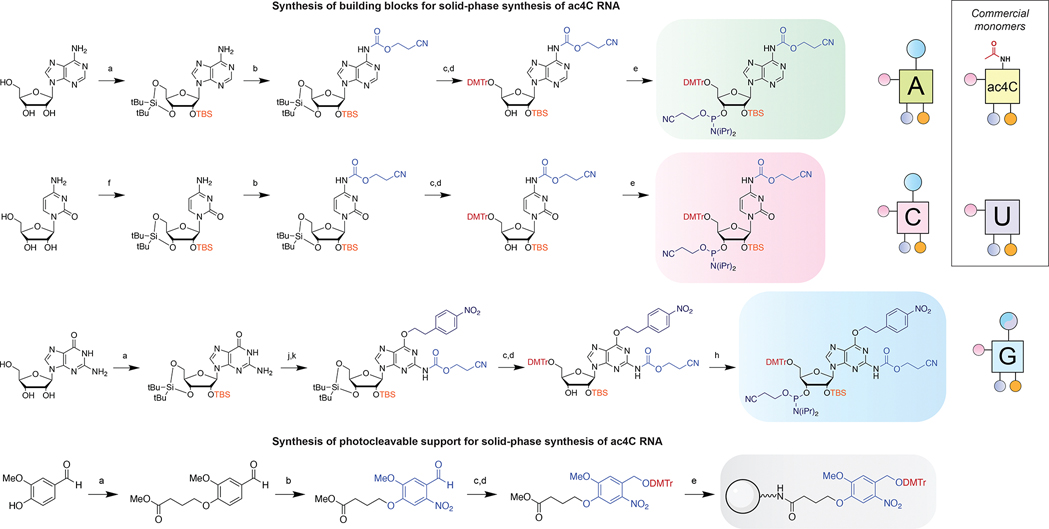
Figure 3.
Synthesis of building blocks for site-specific ac4C synthesis. Top: synthesis of N-ceoc-protected phosphoramidites. a) (i) (O-tBu)2Si(OTf)2, DMF, 0 °C, (ii) TBS-Cl, imidazole, DMF, 60 °C; b) ceoc-carbonyl-N-methylimidazolium chloride, DCM, 23 °C; c) HF-pyridine, pyridine, DCM, 0 °C; d) DMTr-Cl, pyridine, 4 °C; e) (OCH2CH2CN)P(iPr2N)2, tetrazole, ACN, 23 °C; f) (O-tBu)2Si(OTf)2, TfOH, DMF, 0 °C, (ii) TBS-Cl, imidazole, DMF, 60 °C; g) H2 (1 atm), Rh/alumina, MeOH, 23 °C; h) (OCH2CH2CN)(iPr2N)PCl, iPr2NEt, THF, 23 °C; j) triphenylphosphine, DIAD, (4-nitrophenyl)ethanol, dioxane, 100 °C; k) ceoc-chloroformate, DCM, 23 °C. Bottom: synthesis of photocleavable solid support. a) Methyl (4-bromobutyrate), KzCO3, DMF, 23 °C; b) TFA, KNO3, THF, 23 °C; c) NaBH4, MeOH, 23 °C, d) DMTr-Cl, pyridine, 4 °C; e) (i) aq. LiOH, THF, 23 °C, (ii) HATU, iPr2NEt, LCAA-CPG, ACN, 23 °C, (iii) Piv-Cl, N-methylimidazole, 2,6-lutidine, THF, 23 °C. Right: graphical abbreviations for monomers used in this study. Full NMR and mass spectra are provided in the Supporting Information.