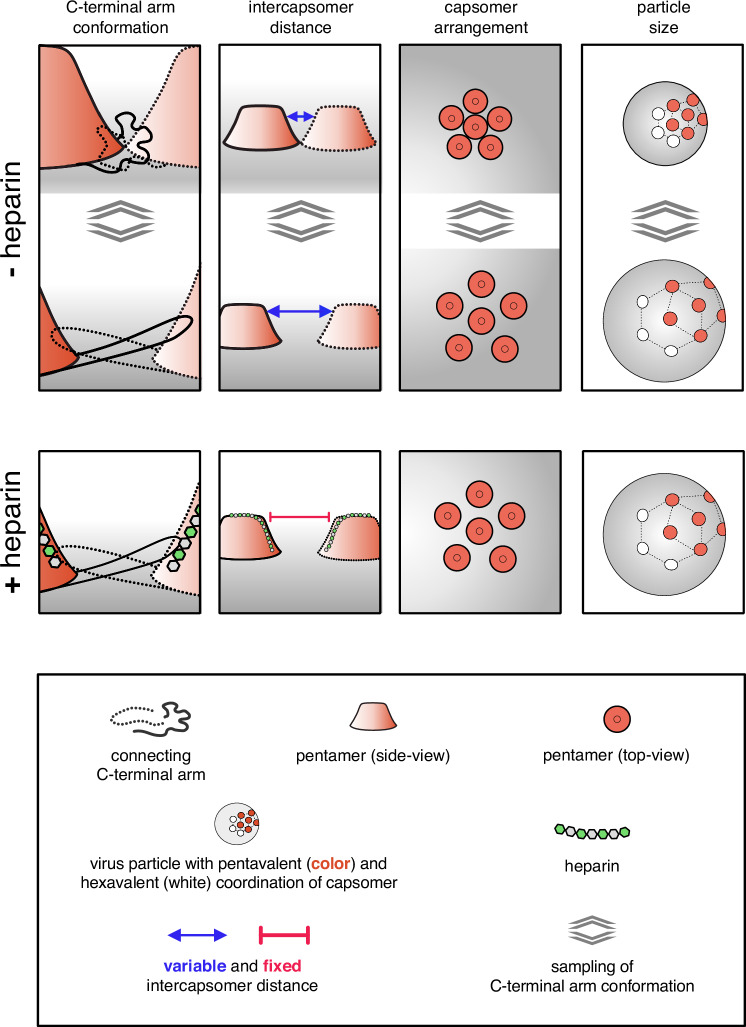
Fig. 6. Model of HPV16 structural activation through HS/heparin.
Schematic of HPV16 pentamer dynamics. The pentamers are viewed from the side in columns one and two and from the top in three. The last column displays particle size. Column one displays the invading arm that forms a disulfide bond in the adjacent pentamer to stabilise the particle. The invading arm is very flexible, which causes the pentamers to move towards and apart from each other (compare top and bottom), i.e. so-called ‘particle breathing’. The binding of a long HS/heparin molecule to several aligned binding sites, including K442/443, stabilises the invading arm in the most extended conformation, which reveals the H16.B20 epitope as well as the KLK8 cleavage site. The maximum interpentameric distance leads to increased particle size and a softening in AFM, while additional hydrogen bonds in the stretched arms decrease HDX in this region.