Abstract
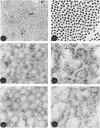
1. Achilles tendons of foetal, newborn, adult and old rabbits were examined by electron microscopy after staining by conventional methods or with the periodate/silver/methenamine technique. 2. The mean diameter of collagen fibrils increased with age whereas silver/methenamine-positivity became less evident. 3. Biochemical analyses showed a great decrease of the concentration of glycoproteins and galactosamine-containing glycosaminoglycans. 4. Collagen content increased with maturation and ageing of the tissue. 5. The extent of glycosylation of collagen hydroxylysine residues was also age-dependent; the total amount of hydroxylysyl glycosides rapidly decreased in the last days of prenatal life and in the first months after birth, corresponding to the rapid growth in collagen fibre diameter. 6. The hydroxylysyl diglycoside concentration decreased more markedly than that of the monoglycoside, thus indicating a possible gradual removal of the monosaccharide units. A role for the extent of glycosylation of tropocollagen molecules in fibre organization was suggested.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. C. Isolation of a glycoprotein and proteodermatan sulphate from bovine achilles tendon by affinity chromatography on concanavalin A-Sepharose. Biochim Biophys Acta. 1975 Feb 27;379(2):444–455. doi: 10.1016/0005-2795(75)90151-8. [DOI] [PubMed] [Google Scholar]
- Anderson J. C., Labedz R. I., Kewley M. A. The effect of bovine tendon glycoprotein on the formation of fibrils from collagen solutions. Biochem J. 1977 Nov 1;167(2):345–351. doi: 10.1042/bj1670345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anttinen H., Hulkko A. Regulation of the glycosylations of collagen hydroxylysine in chick embryo tendon and cartilage cells. Biochim Biophys Acta. 1980 Oct 15;632(3):417–427. doi: 10.1016/0304-4165(80)90237-8. [DOI] [PubMed] [Google Scholar]
- BITTER T., MUIR H. M. A modified uronic acid carbazole reaction. Anal Biochem. 1962 Oct;4:330–334. doi: 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- Cetta G., Tenni R., Castellani A. A. A simple method for quantitative estimation of collagen type III to type I ratio in soft tissues. Ital J Biochem. 1979 May-Jun;28(3):163–172. [PubMed] [Google Scholar]
- De Martino C., Zamboni L. Silver methenamine stain for electron microscopy. J Ultrastruct Res. 1967 Aug;19(3):273–282. doi: 10.1016/s0022-5320(67)80221-1. [DOI] [PubMed] [Google Scholar]
- Furthmayr H., Timpl R. Characterization of collagen peptides by sodium dodecylsulfate-polyacrylamide electrophoresis. Anal Biochem. 1971 Jun;41(2):510–516. doi: 10.1016/0003-2697(71)90173-4. [DOI] [PubMed] [Google Scholar]
- Hamazaki H., Hotta K. Enzymatic hydrolysis of disaccharide unit of collagen. Isolation of 2-O-alpha-D-glucopyranosyl-O-beta-D-galactopyranosyl-hydroxylysine glucohydrolase from rat spleens. Eur J Biochem. 1980 Oct;111(2):587–591. doi: 10.1111/j.1432-1033.1980.tb04975.x. [DOI] [PubMed] [Google Scholar]
- Huszar G., Maiocco J., Naftolin F. Monitoring of collagen and collagen fragments in chromatography of protein mixtures. Anal Biochem. 1980 Jul 1;105(2):424–429. doi: 10.1016/0003-2697(80)90481-9. [DOI] [PubMed] [Google Scholar]
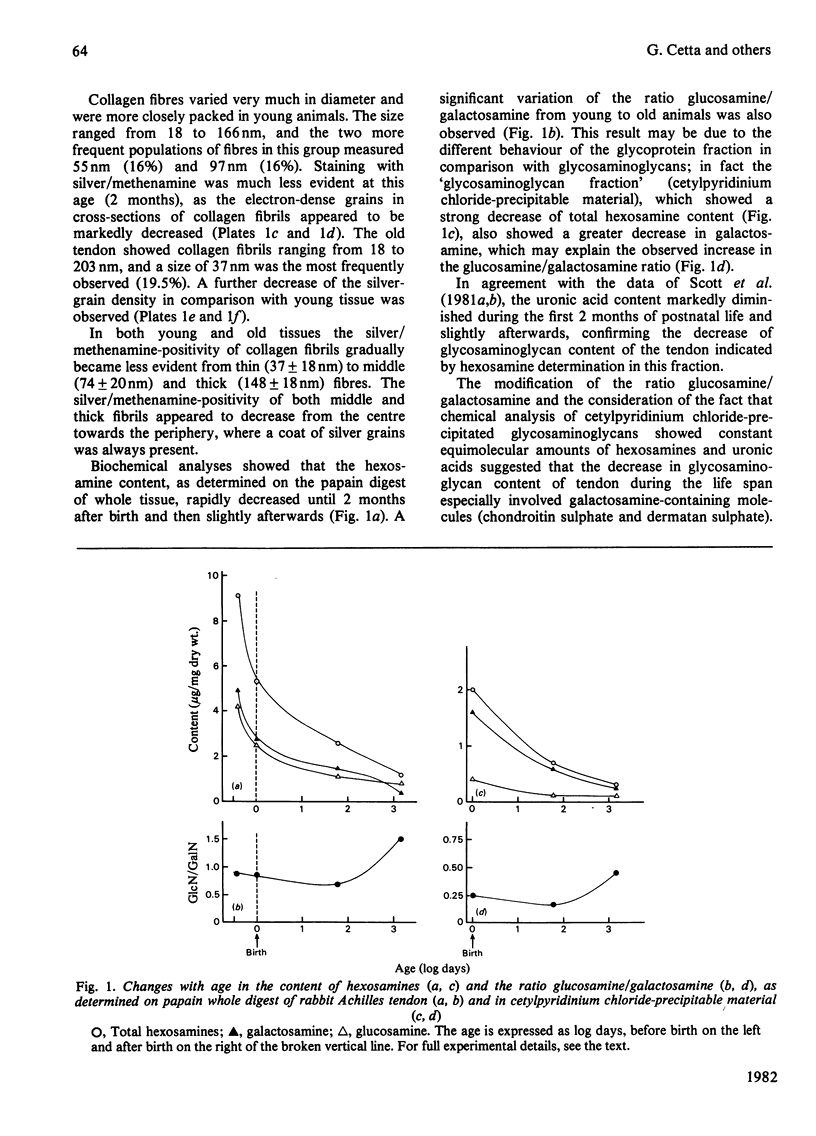
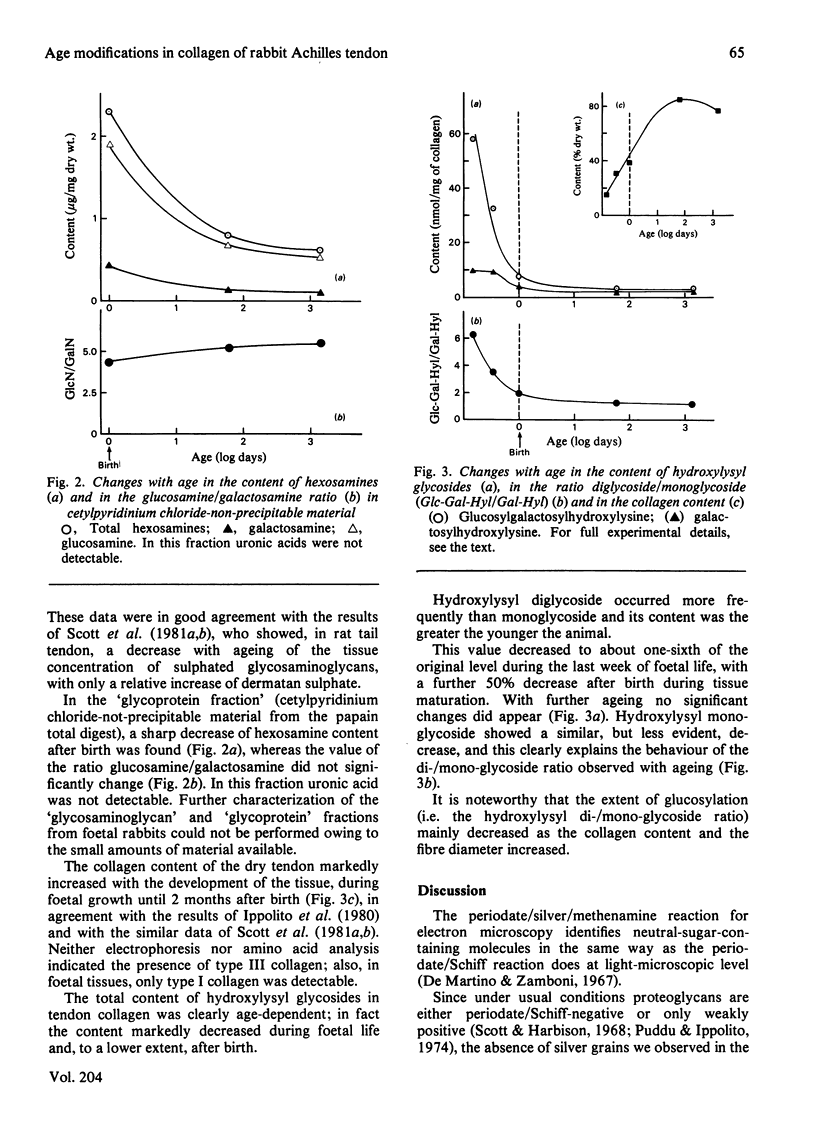
- Ippolito E., Natali P. G., Postacchini F., Accinni L., De Martino C. Morphological, immunochemical, and biochemical study of rabbit achilles tendon at various ages. J Bone Joint Surg Am. 1980;62(4):583–598. [PubMed] [Google Scholar]
- Isemura M., Takahashi K., Ikenaka T. Comparative study of carbohydrate-protein complexes. II. Determination of hydroxylysine and its glycosides in human skin and scar collagens by an improved method. J Biochem. 1976 Oct;80(4):653–658. doi: 10.1093/oxfordjournals.jbchem.a131324. [DOI] [PubMed] [Google Scholar]
- JACKSON S. F. The fine structure of developing bone in the embryonic fowl. Proc R Soc Lond B Biol Sci. 1956 Mar 26;146(923):270–280. doi: 10.1098/rspb.1957.0010. [DOI] [PubMed] [Google Scholar]
- KEECH M. K. The formation of fibrils from collagen solutions. IV. Effect of mucopolysaccharides and nucleic acids: an electron microscope study. J Biophys Biochem Cytol. 1961 Jan;9:193–209. doi: 10.1083/jcb.9.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan P. H., Jacobs H. G., Segrest J. P., Cunningham L. W. A comparative study of glycopeptides derived from selected vertebrate collagens. A possible role of the carbohydrate in fibril formation. J Biol Chem. 1970 Oct 10;245(19):5042–5048. [PubMed] [Google Scholar]
- Obrink B. A study of the interactions between monomeric tropocollagen and glycosaminoglycans. Eur J Biochem. 1973 Mar 1;33(2):387–400. doi: 10.1111/j.1432-1033.1973.tb02695.x. [DOI] [PubMed] [Google Scholar]
- Parry D. A., Craig A. S. Collagen fibrils and elastic fibers in rat-tail tendon: an electron microscopic investigation. Biopolymers. 1978 Apr;17(4):843–845. doi: 10.1002/bip.1978.360170404. [DOI] [PubMed] [Google Scholar]
- SCOTT J. E. Aliphatic ammonium salts in the assay of acidic polysaccharides from tissues. Methods Biochem Anal. 1960;8:145–197. doi: 10.1002/9780470110249.ch4. [DOI] [PubMed] [Google Scholar]
- Scott J. E. Collagen--proteoglycan interactions. Localization of proteoglycans in tendon by electron microscopy. Biochem J. 1980 Jun 1;187(3):887–891. doi: 10.1042/bj1870887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J. E., Harbinson R. J. Periodate oxidation of acid polysaccharides inhibition by the electrostatic field of the substrate. Histochemie. 1968;14(3):215–220. doi: 10.1007/BF00306317. [DOI] [PubMed] [Google Scholar]
- Scott J. E., Orford C. R. Dermatan sulphate-rich proteoglycan associates with rat tail-tendon collagen at the d band in the gap region. Biochem J. 1981 Jul 1;197(1):213–216. doi: 10.1042/bj1970213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J. E., Orford C. R., Hughes E. W. Proteoglycan-collagen arrangements in developing rat tail tendon. An electron microscopical and biochemical investigation. Biochem J. 1981 Jun 1;195(3):573–581. doi: 10.1042/bj1950573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykes B., Puddle B., Francis M., Smith R. The estimation of two collagens from human dermis by interrupted gel electrophoresis. Biochem Biophys Res Commun. 1976 Oct 18;72(4):1472–1480. doi: 10.1016/s0006-291x(76)80180-5. [DOI] [PubMed] [Google Scholar]
- WOOD G. C. The formation of fibrils from collagen solutions. 2. A mechanism of collagen-fibril formation. Biochem J. 1960 Jun;75:598–605. doi: 10.1042/bj0750598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood G. C. The precipitation of collagen fibers from solution. Int Rev Connect Tissue Res. 1964;2:1–31. doi: 10.1016/b978-1-4831-6751-0.50007-0. [DOI] [PubMed] [Google Scholar]