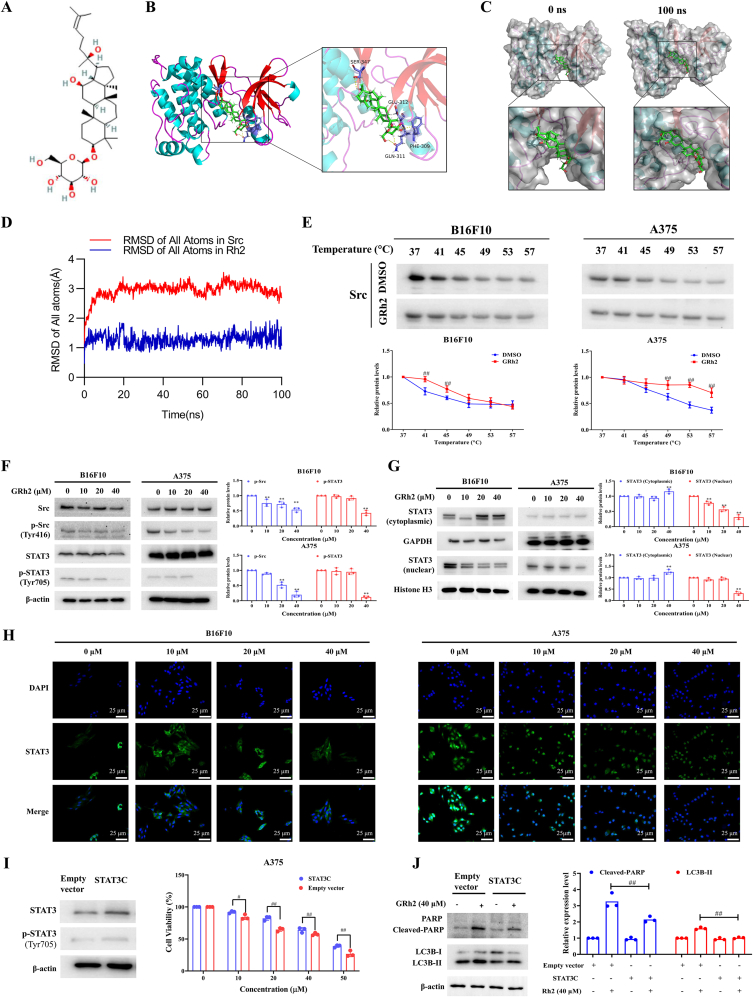
Fig. 5.
GRh2 exerts anti-melanoma effects via suppressing Src/STAT3 signaling. (A) GRh2's chemical structure. (B) Three-dimensional binding mode of GRh2 (green) in complex with Src. (C) Surface crystal structure of Src-GRh2 complex at 0 ns (left panels) and 100 ns (right panels). (D) RMSD of heavy atoms of unbound Src (blue) and the Rh2-Src complex (red). (E) CETSA for the binding of GRh2 to Src protein in melanoma cell lysate. ##P < 0.01 vs. DMSO treatment. (F) GRh2 prohibits Src/STAT3 activation in melanoma cells. (G) GRh2 lowers nuclear STAT3 protein level in melanoma cells. (H) Immunofluorescence assay was performed to analyze the effects of GRh2 on STAT3 nuclear translocation in B16F10 and A375 cells. Cells were incubated with GRh2 for 24 h, then immunofluorescence of STAT3 was labeled with green, and the nuclei was labeled with DAPI. Scale bar, 25 μm. (I) STAT3 over-activation attenuated the inhibitory effects of GRh2 on A375 cell viability. (J) Effects of GRh2 on LC3B and PARP protein expression in empty vector and STAT3C transfected A375 cells. ∗∗P < 0.01 vs. control group. #P < 0.05, ##P < 0.01.