Fig. 7. Lyn protects LCMV-infected mice from GI bleeding.
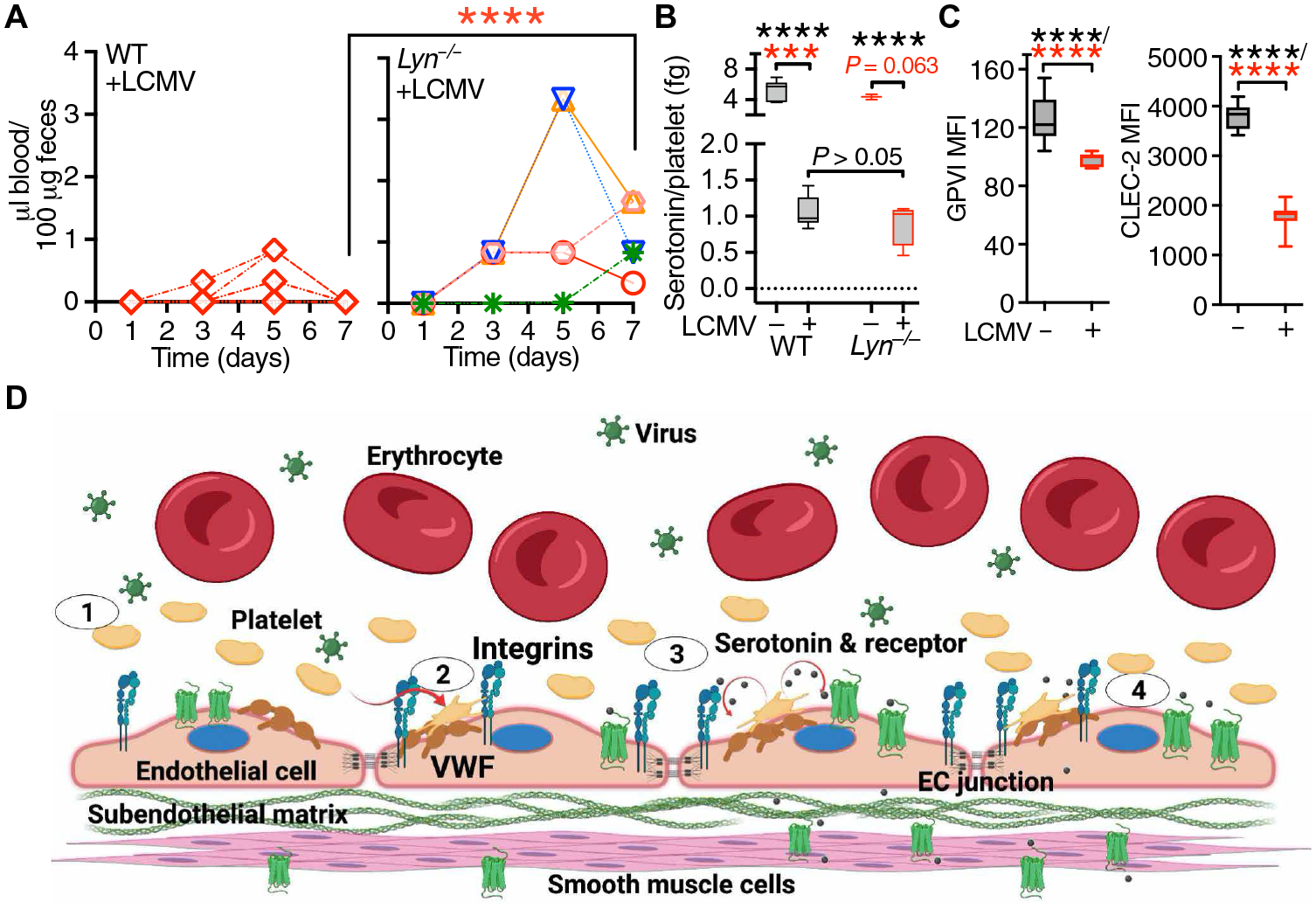
(A) Time course of FOB appearance in WT (n = 9) and Lyn−/− mice (n = 5) after LCMV infection; each symbol represents an individual mouse. Statistical analysis with the Kruskal-Wallis test followed by Dunn’s test for multiple comparisons (red asterisks). (B) Platelet serotonin release in WT (n = 7) and Lyn−/− (n = 3) noninfected mice (−LCMV) or WT (n = 14) and Lyn−/− (n = 4) mice 7 days after LCMV infection (+LCMV). Data, shown as 25th to 75th percentile boxes with minimum-maximum whiskers and median, were analyzed as in (A) (red asterisks) or by one-way ANOVA with Tukey’s posttest (black asterisks). (C) Platelet GPVI and CLEC-2 expression in uninfected WT mice (n = 15) or WT mice 7 days after LCMV infection (n = 9). Data were analyzed by two-tailed unpaired t test with Welch’s correction (black asterisks) and Mann-Whitney test (red asterisks). ***P < 0.001; ****P < 0.0001. (D) Schematic representation (created with BioRender.com) of the prohemostatic role of platelets during LCMV infection. Step 1: Inflammatory stimuli during LCMV infection induce endothelial cell (EC) activation, thereby promoting the release and up-regulation of mediators including von Willebrand factor (VWF) and P-selectin. Step 2: Platelets adhere to VWF on the surface of activated ECs through GPIbα and/or integrin αIIbβ3. These interactions represent the first necessary step for the control of endothelial barrier function by platelets. Step 3: Activated platelets release serotonin from δ-granules; released serotonin activates a subset of 5-hydroxytryptamine (5HT) receptors potentially linked to the activation of the Src kinase Lyn. Step 4: Activated Lyn leads to the tightening of endothelial cell junctions. Platelet-released serotonin can also induce vasoconstriction through effects on smooth muscle cells, further promoting control of vascular permeability.
