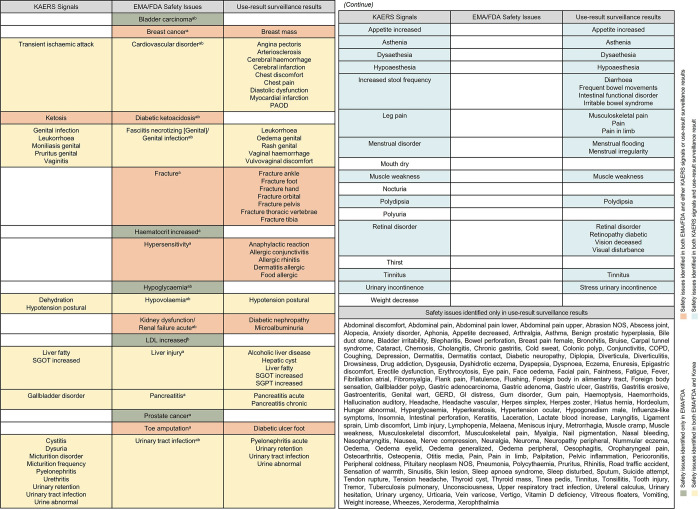
Fig 2. Matching the 17 EMA/FDA safety issues with Korean safety issues.
AE, adverse event; COPD, Chronic obstructive pulmonary disease; EMA, European Medicines Agency; FDA, Food and Drug Administration; GERD, gastroesophageal reflux disease; GI, gastrointestinal; KAERS, Korea adverse event reporting system; LDL, low-density lipoprotein; NOS, not otherwise specified; PAOD, Peripheral arteriosclerotic occlusive disease; SGOT, serum glutamic oxaloacetic; SGPT, serum glutamic pyruvate transaminase. aSafety concerns in the European Union Risk Management Plan. bAdverse events noted in the Warning and Precautions section of the FDA drug label.