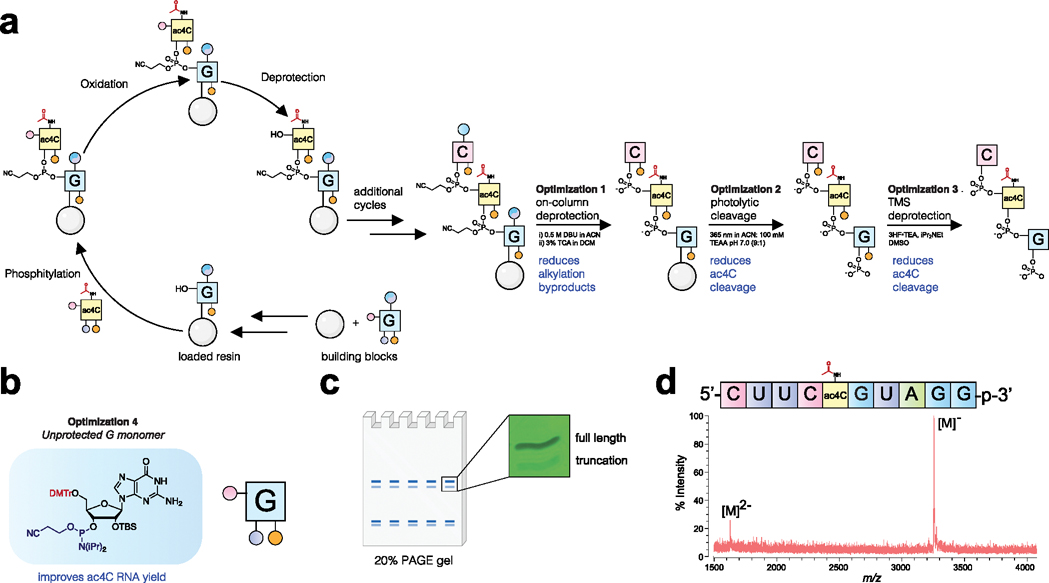
Figure 4.
(a) Solid-phase synthesis of ac4C-containing RNA oligonucleotides. Standard phosphoramidite synthesis conditions were employed, with the exception that no 5′-capping step was used. Optimizations critical for ac4C synthesis included on-column deprotection (#1), buffered photolytic cleavage (#2), and buffered desilylation condition (#3) to minimize alkylation and ac4C cleavage byproducts during release of the deprotected oligomer. (b) Ac4C synthesis is improved by the use of unprotected G monomer (#4). (c) Schematic for polyacrylamide gel electrophoresis (PAGE) purification and UV image of full-length and truncation products formed by optimized synthesis. (d) MALDI-TOF mass spectra of purified ac4C-containing 10-mer RNA. Full gels and MALDI-TOF spectra are provided in the Supporting Information.