Abstract
Background
There is a paucity of Canadian studies using patient-level data to analyze the costs of catheter ablation (CA) for atrial fibrillation (AF). We sought to identify the health care resource use, costs, and cost predictors of CA.
Methods
A cost analysis was performed in a population of AF patients treated with CA in Central Zone Nova Scotia from 2010 to 2018. Costs were compared 2 years before ablation (pre-CA) with costs 2 years after (post-CA); the 3-month period post-CA was defined as the treatment window. Costs were also compared according to CA technology defined as before 2015 for patients treated with non-contact force sensing CA and after 2015 for patients treated with contact force sensing CA.
Results
Heart failure hospitalizations, AF-related emergency department visits, acute inpatient admissions, and cardioversions all decreased after ablation. The cost difference post-CA vs pre-CA was CAD$18,869 (95% confidence interval [CI], $15,570-$22,168). This increase in costs was driven by costs incurred during the treatment window, which was $21,439 (95% CI, $20,468-$22,409). After excluding treatment window costs, the mean year 1 post-CA cost was $11,223 (95% CI, $9113-$13,334) and year 2 post-CA cost was $4555 (95% CI, $3145-$5965); both were lower than the pre-CA costs. Costs remained stable over the time frame of the study period, with no influence from new technologies on cost. The post-CA cost difference between the post-2015 and pre-2015 groups was $2573 (95% CI, -$2336 to $7481).
Conclusions
We showed that although CA is expensive, it might be a cost-effective treatment modality for AF because of the associated reduction in costs and health care resource use.
RÉsumÉ
Contexte
Il existe peu d’études canadiennes utilisant des données sur les patients pour analyser les coûts de l’ablation par cathéter (AC) de la fibrillation auriculaire (FA). Notre étude visait à déterminer l’utilisation des ressources de santé, les coûts et les facteurs prédictifs du coût de l’AC.
Méthodologie
Une analyse des coûts a été réalisée au sein d’une population de patients atteints de FA traités par AC dans la région sociosanitaire Central de la Nouvelle-Écosse entre 2010 et 2018. Nous avons comparé les coûts 2 ans avant l’ablation (pré-AC) aux coûts 2 ans après l’ablation (post-AC); la période de 3 mois suivant l’AC était définie comme l’intervalle thérapeutique. Nous avons ensuite comparé les coûts en fonction de la technologie d’AC utilisée avant 2015 chez les patients traités par AC sans capteur de force de contact et après 2015 chez les patients traités par AC avec capteur de force de contact.
Résultats
Le nombre d’hospitalisations pour insuffisance cardiaque, de visites à l’urgence liées à la FA, d’admissions aux soins intensifs et de cardioversions a diminué après l’ablation. La différence de coûts entre la post-AC et la pré-AC était de 18 869 $ CA (intervalle de confiance [IC] à 95 %, 15 570 $ à 22 168 $). Cette hausse est attribuable aux coûts engagés durant l’intervalle thérapeutique, qui se sont chiffrés à 21 439 $ (IC à 95 %, 20 468 $ à 22 409 $). Une fois les coûts liés à l’intervalle thérapeutique exclus, le coût moyen post-AC à 1 an était de 11 223 $ (IC à 95 %, 9 113 $ à 13 334 $) et à 2 ans, de 4 555 $ (IC à 95 %, 3 145 $ à 5 965 $) : ces deux montants étaient inférieurs aux coûts pré-AC. Les coûts sont demeurés stables au cours de la période de l’étude et n’ont pas été influencés par les nouvelles technologies. La différence de coûts post-AC entre les groupes traités après 2015 et les groupes traités avant 2015 était de 2 573 $ (IC à 95 %, -2 336 $ à 7 481 $).
Conclusions
Nous avons démontré que même si l’AC est une intervention coûteuse, elle pourrait être une modalité de traitement rentable de la FA en raison de la réduction des coûts et de l’utilisation moindre des ressources de santé.
Atrial fibrillation (AF) is a common cardiac arrhythmia treated with the intent to prevent morbidity and improve quality of life.1 Observational studies have shown that patients with AF are high users of health care resources as a result of increased morbidity and AF progression.2, 3, 4 There is also mounting evidence that catheter ablation (CA) leads to reductions in health care resource use (HCRU) thereafter.5, 6, 7, 8
CA is often a part of the standard of care for the treatment of AF because of its improved efficacy in providing symptom relief and reducing arrhythmia burden compared with antiarrhythmic drugs (AADs).9 CA is a costly procedure, but can reduce progression and complications of AF, and might thus provide cost-effectiveness benefits.10 Berman et al. estimated a 21%-26% reduction in cardiovascular-related health care encounters in AF patients treated with CA.11 Furthermore, their model identified that CA could provide greater cost savings and quality-adjusted life years (QALYs) for patients in early AF.
Previous Canadian studies on the cost-effectiveness of CA in patients with AF have shown that CA is cost-effective in patients with heart failure (HF)12 and patients at low risk of stroke13 compared with AADs. Several modelling studies in other jurisdictions have also been performed to assess the cost-effectiveness of CA compared with AADs.14, 15, 16, 17, 18, 19, 20 Although the willingness-to-pay threshold varies among studies, they show greater costs associated with CA and QALY improvements ranging from 1.1 to 0.1 QALYs compared with AADs. These studies used economic modelling methods on the basis of published observational studies to arrive at these conclusions. A more recent analysis of the Catheter Ablation vs Antiarrhythmic Drug Therapy for Atrial Fibrillation (CABANA) trial population showed that CA is cost-effective on the basis of the incremental benefit in QALYs but not in life years provided compared with AADs.21 Although the current evidence points to CA as a cost-effective treatment modality, there is a paucity of Canadian studies using patient-level data to ascertain the value of CA compared with AADs.
The goal of this study was to build on the current evidence and further assess the HCRU and costs associated with CA. This was assessed by comparing the costs associated with AF management 2 years before CA (pre-CA) with 2 years after CA (post-CA). Additionally, the costs of patients treated with contact force sensing (CFS) technology were compared with those treated with non-CFS CA.
Methods
A cost analysis was conducted from a health care payer perspective.22 The study population included AF patients treated with CA at the QEII heart rhythm lab between 2010 and 2018. All patients residing outside of Nova Scotia Health Central Zone were excluded from the study using postal codes documented in the Heart Rhythm database. This subset of the patient population was excluded to permit acquisition of reliable emergency department (ED) visit data.
Study design, setting, and location
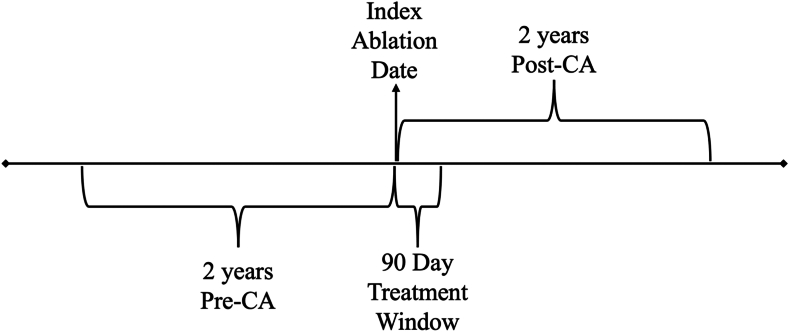
The study was designed as a before-and-after analysis of AF patients treated with CA at the QEII Health Sciences Centre in Halifax, Nova Scotia. The 2 years pre-CA when patients were receiving medications was considered the control and the 2 years post-CA as the intervention in the primary analysis. For each patient, 3 time points were identified: 2 years before ablation, a treatment window of 3 months from the index ablation date, and 2 years after ablation. In a secondary analysis, costs were compared according to the use of CFS catheters, which were introduced in 2015. Patients who had CA before 2015 were considered to have been treated with non-CFS technology and those treated after 2015 were considered to have been treated with CFS. CFS was the intervention and non-CFS the control for the analysis. Any CA taking place after the index CA date was considered a reablation. The study timeline is summarized in Figure 1.
Figure 1.
Study time line. CA, catheter ablation.
Health resource use and costs
Patient-level data on the number of HF hospitalizations, AF-related ED visits, AF-related hospitalizations, and same-day cardioversions were obtained from data linkage to the Emergency Department Information System (EDIS) and the Discharge Abstract Database. The EDIS is similar to the National Ambulatory Care System database. The QEII case costing centre compiles resource use data (inpatient admission, surgery, medical imaging, medication, laboratory tests, allied health labour, outpatient visits, and ED visits), assigns costs, and estimates the cost per case of patients assigned to a given case mix group. Physician fees are not included. This database was used to determine hospitalization costs. The EDIS and Discharge Abstract Database collect demographic, administrative, and clinical data from patients discharged from acute inpatient facilities and qualify them under case-mix group designations. The cost per HF hospitalization was the mean of case-mix groups; 195 (HF with coronary angiogram) and 196 (HF without coronary angiogram). The cost per ED visit comprised cost per case, ground ambulance, and specialist consultation fees. The cost per case came from the QEII case costing centre, and the specialist consultation fees were derived from the Nova Scotia Medical Services Insurance Physician Manual.23 The acute care inpatient cost per event was a mean of case-mix groups; 179 (cardiac conduction system intervention), 201(arrhythmia with coronary angiogram), and 202 (arrhythmia without coronary angiogram), also from the case costing centre. The CA procedure costs came from the QEII case costing centre. The relevant specialist costs associated with the CA procedure were also extracted from the Nova Scotia Physician manual. The cost per same-day AF cardioversion was on the basis of the Comprehensive Ambulatory Classification System code 201 and came from the case costing centre. The Comprehensive Ambulatory Classification System is another case-mix grouping system that is used to assign costs to patients admitted to ambulatory care settings using a unifying classification code. All costs were measured in 2021 Canadian dollars and are summarized in Supplemental Table S1.
Patient characteristics
Patient characteristics included age at ablation, age at first AF-related ED admission, sex, and time from first ED admission for AF to ablation.
Statistical analysis
Categorical variables were reported as frequencies and percentages for the entire sample and stratified according to ablation technology. Continuous variables were summarized by calculating the mean, standard deviation, median, and interquartile range where appropriate. Patient characteristics were compared according to ablation technology using the Pearson χ2 test, 2-sample t test with unequal variances, and the median test. The uncertainty around calculated estimates was quantified using 95% confidence intervals (CIs) constructed from bootstrapping (1000 replications). A 2-sample t test with nonparametric bootstrapping was used to compare the mean cost per case for all AF patients for AF management pre- and post-CA.
An augmented inverse probability weighting model with a lasso variable selection and 10-fold cross-validation was used to compare the mean cost per case according to CA technology in the pre- and post-2015 subgroups.24, 25, 26, 27 Augmented inverse probability weighting is used to estimate differences in outcomes in observational studies. It combines inverse probability weighting with augmentation to adjust for confounding variables. This method identifies the most relevant variables that contribute to outcome differences while avoiding overfitting and improving the model’s generalizability.25, 26, 27 The covariates in the outcome model included pre-CA costs (where relevant), age, sex, and repeat ablation. Age and sex were the covariates included in the treatment model, with the type of CA technology (contact force = 1, otherwise = 0) being the dependent variable. A generalized linear model with a log link function and gamma distribution to estimate the model was used to identify the demographic characteristics that predict higher costs.28 A 95% CI that excludes zero in all cases signifies a statistically significant difference at P < 0.05. All statistical analyses were performed using Stata, version 18 (StataCorp, College Station, TX).
Results
Study population demographic characteristics
Of the 346 patients who met inclusion criteria, 211 (61%) underwent ablation before 2015, and 135 (39%) after 2015 using CFS technology (Table 1). There was no statistically significant difference in age at the time of ablation, sex, or age at the time of first AF-related ED admission according to CA type. However, the mean time in months from the first ED presentation for AF to ablation was higher in the post-2015 group (20 ± 32 months) compared with those who underwent ablation before 2015 (15 ± 20 months; Table 2).
Table 1.
Cost∗ differences according to ablation technology
| Full sample, mean (95% CI) | Post-2015, mean (95% CI) | Pre-2015, mean (95% CI) | Cost difference, mean (95% CI)† | |
|---|---|---|---|---|
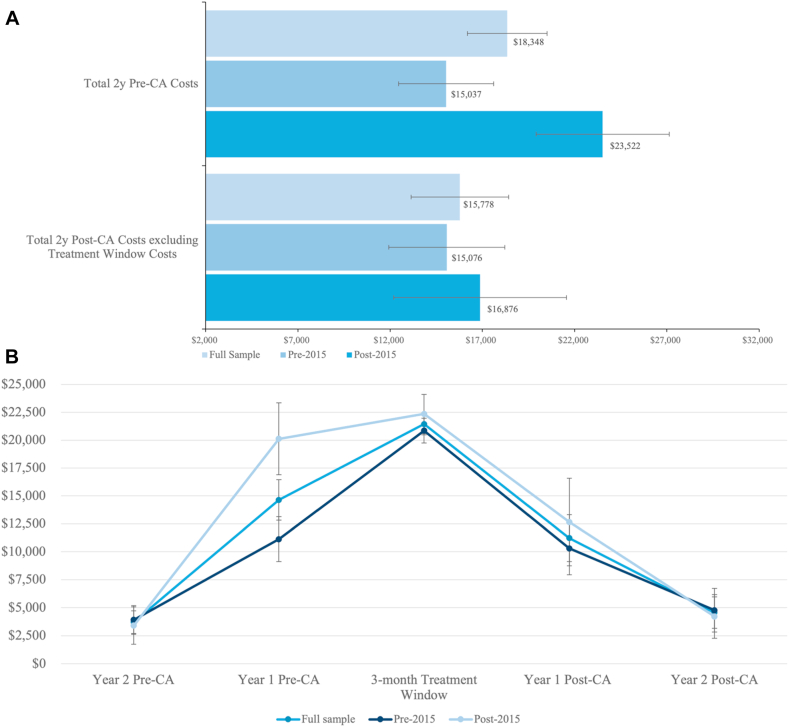
| Pre-CA (pharmacological management) | 18,348 (16,188; 20,509) | 23,522 (19,880-27,165) | 15,037 (12,442-17,632) | 9054 (4663-13,446) |
| Post-CA | 37,217 (34,235; 40,199) | 39,226 (33,742; 44,709) | 35,932 (32,498; 39,366) | 2573 (-2336 to 7481) |
| Difference: post-CA–pre-CA | 18,869 (15,570-22,168)‡ | 15,703 (9886-21,521)‡ | 20,895 (17,233; 24,555)‡ | - |
The pre-2015 sample represents patients who were treated with non-contact force sensing CA and the post-2015 group represents patients treated with contact force sensing CA.
CA, catheter ablation; CI, confidence interval.
All values are CAD$.
Differences in costs estimated using an augmented inverse probability of treatment weighting with lasso variable selection (10-fold cross-validation) model with 95% CI constructed from robust standard errors.
95% CI constructed from bootstrapped (1000 replications) standard errors.
Table 2.
Study population demographic characteristics
| Full sample (N = 346) | Pre-2015 (n = 211; 61%) | Post-2015 (n = 135; 39%) | P | |
|---|---|---|---|---|
| Female sex, n (%) | 106 (31) | 58 (27) | 48 (36) | 0.112 |
| Age at the time of CA | ||||
| Mean (SD), years | 58 (10) | 58 (10) | 58 (10) | 0.826 |
| 65 Years or older, n (%) | 106 (31) | 69 (33) | 37 (27) | 0.297 |
| Mean age at first AF-related ED visit (SD), years | 57 (10) | 57 (10) | 57 (10) | 0.599 |
| Mean time from first ED admission for AF to CA (SD), months | 17 (26) | 15 (20) | 20 (32) |
The pre-2015 sample represents patients who were treated with non-contact force sensing CA and the post-2015 group represents patients treated with contact force sensing CA.
AF, atrial fibrillation; CA, catheter ablation; ED, emergency department; SD, standard deviation.
HCRU
Overall, 138 (39.8%) patients underwent repeat ablation procedures; however, there was no statistical difference in the number of repeat ablations over time (Supplemental Table S2). The number of patients with AF-related ED visits post-CA was 3 in the pre-2015 group and 13 in the post-2015 group (Supplemental Table S3). Within each ablation technology, the number of patients requiring HF hospitalizations, AF-related ED visits, acute care inpatient stays, and same-day cardioversions decreased post-CA (Supplemental Table S3).
Cost differences
The mean cost in the pre-CA period for the total sample was CAD$18,348 (95% CI, $16,188-$20,509). The cost was higher in the 1 year pre-CA than in the 2 years pre-CA (Fig. 2A; Supplemental Table S4). However, the mean costs (95% CI) increased in the post-CA period to $37,217 ($34,235; $40,199), driven mainly by the mean costs incurred in the treatment window of $21,439 (95% CI, $20,468-$22,409). The mean cost in the post-CA period, excluding the treatment window, was $15,778 (95% CI, $13,122-18,434; Fig. 2B). The estimated cost difference pre-post was $18,869 (95% CI, $15,570-$22,168), and there was no statistically significant difference in the post-CA costs according to time period (Table 1).
Figure 2.
Summary cost (in CAD$) statistics (A) Pre- vs post-CA and (B) over time according to time period of CA. CA, catheter ablation.
Predictors of high costs
Patients who had repeat ablations had higher post-CA costs than those who did not, with a statistically significant cost difference of $33,710 (95% CI, $28,891- $38,529; Table 3). Similarly, patients aged 65 years or older at the time of ablation had higher costs post ablation than those younger than 65 years, with a cost difference of $6041 (95% CI, $1397-$10,685).
Table 3.
Patient characteristics influencing post-CA costs∗
| Variable | Post-CA cost difference | P |
|---|---|---|
| CA Time Period | ||
| Pre-2015 | Reference | |
| Post-2015 | 83 (95% CI, -4246 to 4411) | 0.970 |
| Sex | ||
| Male | Reference | |
| Female | -219 (95% CI, -4038 to 3600) | 0.911 |
| Age category at time of CA | ||
| Younger than 65 years | Reference | |
| 65 Years or older | 6041 (95% CI, 1397-10,685) | 0.011 |
| Repeat ablation | ||
| No repeat | Reference | |
| Repeat CA | 33,710 (95% CI, 28,891-38,529) | < 0.001 |
The results are from an adjusted generalized linear model with a gamma family and a log link function with bootstrapping. The variables included in the model were pre-CA costs, age at time of CA, CA technology, repeat ablation, and sex. The pre-2015 sample represents patients who were treated with non-contact force sensing CA and the post-2015 group represents patients treated with contact force sensing CA. P-values that are bolded highlight significance.
CA, catheter ablation; CI, confidence interval.
All cost values are CAD$.
Discussion
The results show that treating AF with CA results in reductions in HCRU. After excluding the cost of CA, this reduction is accompanied by reductions in costs that are sustained for the first and second years post-CA compared with the costs incurred in the year pre-CA. There was no significant difference in costs in the post-CA period before and after 2015; however, there was a significant difference in the pre-CA period.
Similar to these results, several observational studies of health care resource utilization post-CA also show significant reductions during the 1-2 years post-CA compared with the 1-2 years pre-CA because of the decrease in arrhythmia recurrence associated with CA compared with AADs.9 Furthermore, CA at a younger age and early in the diagnosis might lead to greater reductions in HCRU because of improved outcomes.29 AF patients who are more comorbid achieve greater benefits from CA, including reductions in hospitalization rates.30,31 The mean time from the first AF-related ED visit to CA in the study sample was 17 months. Therefore, the benefits of reducing recurrences and disease progression might not have been fully realized in the study sample. Because there were reductions in resource use, reduced costs post-CA were identified after the exclusion of the treatment window costs. This decrease in costs was in the CFS and non-CFS groups. There are reports in the literature regarding the association of higher costs and increased age and repeat ablation.6,32 Long-term outcomes of AF ablation have shown that younger age at the time of CA is a significant predictor of freedom from AF.33 It is possible that older patients are less likely to be free of AF post-CA and require repeat CA for adequate AF control.
The main determinants of costs and cost-effectiveness of CA are the costs incurred during the treatment window and those after ablation. Ablation itself requires preprocedural imaging, staffing, specialist fees, specialized equipment and technologies, and hospitalization post-CA. It is expected that the cost and cost-effectiveness of CA will improve as these aspects of CA are optimized. Improvements in each of these aspects are required to sustain the high number of AF ablations nationwide.
CA has consistently been shown to be superior to AADs in improving the quality of life of patients,5 regardless of AF recurrence or the need for reablation.34 It is likely that CA is cost-effective on the basis of previous analyses that show a significant QALY benefit that justifies the high cost of CA. The willingness-to-pay threshold is the national standard at which a medical intervention is deemed cost-effective. This is optimally a shared decision between those who contribute to the funds allocated for health care spending and those who benefit from the treatment in question.35 The Canadian Agency for Drugs and Technologies in Health does not publish willingness-to-pay or incremental cost effectiveness ratio (ICER) thresholds for new drug and technology assessments. Meanwhile, the Ontario Ministry of Health recommended an ICER threshold of $40,000-$60,000 per QALY in its letter to the Ombudsman of Ontario’s economic analysis of bevacizumab for colorectal cancer.36 These points highlight the complexity of these decisions as a single-payer health system in Canada, where health spending must balance the needs of those affected while allocating funds for all. The Canadian health system, however, does accept the cost-effectiveness of facility-based hemodialysis with an incremental cost-utility ratio of $103,779 per QALY37 and if this is an acceptable threshold then CA might also be similarly acceptable to Canadian taxpayers.
The only previous Canadian study to assess the cost-effectiveness of CA was performed by Lau et al.,12 in which the costs of CA were simulated in a population of AF patients with HF. Their results showed an incremental QALY gain of 0.45, an incremental cost of $15,095, and an ICER of $35,360 per QALY from CA. Their analysis yielded lower incremental costs because of the lower unit costs of several factors including admissions and the cost of CA. The QALY benefit estimated in their study might have been a product of the population they simulated. Their population, selected for HF, might have had a baseline quality of life that was poorer and subject to greater improvements from CA. These differences might explain the variability in ICER estimates in the literature; however, they continue to show that CA is a cost-effective treatment modality for AF. As a single-payer health care system in Canada, this cost analysis can continue to guide judicious public health investment into the treatments that yield the greatest benefit for Canadians. Although CA is a greater initial investment compared with coverage for AADs, the evidence presented suggests that there might be substantial future costs avoided by treating patients with CA. Moreover, patient quality of life is likely to improve even more with CA. Studies of patient outcomes show continued freedom from AF up to 15 years post-CA.33 It is possible that with long-term freedom from AF post-CA that HCRU, costs, QALY improvements and cost-effectiveness would all be improved by diverting patients away from AF progression.
Study limitations
There are limitations to this study. This analysis is limited in estimating the long-term costs of CA because of limited follow-up time. As a pre-post study design, the study sample was retrospectively selected on the basis of a registry of AF patients treated with CA. Therefore patients who used AADs with success and did not require CA were not included. This selection biased the sample to be one that was less comorbid and with more severe AF destined for CA. The database did not have a record of which ablation patients were paroxysmal or persistent AF phenotype. However, many patients in this study had persistent AF or longstanding persistent AF, because almost 40% of the total population underwent at least 1 reablation, and the mean time from the first ED visit for AF to ablation was 17 months. This might have biased the population to a subset of AF patients who required repeat ablation for adequate AF control, making their management more costly. Finally, the analysis could not be used to assess cost-effectiveness because there were no QALY data available. However, because of the evidence in the literature that shows QALY improvements from CA, it is expected that CA is a cost-effective treatment modality for AF.
Conclusion
CA might be a cost-effective treatment modality compared with pharmacological management in Canada on the basis of our analysis, which shows improvements in costs post-CA and reductions in HCRU. Further, CA, in the long term, might offer continued freedom from AF for a subset of patients and prevent disease progression. These advantages might justify the greater initial investment of treating AF patients with CA earlier, which could be offset by long-term savings.
Acknowledgements
The authors acknowledge the Case Costing Centre at Nova Scotia Health for supporting our research team with the cost data to perform our analysis.
Ethics Statement
The Nova Scotia Health Research Ethics Board approved this study effective December 30, 2022. Research Ethics Board file number: 1026996.
Patient Consent
A waiver of consent was granted by the Nova Scotia Health Research Ethics Board (file number: 1026996) to perform this research because this study was performed using patient identifiers for the purpose of data linkage. As such, patient consent is not applicable to this article.
Funding Sources
This study was not funded, however, it was supported by the Dalhousie Department of Medicine fund for statistical support from the Research Methods Unit.
Disclosures
The authors have no conflicts of interest to disclose.
Footnotes
See page 1377 for disclosure information.
To access the supplementary material accompanying this article, visit CJC Open at https://www.cjcopen.ca/ and at https://doi.org/10.1016/j.cjco.2024.07.016.
Supplementary Material
References
- 1.Calkins H., Hindricks G., Cappato R., et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm. 2017;14:e275–e444. doi: 10.1016/j.hrthm.2017.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chew D.S., Sacks N.C., Emden M.R., et al. Trends in health care resource use and expenditures in patients with newly diagnosed paroxysmal supraventricular tachycardia in the United States. Am Heart J. 2021;233:132–140. doi: 10.1016/j.ahj.2020.12.012. [DOI] [PubMed] [Google Scholar]
- 3.Kim M.H., Johnston S.S., Chu B.C., Dalal M.R., Schulman K.L. Estimation of total incremental health care costs in patients with atrial fibrillation in the United States. Circ Cardiovasc Qual Outcomes. 2011;4:313–320. doi: 10.1161/CIRCOUTCOMES.110.958165. [DOI] [PubMed] [Google Scholar]
- 4.Tripathi B., Atti V., Kumar V., et al. Outcomes and resource utilization associated with readmissions after atrial fibrillation hospitalizations. J Am Heart Assoc. 2019;8 doi: 10.1161/JAHA.119.013026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gupta D., Vijgen J., Potter T.D., et al. Quality of life and healthcare utilisation improvements after atrial fibrillation ablation. Heart. 2021;107:1296–1302. doi: 10.1136/heartjnl-2020-318676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Degli Esposti L., Dovizio M., Leogrande M., Perrone V., De Ponti R. Evaluation of the impact of catheter ablation procedure on outcomes and economic burden in patients with atrial fibrillation: real-world data from Italian administrative databases. Healthcare (Basel) 2022;10:2561. doi: 10.3390/healthcare10122561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Friedman D.J., Field M.E., Rahman M., et al. Catheter ablation and healthcare utilization and cost among patients with paroxysmal versus persistent atrial fibrillation. Heart Rhythm O2. 2020;2:28–36. doi: 10.1016/j.hroo.2020.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mark D.B., Anstrom K.J., Sheng S., et al. Effect of catheter ablation vs medical therapy on quality of life among patients with atrial fibrillation: the CABANA randomized clinical trial. JAMA. 2019;321:1275–1285. doi: 10.1001/jama.2019.0692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deshpande R., AlKhadra Y., Singanallur P., Botchway A., Labedi M. Outcomes of catheter ablation versus antiarrhythmic therapy in patients with atrial fibrillation: a systematic review and meta-analysis. J Interv Card Electrophysiol. 2022;65:773–802. doi: 10.1007/s10840-022-01365-z. [DOI] [PubMed] [Google Scholar]
- 10.Reynolds M.R. Addition by subtraction: disease progression and the value of atrial fibrillation ablation. Circ Arrhythm Electrophysiol. 2023;16 doi: 10.1161/CIRCEP.123.011918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berman A.E., Kabiri M., Wei T., et al. Economic and health value of delaying atrial fibrillation progression using radiofrequency catheter ablation. Circ Arrhythm Electrophysiol. 2023;16 doi: 10.1161/CIRCEP.122.011237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lau D., Sandhu R.K., Andrade J.G., et al. Cost-utility of catheter ablation for atrial fibrillation in patients with heart failure: an economic evaluation. J Am Heart Assoc. 2021;10 doi: 10.1161/JAHA.120.019599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khaykin Y., Mallow P.J., Rizzo J.A., et al. Cost-effectiveness of catheter ablation versus antiarrhythmic drug therapy for the treatment of atrial fibrillation: a Canadian perspective. J Health Econ Outcomes Res. 2016;3:1–12. doi: 10.36469/9837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leung L.W.M., Imhoff R.J., Marshall H.J., et al. Cost-effectiveness of catheter ablation versus medical therapy for the treatment of atrial fibrillation in the United Kingdom. J Cardiovasc Electrophysiol. 2022;33:164–175. doi: 10.1111/jce.15317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gao L., Moodie M. Modelling the lifetime cost-effectiveness of catheter ablation for atrial fibrillation with heart failure. BMJ Open. 2019;9 doi: 10.1136/bmjopen-2019-031033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Du X., He X., Jia Y., et al. A long-term cost-effectiveness analysis comparing radiofrequency catheter ablation with antiarrhythmic drugs in treatment of Chinese patients with atrial fibrillation. Am J Cardiovasc Drugs. 2019;19:569–577. doi: 10.1007/s40256-019-00349-1. [DOI] [PubMed] [Google Scholar]
- 17.Aronsson M., Walfridsson H., Janzon M., et al. The cost-effectiveness of radiofrequency catheter ablation as first-line treatment for paroxysmal atrial fibrillation: results from a MANTRA-PAF substudy. Europace. 2015;17:48–55. doi: 10.1093/europace/euu188. [DOI] [PubMed] [Google Scholar]
- 18.Reynolds M.R., Lamotte M., Todd D., et al. Cost-effectiveness of cryoballoon ablation for the management of paroxysmal atrial fibrillation. Europace. 2014;16:652–659. doi: 10.1093/europace/eut380. [DOI] [PubMed] [Google Scholar]
- 19.Blackhouse G., Assasi N., Xie F., et al. Cost-effectiveness of catheter ablation for rhythm control of atrial fibrillation. Int J Vasc Med. 2013;2013 doi: 10.1155/2013/262809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eckard N., Davidson T., Walfridsson H., Levin L.Å. Cost-effectiveness of catheter ablation treatment for patients with symptomatic atrial fibrillation. J Atr Fibrillation. 2009;2:195. doi: 10.4022/jafib.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chew D.S., Li Y., Cowper P.A., et al. Cost-effectiveness of catheter ablation versus antiarrhythmic drug therapy in atrial fibrillation: the CABANA randomized clinical trial. Circulation. 2022;146:535–547. doi: 10.1161/CIRCULATIONAHA.122.058575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jönsson B. Ten arguments for a societal perspective in the economic evaluation of medical innovations. Eur J Health Econ. 2009;10:357–359. doi: 10.1007/s10198-009-0173-2. [DOI] [PubMed] [Google Scholar]
- 23.Nova Scotia Medical Services Insurance MSI. Physician’s Manual. https://msi.medavie.bluecross.ca/wp-content/uploads/sites/3/2024/01/Physicians-Manual-2024.pdf Available at:
- 24.Glynn A.N., Quinn K.M. An introduction to the augmented inverse propensity weighted estimator. Political Analysis. 2010;18:36–56. [Google Scholar]
- 25.Koch B., Vock D.M., Wolfson J. Covariate selection with group lasso and doubly robust estimation of causal effects. Biometrics. 2018;74:8–17. doi: 10.1111/biom.12736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Drukker D. Stata Conference; Chicago, IL: July 11-12, 2019. Inference after lasso model selection. Presented at the. [Google Scholar]
- 27.Ahrens A., Hansen C.B., Schaffer M.E. Lassopack: model selection and prediction with regularized regression in Stata. The Stata Journal. 2020;20:176–235. [Google Scholar]
- 28.Lee C.S., Conway C. The role of generalized linear models in handling cost and count data. Eur J Cardiovasc Nurs. 2022;21:392–398. doi: 10.1093/eurjcn/zvac002. [DOI] [PubMed] [Google Scholar]
- 29.Andrade J.G., Deyell M.W., Macle L., et al. Progression of atrial fibrillation after cryoablation or drug therapy. N Engl J Med. 2023;388:105–116. doi: 10.1056/NEJMoa2212540. [DOI] [PubMed] [Google Scholar]
- 30.Dickow J., Kany S., Roth Cardoso V., et al. Outcomes of early rhythm control therapy in patients with atrial fibrillation and a high comorbidity burden in large real-world cohorts. Circ Arrhythm Electrophysiol. 2023;16 doi: 10.1161/CIRCEP.122.011585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rillig A., Borof K., Breithardt G., et al. Early rhythm control in patients with atrial fibrillation and high comorbidity burden. Circulation. 2022;146:836–847. doi: 10.1161/CIRCULATIONAHA.122.060274. [DOI] [PubMed] [Google Scholar]
- 32.Mansour M., Karst E., Heist E.K., et al. The impact of first procedure success rate on the economics of atrial fibrillation ablation. JACC Clin Electrophysiol. 2017;3:129–138. doi: 10.1016/j.jacep.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 33.Winkle R.A., Mead R.H., Engel G., et al. Very long term outcomes of atrial fibrillation ablation. Heart Rhythm. 2023;20:680–688. doi: 10.1016/j.hrthm.2023.02.002. [DOI] [PubMed] [Google Scholar]
- 34.Wokhlu A., Monahan K.H., Hodge D.O., et al. Long-term quality of life after ablation of atrial fibrillation the impact of recurrence, symptom relief, and placebo effect. J Am Coll Cardiol. 2010;55:2308–2316. doi: 10.1016/j.jacc.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 35.Menzel P.T. How should willingness-to-pay values of quality-adjusted life-years be updated and according to whom? AMA J Ethics. 2021;23:601–606. doi: 10.1001/amajethics.2021.601. [DOI] [PubMed] [Google Scholar]
- 36.Martin A. A vast injustice: investigation into the Ministry of Health and Long-Term Care’s decision-making concerning the funding of avastin for colorectal cancer patients. https://www.ombudsman.on.ca/Files/sitemedia/Documents/Investigations/SORT Investigations/avastinweb-en_1.pdf Available at:
- 37.Ferguson T.W., Whitlock R.H., Bamforth R.J., et al. Cost-utility of dialysis in Canada: hemodialysis, peritoneal dialysis, and nondialysis treatment of kidney failure. Kidney Med. 2020;3:20–30.e1. doi: 10.1016/j.xkme.2020.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.