 h)/(2 g/d) following 2 postoperative days | Kidney transplant recipients receiving cyclosporin | 16 (8/8) | Not specified | 45 (5)/42 (4) | 14 | AKIb |
| Markewitz et al45 (1994), Germany | RCT | IC vs placebo control | (500 mg/500 mg every 12 h) preoperatively followed by 7 d postoperatively | Heart transplant patients receiving cyclosporin | 20 (10/10) | Not specified | 51 (9.3)/5 (9.3) | 10 | Scrc and AKId |
| Schmitt et al27 (2006), Multicountry | RCT | IC vs piperacillin/tazobactam | (1 g/1 g)/(4 g/500 mg) every 8 h for 5-21 d | Hospitalized patients with nosocomial infection | 217 (110/107) | (64:47)/(77:33) | 65.7 (13.8)/68.4 (13.7) | 21 | Mortality |
| Zanetti et al28 (2003), Multicountry | RCT | IC vs cefepime | (2 g/2 g every 8 h)/(500 mg every 6 h) | Hospitalized patients with nosocomial pneumonia | 209 (101/108) | (67:34)/(72:36) | 53 (18)/55 (18) | 14 | AKIe, mortality |
| Observational studies | |||||||||
| Baghai et al22 (1995), United States | Observational | IC vs control | (500 mg/500 mg every 6 h) for 5 d | Heart and lung transplant recipients receiving cyclosporin | 20 (10/10) | Not specified | Not specified | 14 | Scrf |
| Gruss et al25 (1996), Spain | Observational | IC vs control | Dose not reported | Bone marrow transplant recipients receiving cyclosporin | 104 (64/40) | Not specified | Not specified | 30 | Scrg and AKIh |
| Hakeam et al46 (2019), Saudi Arabia | Observational | IC vs meropenem | 0.5 g every 6 h in 109 patients and 1 g every 8 h in 12 patients/1 g every 8 h in 98 patients and 0.5 every 8 h in 8 patients | Hospitalized patients being treated for various infections with vancomycin | 227 (106/121) | (62:59)/(63:43) | 50.7 (17.4)/50.7 (17.4) | 7 | Scri, AKIj, and mortality |
| Hornik et al29 (2014), United States | Observational | IC vs meropenem | Dose not specified | Hospitalized infants treated with carbapenem antibiotics | 5566 (2087 /3256)k | Not specified | First 120 d of life | 120 | AKIl and mortality |
| Zaballos et al26 (2021), Spain | Observational | IC vs control | (500 mg/500 mg every 8 h) | Patients with peritoneal carcinomatosis receiving surgery and intraperitoneal cisplatin; 9.4% in the IC group versus 53.5% in the control group received cotreatment with doxorubicin | 181 (83/98) | (5:80)/(7:91) | 56.79 (11.42)/53.22 (10.94) | 7 | Scrm, AKIn, and mortality |
Notes: All studies included imipenem/cilastatin (IC) in the intervention group.
Abbreviations: IC, imipenem/cilastatin; M:F, male:female; RCT, randomized clinical trial; RIFLE, Risk, Injury, Failure Loss, End-stage renal disease; Scr, serum creatinine; SD, standard deviation.
oDefined by Scr measurement >1.7 mg/dL.
Measured on postoperative day 30.
Defined by Scr and urinary output changes over 14 d of follow-up.
Measured on postoperative days 1-10 consecutively.
Defined by receipt of kidney replacement therapy in the postoperative period.
Defined as Scr >200 μmol/L, with measurements taken at baseline, then twice weekly, and within 48 h after the completion of drug therapy and/or patient identified as a possible case of interstitial nephritis.
Measured on postoperative days 1-5 consecutively.
Measured posttransplant, days not specified.
Definition not specified.
Measured day 1 and day 4 following initiation of antibiotics.
Defined according to the RIFLE criteria based on changes in Scr and urinary output.
223 infants received both carbapenems at different times in the study.
Defined according to the RIFLE criteria criteria based on changes in Scr and urinary output.
Measured at baseline and post-intervention day 1 to day 7 consecutively.
Defined according to the RIFLE criteria.
Outcomes of interest included AKI reported in 7 studies using varying definitions,24, 25, 26,28,29,45,46 short-term changes in serum creatinine concentration reported in 6 studies with the last time point of measurement ranging from 5-30 days of follow-up,22,23,25,26,45,46 and all-cause mortality reported in 5 studies.23,26, 27, 28, 29,46 We identified no studies examining the outcomes of development or progression of CKD, long-term kidney function, kidney failure, or cardiovascular events.
Effect of Imipenem-Cilastatin on AKI
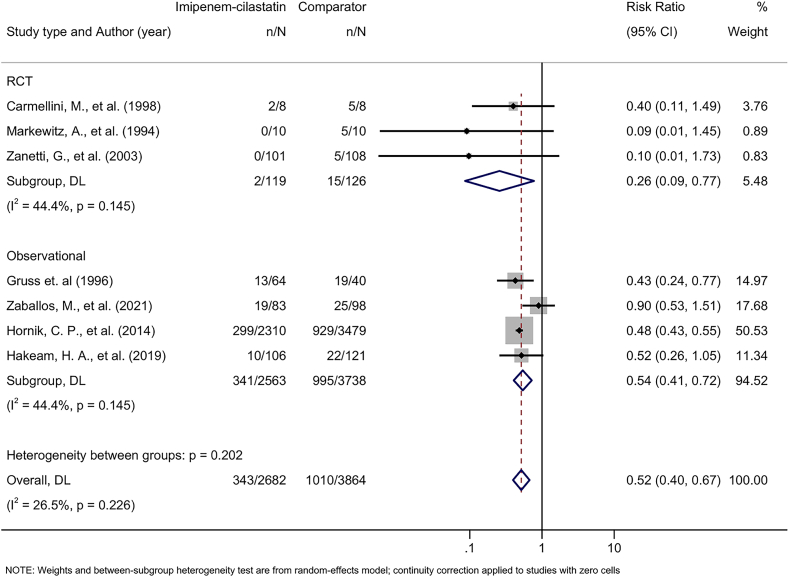
Based on results from 7 studies24, 25, 26,28,29,45,46 including a total of 6,301 participants, patients treated with imipenem-cilastatin had a lower risk of AKI than patients treated with comparators (pooled RR, 0.52; 95% CI, 0.40-0.67), with moderate heterogeneity observed between studies (I2 = 26.5%). Treatment effects of imipenem-cilastatin on AKI were consistent by study design (meta-regression P = 0.24); among 3 RCTs the pooled RR was 0.26 (95% CI, 0.09-0.77), I2 = 44.4%, while among 4 observational studies the pooled RR was 0.54 (95% CI, 0.41-0.72), I2 = 44.4% (Fig 2). Findings were also consistent between studies that compared participants treated with imipenem-cilastatin to a control group (3 studies, pooled RR, 0.55; 95% CI, 0.25-1.18) and those comparing imipenem-cilastatin treatment to an active comparator antibiotic (4 studies, pooled RR, 0.48; 95% CI, 0.43-0.54) (meta-regression P = 0.25) (Fig S1).
Figure 2.
Forest plot demonstrating pooled effect of imipenem-cilastatin on acute kidney injury. The subgroup refers to the pooled risk ratio, with a 95% confidence interval, stratified by study design: RCT and observational study. Note: The study by Hornik et al29 included 223 infants who received imipenem-cilastatin and the comparator carbapenem antibiotics at different times. Abbreviations: CI, confidence interval; DL, DerSimonian and Laird; n, number of acute kidney injury events; N, total number of study participants; RCT, randomized controlled trial.
Effect of Imipenem-Cilastatin on Serum Creatinine Concentration
Results from 6 studies showed that patients treated with imipenem-cilastatin had lower subsequent serum creatinine concentrations than those treated with comparators22,23,25,26,45,46; the weighted mean difference in serum creatinine was −0.14 mg/dL (95% CI, −0.21 to −0.07) with no evidence of statistical heterogeneity observed between studies (I2 = 0%). Results remained consistent between RCTs and observational studies (meta-regression P = 0.46) (Fig 3).
Figure 3.
Forest plot demonstrating pooled effect of imipenem-cilastatin on serum creatinine concentrations. The subgroup refers to the pooled WMD with a 95% CI stratified by study design: RCT and observational study. Abbreviations: CI, confidence interval; N, total number of study participants in imipenem-cilastatin or comparator group for individual study; RCT, randomized controlled trial; REML, restricted maximum likelihood; SD, standard deviation; WMD, weighted mean difference.
Effect of Imipenem-Cilastatin on All-Cause Mortality
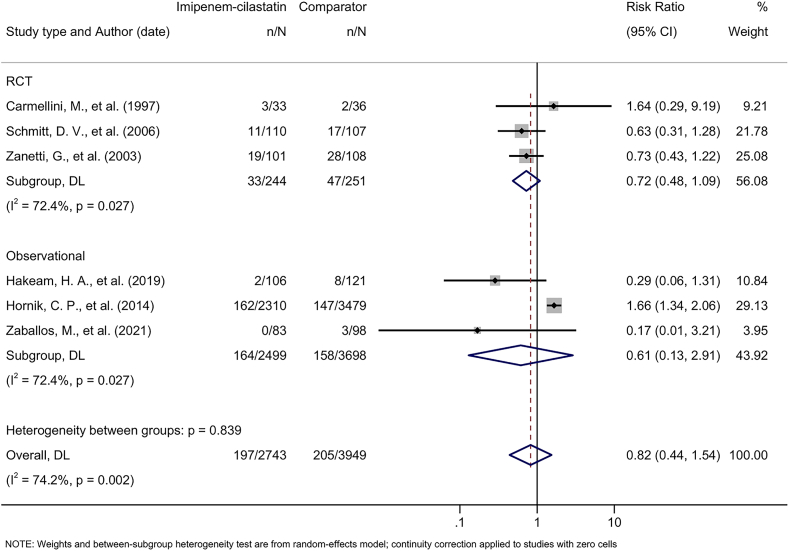
Six studies reported on all-cause mortality.23,26, 27, 28, 29,46 Patients treated with imipenem-cilastatin experienced no statistically significant difference in all-cause mortality than patients treated with comparators (pooled RR, 0.82; 95% CI, 0.44-1.54), with a high degree of heterogeneity across studies (I2 = 74.2%) (Fig 4). Pooled estimates were consistent between RCTs and observational studies (meta-regression P = 0.916).
Figure 4.
Forest plot demonstrating pooled effect of imipenem-cilastatin on all-cause mortality. The subgroup refers to the pooled risk ratio, with a 95% CI, stratified by study design: randomized controlled trial (RCT) and observational study. Note: The study by Hornik et al29 included 223 infants who received imipenem-cilastatin and the comparator carbapenem antibiotics at different times. Abbreviations: CI, confidence interval; DL, DerSimonian and Laird; n, number of acute kidney injury events; N, total number of study participants; RCT, randomized controlled trial.
Risk of Bias
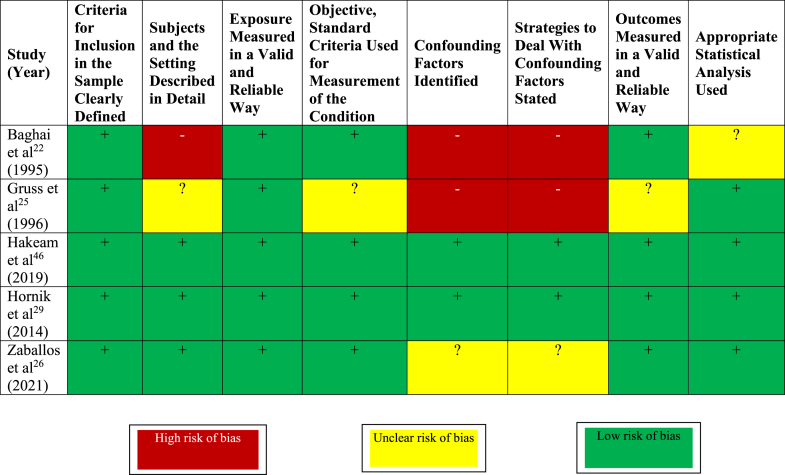
The risk of bias of RCTs according to the RoB 2.0 tool is shown in Figure 5.23,24,27,28,45 Four of the 5 trials had unclear or high risk of bias due to lack of or unclear allocation concealment. All trials were at high or unclear risk of detection bias due to lack of or unclear blinding, and 3 were at unclear risk of attrition bias due to lack of reporting of losses to follow-up. The risk of bias of observational studies according to the JBI critical appraisal tool for observational studies is shown in Figure 6.22,25,26,29,46 Three of the 5 observational studies were at unclear or high risk of bias due to unclear or inadequate strategies to address confounding.
Figure 5.
Risk of bias of randomized trials of imipenem-cilastatin. Adapted from the Cochrane Risk of Bias Tool (RoB Tool Version 2).
Figure 6.
Risk of bias assessment of observational studies of imipenem-cilastatin. Adapted from the Joanna Briggs Institute Critical Appraisal Checklist.
Publication Bias
Funnel plots for AKI, serum creatinine, and mortality showed asymmetry, consistent with small study effects suggestive of publication bias (Fig S2). However, there was no statistical evidence of small study effects based on Egger’s test for the outcomes of AKI (P = 0.601), serum creatinine (P = 0.079), or all-cause mortality (P = 0.093), although the number of studies limited the power of these tests.
Certainty of the Evidence
The overall certainty of the evidence was graded as low due to moderate and high statistical heterogeneity for the outcomes of AKI and mortality, high risk of bias for most of the individual studies, and indirectness (use of surrogate outcomes).
Discussion
In this systematic review and meta-analysis, we found that the risk of developing AKI was 48% lower with imipenem-cilastatin treatment, although there was heterogeneity in the effect size between studies. We also found that serum creatinine measured after short-term follow-up ranging from 5-30 days was lower among participants who received imipenem-cilastatin than those who received comparators. There was no statistically significant difference in all-cause mortality among participants who received imipenem-cilastatin that in those who received comparators. Results were similar among RCTs and observational studies and studies that made comparisons to alternative antibiotics and those that used a nonactive control group. It is important to note that many of the studies were at high risk of bias, and funnel plots suggested that publication bias may exist. Given the uncertainty of these results, our findings indicate that further high-quality trials are required to establish the effectiveness of cilastatin for AKI prevention.
The nephroprotective effects of cilastatin have been demonstrated in several preclinical studies,16, 17, 18, 19,21 and mechanistic effects have been further examined in several human studies.22, 23, 24, 25 Cilastatin inhibits dehydropeptidase 1 within the brush border of the renal proximal tubule thereby inhibiting dehydropeptidase 1-mediated hydrolysis of drugs before they are taken up into tubular epithelial cells via megalin where they can cause cell necrosis through several mechanisms, including via reactive oxygen species, inflammation, and apoptosis.16,47,48 Cilastatin can also block megalin-mediated uptake of endogenous nephrotoxic substances, as observed in animal models of pigment nephropathy.49,50 Cilastatin also appears to have nephroprotective effects distinct from effects on tubular uptake of nephrotoxic agents.51 Cilastatin blocks dehydropeptidase 1-mediated leukocyte recruitment in the tubulointerstitial space, thereby reducing renal inflammation in response to injury.16,31,52,53 Animal studies have demonstrated that cilastatin exerts nephroprotective effects in animal models of ischemia-reperfusion injury.54 The reported nephroprotective effects of cilastatin from preclinical studies have generated interest in its use as an agent for prevention of AKI caused by nephrotoxic medication exposures, and the availability of the imipenem-cilastatin formulation has enabled comparative studies that have evaluated kidney outcomes in several clinical settings.31
Our study findings align with those from a previous meta-analysis conducted by Tejedor et al30 in 2007, which included 5 studies comparing patients with organ transplantation receiving imipenem-cilastatin to those receiving cyclosporine. They also reported lower serum creatinine concentrations for patients treated with imipenem-cilastatin and a 76% reduction in the odds of developing acute kidney injury. Our updated review identified 5 additional studies published since their review and provides an updated and consolidated evidence base including additional studies with comparisons to alternative antibiotics demonstrating the nephroprotective effects of imipenem-cilastatin. In this review, the study participants were from diverse clinical settings in which they were exposed to pharmacological agents or procedures that confer risk of AKI. These interventions involved antibiotic therapy for bacterial infections, cancer chemotherapy, as well as the administration of calcineurin inhibitors in patients undergoing solid organ transplantation, suggesting cilastatin might be an AKI prevention approach that could be applied in several clinical settings.
This review has several limitations that are important to acknowledge. First, the studies included had a large degree of clinical heterogeneity, not only in the clinical populations and settings, but also in the way outcomes were measured, including the definition used for AKI and the timing and methodology of serum creatinine measurement. Studies did not consistently define AKI using the KDIGO definition, requiring the use of a variety of definitions as reported by the authors that differed in their incorporation of serum creatinine thresholds, incorporation of urine output, and identification of treatment with dialysis across the studies. Furthermore, measurements of serum creatinine were made at different time points after treatment in these studies, and all were within short-term periods of follow-up. We were able to address this in our meta-analysis by selecting the last available serum creatinine measurement reported by each study; however, this makes interpretation of the pooled difference challenging because changes in serum creatinine may vary (with both worsening and improvement) with time following AKI. Furthermore, serum creatinine has limitations for assessing kidney function, as it may be influenced by dietary factors,55 medications,56 body composition,57 and hemodilution.58 Second, the number of studies identified was small, and the existing RCTs that were identified had small sample sizes and were at high risk of bias. The small number of studies limited our ability to explore reasons for statistical heterogeneity and detect publication bias. Third, the comparator groups varied across studies, with a number including an active comparator including an alternative broad-spectrum antibiotic. It is thus possible that these comparisons are confounded by differences in the risk of AKI with imipenem versus meropenem or β-lactam antibiotics, rather than being attributable to an independent effect of cilastatin itself. Other studies have suggested that piperacillin-tazobactam carries a higher risk of AKI than meropenem, particularly when used in conjunction with vancomycin, which may contribute to overestimation of the nephroprotective effects of cilastatin obtained from studies comparing imipenem-cilastatin to piperacillin-tacobactum.59,60 We did not identify published results from trials of cilastatin alone, which may have a more favorable safety and efficacy profile than imipenem-cilastatin when used for AKI prevention alone. New trials testing formulations of cilastatin for AKI in the absence of confounding effects from comparator antibiotics are needed to test this hypothesis. Finally, the studies identified largely relied on surrogate endpoints such as AKI and serum creatinine differences, rather than patient-centered clinical outcomes such as major adverse kidney outcomes.
In conclusion, this systematic review and meta-analysis suggests that cilastatin may reduce the risk of AKI; however, the existing evidence base is derived from studies of imipenem-cilastatin with a high risk of bias, and efficacy is uncertain due to the statistical heterogeneity of findings, indirectness of the evidence base, and potential detection of publication biases. Further large-scale randomized placebo-controlled trials of cilastatin with appropriate allocation concealment and blinding and focused on clinically important outcomes are needed to determine the efficacy and safety of cilastatin used for AKI prevention among patients receiving contemporary nephrotoxic exposures.
Article Information
Authors’ Full Names and Academic Degrees
Dilaram Acharya, MPH, PhD, Fanar Ghanim, BSc, Tyrone G. Harrison, MD, PhD, Tayler Dawn Scory, MSc, Nusrat Shommu, PhD, Paul E. Ronksley, PhD, Meghan J. Elliott, MD, MSc, David Collister, MD, PhD, Neesh Pannu, MD, SM, and Matthew T. James, MD, PhD
Authors’ Contributions
Research idea and study design: DA, MTJ; data acquisition: DA, FG, TGH, TDS, NS, PER, MJE, DC, and NP; data analysis/interpretation: DA, MTJ, FG, TGH, TDS, NS, PER, MJE, DC, and NP; statistical analysis: DA, MTJ; supervision or mentorship: MTJ. Each author contributed important intellectual content during article drafting or revision and accepts accountability for the overall work by ensuring that questions about the accuracy or integrity of portion of the work are appropriately investigated and resolved.
Support
The study was funded by the Canadian Institutes of Health Research (CIHR) Team Grant: Intervention Trial in Inflammation for Chronic Conditions - Evidence to Impact; Funding Reference Number LI3 189373.
Financial Disclosure
The authors report research funding from the CIHR Accelerating Clinical Trials Consortium for a clinical trial of cilastatin for AKI prevention. Dr Harrison reports support from a Kidney Research Scientist Core Education and National Training Program New Investigator Award (KRESCENT cosponsored by the Kidney Foundation of Canada and Canadian Institutes of Health Research) and as a new investigator by the Roy and Vi Baay Chair for Kidney Research and the Kidney Health and Wellness Institute at the University of Calgary. Dr Collister reports support from a KRESCENT New Investigator Award and has received funding from the Canadian Institutes of Health Research, Kidney Foundation of Canada, and CSL Behring outside the submitted work. The authors report no other conflicts of interest.
Acknowledgements
The authors thank Diane L. Lorenzetti, from the Health Sciences Library, University of Calgary for her assistance in developing the electronic bibliographic database search strategy.
Data Sharing
All data produced in the present study are available on reasonable request to the authors.
Prior Presentation
A preprint version of this article is available at medRxiv: https://www.medrxiv.org/content/10.1101/2024.03.06.24303823v1.
Peer Review
Received May 28, 2024 as a submission to the expedited consideration track with 2 external peer reviews. Direct editorial input from the Editor-in-Chief. Accepted in revised form June 18, 2024.
Footnotes
Complete author and article information provided before references.
Figure S1: Forest plot demonstrating pooled effect of imipenem-cilastatin on acute kidney injury from all studies and stratified by treatment type: Imipenem-cilastatin vs control and imipenem-cilastatin vs comparator antibiotic. Note: The study by Hornik et al29 included 223 infants who received imipenem-cilastatin and the comparator carbapenem antibiotics at different times. Abbreviations: CI, confidence interval; DL, DerSimonian and Laird; n, number of acute kidney injury events; N, total number of study participants.
Figure S2: Funnel plots for small study effects suggesting publication bias. (A) Funnel plot for AKI. (B) Funnel plot for serum creatinine. (C) Funnel plot for all-cause mortality.
Table S1: Literature Search Strategy Applied to Electronic Bibliographic Databases.
Supplementary Material
Figures S1, S2; Table S1.
References
- 1.Makris K., Spanou L. Acute kidney injury: definition, pathophysiology and clinical phenotypes. Clin Biochem Rev. 2016;37(2):85–98. [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang S., Ren H.F., Du R.X., Sun W.L., Fu M.L., Zhang X.C. Global, regional, and national burden of kidney dysfunction from 1990 to 2019: a systematic analysis from the global burden of disease study 2019. BMC Public Health. 2023;23(1):1218. doi: 10.1186/s12889-023-16130-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sarnak M.J., Levey A.S., Schoolwerth A.C., et al. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation. 2003;108(17):2154–2169. doi: 10.1161/01.CIR.0000095676.90936.80. [DOI] [PubMed] [Google Scholar]
- 4.Saran R., Pearson A., Tilea A., et al. Burden and cost of caring for US Veterans with CKD: initial findings from the VA Renal Information System (VA-REINS) Am J Kidney Dis. 2021;77(3):397–405. doi: 10.1053/j.ajkd.2020.07.013. [DOI] [PubMed] [Google Scholar]
- 5.Chronic Kidney Disease Prognosis Consortium. Matsushita K., van der Velde M., et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010;375(9731):2073–2081. doi: 10.1016/s0140-6736(10)60674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matsushita K., Coresh J., Sang Y., et al. Estimated glomerular filtration rate and albuminuria for prediction of cardiovascular outcomes: a collaborative meta-analysis of individual participant data. Lancet Diabetes Endocrinol. 2015;3(7):514–525. doi: 10.1016/s2213-8587(15)00040-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Awdishu L., Mehta R.L. The 6R’s of drug induced nephrotoxicity. BMC Nephrol. 2017;18(1):124. doi: 10.1186/s12882-017-0536-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sharma V., Singh T.G. Drug induced nephrotoxicity- a mechanistic approach. Mol Biol Rep. 2023;50(8):6975–6986. doi: 10.1007/s11033-023-08573-4. [DOI] [PubMed] [Google Scholar]
- 9.Kane-Gill S.L., Goldstein S.L. Drug-induced acute kidney injury: a focus on risk assessment for prevention. Crit Care Clin. 2015;31(4):675–684. doi: 10.1016/j.ccc.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 10.Mendoza S.A. Nephrotoxic drugs. Pediatr Nephrol. 1988;2(4):466–476. doi: 10.1007/bf00853443. [DOI] [PubMed] [Google Scholar]
- 11.Patel J.B., Sapra A. StatPearls Publishing; 2023. Nephrotoxic Medications. [PubMed] [Google Scholar]
- 12.Perazella M.A., Rosner M.H. Drug-induced acute kidney injury. Clin J Am Soc Nephrol. 2022;17(8):1220–1233. doi: 10.2215/cjn.11290821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Calandra G.B., Ricci F.M., Wang C., Brown K.R. The efficacy results and safety profile of imipenem/cilastatin from the clinical research trials. J Clin Pharmacol. 1988;28(2):120–127. doi: 10.1002/j.1552-4604.1988.tb05735.x. [DOI] [PubMed] [Google Scholar]
- 14.Drusano G.L., Standiford H.C. Pharmacokinetic profile of imipenem/cilastatin in normal volunteers. Am J Med. 1985;78(6A):47–53. doi: 10.1016/0002-9343(85)90101-9. [DOI] [PubMed] [Google Scholar]
- 15.Kahan F.M., Kropp H., Sundelof J.G., Birnbaum J. Thienamycin: development of imipenem-cilastatin. J Antimicrob Chemother. 1983;12(suppl D):1–35. doi: 10.1093/jac/12.suppl_D.1. [DOI] [PubMed] [Google Scholar]
- 16.Humanes B., Camaño S., Lara J.M., et al. Cisplatin-induced renal inflammation is ameliorated by cilastatin nephroprotection. Nephrol Dial Transplant. 2017;32(10):1645–1655. doi: 10.1093/ndt/gfx005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Humanes B., Jado J.C., Camaño S., et al. Protective effects of cilastatin against vancomycin-induced nephrotoxicity. Biomed Res Int. 2015;2015 doi: 10.1155/2015/704382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Humanes B., Lazaro A., Camano S., et al. Cilastatin protects against cisplatin-induced nephrotoxicity without compromising its anticancer efficiency in rats. Kidney Int. 2012;82(6):652–663. doi: 10.1038/ki.2012.199. [DOI] [PubMed] [Google Scholar]
- 19.Hammer C., Thies J.C., Mraz W., Mihatsch M. Reduction of cyclosporine (CSA) nephrotoxicity by imipenem/cilastatin after kidney transplantation in rats. Transplant Proc. 1989;21(1 Pt 1):931. [PubMed] [Google Scholar]
- 20.Jado J.C., Humanes B., González-Nicolás M.Á., et al. Nephroprotective effect of cilastatin against gentamicin-induced renal injury in vitro and in vivo without altering its bactericidal efficiency. Antioxidants (Basel) 2020;9(9):821. doi: 10.3390/antiox9090821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sido B., Hammer C., Mraz W., Krombach F. Nephroprotective effect of imipenem/cilastatin in reducing cyclosporine toxicity. Transplant Proc. 1987;19(1 Pt 2):1755–1758. [PubMed] [Google Scholar]
- 22.Ali B., Maryam B., Cyril A., et al. The effect of imipenem/ cilastatin on acute cyclosporine nephrotoxicity in heart/lung transplant patients. Crit Care Med. 1995;23(1 suppl):A241. [Google Scholar]
- 23.Carmellini M., Frosini F., Filipponi F., Boggi U., Mosca F. Effect of cilastatin on cyclosporine-induced acute nephrotoxicity in kidney transplant recipients. Transplantation. 1997;64(1):164–166. doi: 10.1097/00007890-199707150-00029. [DOI] [PubMed] [Google Scholar]
- 24.Carmellini M., Matteucci E., Boggi U., Cecconi S., Giampietro O., Mosca F. Imipenem/cilastatin reduces cyclosporin-induced tubular damage in kidney transplant recipients. Transplant Proc. 1998;30(5):2034–2035. doi: 10.1016/s0041-1345(98)00523-5. [DOI] [PubMed] [Google Scholar]
- 25.Gruss E., Tomás J.F., Bernis C., Rodriguez F., Traver J.A., Fernández-Rañada J.M. Nephroprotective effect of cilastatin in allogeneic bone marrow transplantation. Results from a retrospective analysis. Bone Marrow Transplant. 1996;18(4):761–765. [PubMed] [Google Scholar]
- 26.Zaballos M., Power M., Canal-Alonso M.I., et al. Effect of cilastatin on cisplatin-induced nephrotoxicity in patients undergoing hyperthermic intraperitoneal chemotherapy. Int J Mol Sci. 2021;22(3):1239. doi: 10.3390/ijms22031239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schmitt D.V., Leitner E., Welte T., Lode H. Piperacillin/tazobactam vs imipenem/cilastatin in the treatment of nosocomial pneumonia - a double blind prospective multicentre study. Infection. 2006;34(3):127–134. doi: 10.1007/s15010-006-5020-0. [DOI] [PubMed] [Google Scholar]
- 28.Zanetti G., Bally F., Greub G., et al. Cefepime versus imipenem-cilastatin for treatment of nosocomial pneumonia in intensive care unit patients: a multicenter, evaluator-blind, prospective, randomized study. Antimicrob Agents Chemother. 2003;47(11):3442–3447. doi: 10.1128/aac.47.11.3442-3447.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hornik C.P., Herring A.H., Benjamin D.K., Jr., et al. Adverse events associated with meropenem versus imipenem/cilastatin therapy in a large retrospective cohort of hospitalized infants. Pediatr Infect Dis J. 2014;32(7):748–753. doi: 10.1097/INF.0b013e31828be70b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tejedor A., Torres A.M., Castilla M., Lazaro J.A., de Lucas C., Caramelo C. Cilastatin protection against cyclosporin A-induced nephrotoxicity: clinical evidence. Curr Med Res Opin. 2007;23(3):505–513. doi: 10.1185/030079906X167633. [DOI] [PubMed] [Google Scholar]
- 31.Shayan M., Elyasi S. Cilastatin as a protective agent against drug-induced nephrotoxicity: a literature review. Expert Opin Drug Saf. 2020;19(8):999–1010. doi: 10.1080/14740338.2020.1796967. [DOI] [PubMed] [Google Scholar]
- 32.Acharya D, Ghanim F, Harrison TG, et al. Nephroprotective effects of cilastatin in people at risk of acute kidney injury: a study protocol for a systematic review and meta-analysis. Kidney Med. https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42023488809. Accessed November 1, 2024
- 33.Page M.J., McKenzie J.E., Bossuyt P.M., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372 doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yaqub S., Hashmi S., Kazmi M.K., Aziz Ali A., Dawood T., Sharif H. A comparison of AKIN, KDIGO, and RIFLE definitions to diagnose acute kidney injury and predict the outcomes after cardiac surgery in a South Asian cohort. Cardiorenal Med. 2022;12(1):29–38. doi: 10.1159/000523828. [DOI] [PubMed] [Google Scholar]
- 35.EndNote Version 21. Clarivate; 2013.
- 36.Sterne J.A.C., Savović J., Page M.J., et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366 doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 37.Aromataris E., Lockwood C., Porritt K., Pilla B., Jordan Z., editors. JBI Manual for Evidence Synthesis. JBI; 2024. [DOI] [Google Scholar]
- 38.IntHout J., Ioannidis J.P.A., Borm G.F. The Hartung-Knapp-Sidik-Jonkman method for random effects meta-analysis is straightforward and considerably outperforms the standard DerSimonian-Laird method. BMC Med Res Methodol. 2014;14(1):25. doi: 10.1186/1471-2288-14-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.DerSimonian R., Laird N. Meta-analysis in clinical trials revisited. Contemp Clin Trials. 2015;45(Pt A):139–145. doi: 10.1016/j.cct.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sterne J.A., Egger M. Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J Clin Epidemiol. 2001;54(10):1046–1055. doi: 10.1016/s0895-4356(01)00377-8. [DOI] [PubMed] [Google Scholar]
- 41.Egger M., Smith G.D., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stata Statistical Software, Release 17. Stata Corporation; 2021. [Google Scholar]
- 43.Schünemann H.J., Higgins J.P.T., Vist G.E., et al. In: Cochrane Handbook for Systematic Reviews of Interventions. Higgins J., Thomas J., editors. Cochrane; 2019. Completing ‘Summary of findings’ tables and grading the certainty of the evidence; pp. 375–402. [Google Scholar]
- 44.Page M.J., McKenzie J.E., Bossuyt P.M., et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. PLOS Med. 2021;18(3) doi: 10.1371/journal.pmed.1003583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Markewitz A., Hammer C., Pfeiffer M., et al. Reduction of cyclosporine-induced nephrotoxicity by cilastatin following clinical heart transplantation. Transplantation. 1994;57(6):865–870. doi: 10.1097/00007890-199403270-00017. [DOI] [PubMed] [Google Scholar]
- 46.Hakeam H.A., AlAnazi L., Mansour R., AlFudail S., AlMarzouq F. Does nephrotoxicity develop less frequently when vancomycin is combined with imipenem-cilastatin than with meropenem? A comparative study. Infect Dis (Lond) 2019;51(8):578–584. doi: 10.1080/23744235.2019.1619934. [DOI] [PubMed] [Google Scholar]
- 47.Bhagunde P., Colon-Gonzalez F., Liu Y., et al. Impact of renal impairment and human organic anion transporter inhibition on pharmacokinetics, safety and tolerability of relebactam combined with imipenem and cilastatin. Br J Clin Pharmacol. 2020;86(5):944–957. doi: 10.1111/bcp.14204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hori Y., Aoki N., Kuwahara S., et al. Megalin blockade with cilastatin suppresses drug-induced nephrotoxicity. J Am Soc Nephrol. 2017;28(6):1783–1791. doi: 10.1681/asn.2016060606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Han J., Cui L., Yu F., Wang R., Yuan H., Hu F. Megalin blockade with cilastatin ameliorates multiple wasp sting-induced acute kidney injury in rats. Toxicon. 2022;220 doi: 10.1016/j.toxicon.2022.106960. [DOI] [PubMed] [Google Scholar]
- 50.Matsushita K., Mori K., Saritas T., et al. Cilastatin ameliorates rhabdomyolysis-induced AKI in mice. J Am Soc Nephrol. 2021;32(10):2579–2594. doi: 10.1681/asn.2020030263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Buckley M.M., Brogden R.N., Barradell L.B., Goa K.L. Imipenem/cilastatin: a reappraisal of its antibacterial activity, pharmacokinetic properties and therapeutic efficacy. Drugs. 1992;44(3):408–444. doi: 10.2165/00003495-199244030-00008. [DOI] [PubMed] [Google Scholar]
- 52.Lau A., Rahn J.J., Chappellaz M., et al. Dipeptidase-1 governs renal inflammation during ischemia reperfusion injury. Sci Adv. 2022;8(5) doi: 10.1126/sciadv.abm0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Luo K., Lim S.W., Jin J., et al. Cilastatin protects against tacrolimus-induced nephrotoxicity via anti-oxidative and anti-apoptotic properties. BMC Nephrol. 2019;20(1):221. doi: 10.1186/s12882-019-1399-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hong Y.A., Jung S.Y., Yang K.J., et al. Cilastatin preconditioning attenuates renal ischemia-reperfusion injury via hypoxia inducible factor-1α activation. Int J Mol Sci. 2020;21(10):3583. doi: 10.3390/ijms21103583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pimenta E., Jensen M., Jung D., Schaumann F., Boxnick S., Truebel H. Effect of diet on serum creatinine in healthy subjects during a phase I study. J Clin Med Res. 2016;8(11):836–839. doi: 10.14740/jocmr2738w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Alhassani R.Y., Bagadood R.M., Balubaid R.N., Barno H.I., Alahmadi M.O., Ayoub N.A. Drug therapies affecting renal function: an overview. Cureus. 2021;13(11) doi: 10.7759/cureus.19924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bartholomae E., Knurick J., Johnston C.S. Serum creatinine as an indicator of lean body mass in vegetarians and omnivores. Front Nutr. 2022;9 doi: 10.3389/fnut.2022.996541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jin J., Xu J., Xu S., et al. Hemodilution is associated with underestimation of serum creatinine in cardiac surgery patients: a retrospective analysis. BMC Cardiovasc Disord. 2021;21(1):61. doi: 10.1186/s12872-021-01879-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rutter W.C., Burgess D.S. Comparative rates of nephrotoxicity in patients treated with piperacillin-tazobactam and meropenem: a retrospective cohort study. Open Forum Infect Dis. 2016;3(suppl 1):1814. doi: 10.1093/ofid/ofw172.1362. [DOI] [Google Scholar]
- 60.Rutter W.C., Burgess D.S. Incidence of acute kidney injury among patients treated with piperacillin-tazobactam or meropenem in combination with vancomycin. Antimicrob Agents Chemother. 2018;62(7) doi: 10.1128/AAC.00264-18. doi:10.1128/aac.00264-18 e00264-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figures S1, S2; Table S1.