Abstract
Background
To evaluate changes in H pylori infection prevalence in Greece during a ten-year period, and to examine its antigenic profile.
Methods
Three groups of patients were studied. Group O-87: Banked serum samples of 200 consecutive adult outpatients, from the Hepato-Gastroenterology clinic of a teaching hospital at Athens, collected in 1987. Group O-97: Serum samples of 201 similarly selected outpatients from the same Unit, collected in 1997. Group BD-97: Serum samples of 120 consecutive blood donors from the same hospital, collected in 1997. H pylori IgG antibody seroprevalence was studied by a quantitative ELISA. Antigenic profile was studied by western-blot IgG assay, in 62 IgG positive patients of O-97 and BD-97. Results were analyzed by conventional statistics and multivariate regression analysis.
Results
The H pylori seroprevalence increased with age in the three tested groups. In O-97, seroprevalence did not differ from that, in BD-97. On the contrary, there was a significant decrease in seropositivity between O-87 and O-97 (59.5% vs 49.2%, p = 0.039). Multiple regression analysis showed that age over 35 years (OR:3.45, 95% CI:1.59–7.49, p = 0.002) and year of patients' selection – that is 1987 or 1997 – (OR:1.73, 95% CI:1.14–2.65 for 1987, p = 0.010), were independent risk factors of H pylori infection. The seroprevalence of CagA+ and VacA+ strains was 77.4% and 58.5%, respectively, and type I(CagA+/VacA+) strains were significantly more common than type II(CagA-/VacA-) strains (59.7% vs 22.6%, p < 0.001).
Conclusions
During a ten-year period, we found a significant decrease of H pylori infection in Greece and our data support the birth cohort phenomenon as an explanation for the age-dependent increase of H pylori infection. The prevalence of CagA and/or VacA positive strains is relatively high, in a country with low incidence of gastric cancer.
Background
It is known that H pylori infection occurs mainly in early childhood and the link between the infection and risk factors such as socioeconomic status and living conditions in childhood is well documented [1-3]. Thus, there is a marked difference in the prevalence of H pylori infection between developing and developed countries during early childhood [4]. However, everywhere in the world, serological data have shown that the prevalence of the infection increases with age [5-7]. In particular in Greece, according to a study carried out in the early '90s [8] seroprevalence increased about 10% per 10 years, from 40% in people aged 21–40 years to 77% in those older than 60 years.
Long-term follow-up studies in developed countries, with low rates of H pylori infection, suggest that the age-dependent increase of seropositivity is mainly due to the decreasing rate of childhood infections [9-13], since many studies have proved that during adulthood the rates of seroconversion and seroreversion are almost equal [9,11,14,15]. This phenomenon, is often referred as a "birth cohort phenomenon". In contrast to this theory, there is also evidence suggesting that a continuous risk of acquisition rather than a cohort effect, best explains the age-dependent increase of seropositivity [16]. However, in populations with higher rates of infection than that observed in occidental and Scandinavian countries, there are no data available to evaluate if the cohort phenomenon or the continuous risk of acquisition could better explain the age dependent increase of H pylori infection.
Nevertheless, it has been shown that some H pylori genes (vacA, cagA) confer different biological properties which could enhance the in vivo pathogenicity of the bacteria [17,18]. Patients infected with cagA-positive strains of H pylori demonstrate enhanced expression of various cytokines [19] and these patients present a higher grade of gastric inflammation and accelerated epithelial damage [20]. Thus, recently, there has been an interest in detecting the H pylori immunophenotype and in particular, the CagA and/or VacA status.
The aim of this study was two-fold: first, to examine the seroprevalence of H pylori infection in two samples of Greek adult population that were collected in 1987 and in 1997, that is ten years apart, in order to find out if the "birth cohort phenomenon" or the continuous risk of acquisition could better explain the age-dependent increase of H pylori seropositivity, in a country of south-east Europe with relatively high rate of H pylori infection; and second, to evaluate the antigenic profile of the H pylori infection (Vag, Cag etc) in a Greek adult population.
Material and Methods
Subjects
The study population included three groups of patients:
Group O-87
Banked serum samples of 200 consecutive adult outpatients, irrespective of their socioeconomic status and the cause of admission, (107 men and 93 women, aged 15–82 years, mean age 44.3 +17 years), from Hepatology Section of the Gastroenterology clinic of 1st Department of Propedeutic Medicine (Athens University School of Medicine, "Laikon" General Hospital). The used serum samples were originally obtained in 1987, for the study of viral hepatitis.
Group O-97
Serum samples of 201 similarly selected outpatients (123 men and 78 women, aged 16–85 years, mean age 45.9 +15.2 years), from the same Section, that were collected in 1997.
Group BD-97
Serum samples of 120 consecutive blood donors from the same hospital (102 men and 18 women, aged 18–62 years, mean age 40.1 +10.8 years), collected in 1997.
Study design and definition of variables
Group O-97 was compared with group BD-97. Subsequently, group O-97 was compared with group O-87. The analyzed variables were age and gender. Both groups (O-87 and O-97) comprised of consecutive outpatients from the same central hospital in Athens. Thus, the demographic data and the average socioeconomic profile of patients could be considered roughly similar in both groups, taking of course into consideration the total development of the socioeconomic and educational level in our country during the last decade.
The antigenic profile of the infection was studied in 62 randomly selected H pylori IgG antibody positive patients of O-97 and BD-97 groups (the first 40 and 22 consecutive H pylori IgG antibody positive samples of O-97 and BD-97 groups, respectively).
Assays
All serum samples were stored at -70°C and had not been thawed before the current analysis, except for the samples of O-87 group which had been thawed once before.
Assay for antibodies to H pylori
H pylori seroprevalence was studied determining IgG antibodies by a quantitative enzyme-linked immunosorbent assay (ELISA, Diasorin Diagnostics Srl-Manufactured by Hycor Biomedical GmbH). The antigen used was extracted by sonication from the H pylori strain NTCC 43054. The main protein fractions which predominantly included in H pylori antigen coated to ELISA microtiter plates, were: p120–130, p83–87, p67, p63/66, p50–59, p28–31, p19 and p14. These immunogenic proteins were enriched and purified by a designated developed biochemical procedure. All assays were performed in duplicate and the intra-assay and inter-assay variations were <5%, as was estimated with the positive and negative control sera. Validation of the ELISA used, was performed, at the same period, in 40 consecutive patients of the Endoscopy Unit of the same hospital. Using histology (modified Giemsa) as "gold-standard", the estimated sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) of that ELISA were 87%, 94%, 95% and 84%, respectively.
Antigenic profile of H pylori infection
The antigenic profile of the infection was studied using Immunoblot Helicobacter pylori IgG (Microgen GmbH, Germany). This test permits the safe identification and localization of specific antibodies on the solid phase because the antigens are electrophoretically separated. The test results were evaluated according to the manufacturer's instructions and the main detected bands were: p120(CagA), p87(VacA), p62(UreB), p58(HspA&B), p54(FlaA) and p29(UreA). Using histology and/or microbiology as "gold standard", the sensitivity and specificity of this immunoblotting test was 95% and 83%, respectively (Mikrogen molekularbiologische Entwicklungs-Gmbh, Immunoblot Helicobacter pylori IgG, Instructions for use version: GIIBHPE004.DOC).
Statistical analysis
Chi-square test was used to test for an association between H pylori infection and year of patients' selection, by age group and overall. Multivariate logistic regression was used to determine risk factors associated with H pylori infection. A model containing all variables was considered. Variables that did not contribute to the model, based on their Wald statistic, were eliminated and the new model was compared to the old through the likelihood ratio statistic. Variables whose exclusion gave a non-significant likelihood ratio statistic (p > 0.05) were omitted from the model.
Results
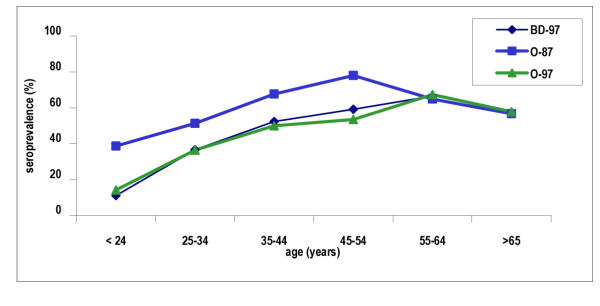
H pylori seropositivity in O-97 and BD-97 groups (table 1, fig. 1)
Table 1.
Seroprevalence of H pylori antibodies (IgG) in O-87, O-97 and BD-97 groups.
| Age | Studied number | H pylori IgG(+) (%) | ||||
| (years) | O-87 | O-97 | BD-97 | O-87 | O-97 | BD-97 |
| 15–24 | 31 | 21 | 9 | 12(38.7) | 3(14.2) | 1(11.1) |
| 25–34 | 39 | 33 | 30 | 20(51.3) | 12(36.4) | 11(36.6) |
| 35–44 | 31 | 42 | 42 | 21(67.7) | 21(50.0) | 22(52.4) |
| 45–54 | 32 | 43 | 27 | 25(78.1)† | 23(53.5)† | 16(59.2) |
| 55–64 | 37 | 43 | 12 | 24(64.8) | 29(67.4) | 8(66.6) |
| > 65 | 30 | 19 | – | 17(56.7) | 11(57.9) | – |
| Total | 200 | 201 | 120 | 119(59.5)‡ | 99(49.2)‡ | 58(48.3) |
† p = 0.032, ‡ p = 0.039
Figure 1.
Seroprevalence of H pylori antibodies (IgG) in O-87, O-97 and BD-97 groups.
In 1997, seroprevalence of H pylori infection in all patients and in the patients' age groups did not differ from that of blood donors Thus in group BD-97, seropositivity was 11% up to 24 years, increased of about 136% per decade up to 44 years and continued to increase at an average of 13% per decade up to 60 years. In group O-97 seropositivity was 14.2% up to 24 years, increased of about 96% per decade up to 44 years and continued to increase at an average of 16.5% per decade up to 64 years.
Prevalence of antibodies in O-87 and O-97 groups of patients (table 1, figure 1)
The prevalence increased with age in both tested groups of patients (O-87 and O-97). More specifically, in 1987, the increase was continuous up to 45–54 years, at an average rate of 26% per decade. After the age of 55 years, a decrease of seropositivity at an average of 10% per decade was observed.
In 1997, the prevalence was increasing up to the 55–64 years group, at an average rate of 50% per decade. The average increasing rate was highest for up to 44 years (96% per decade) and continued at an average of 16.5% per decade up to the 55–64 years group. In the oldest age group (>65 years), a decrease of seropositivity of about 14% per decade was noticed.
Logistic regression analysis in the group O-87 with independent variables such as age and gender, showed that age over 35 years was the only independent risk factors of H pylori infection (table 2). Gender was not associated with positivity, but there was a small but insignificant increase of seropositivity in males (OR: 1.24, 95% CI: 0.68–2.25, p = 0.47).
Table 2.
Independent risk factors of H pylori seropositivity in each group of tested patients (O-87, O-97) (multivariate logistic regression analysis).
| O-87 | O-97 | |||||
| Variables Age(years) | Odds ratio | 95% CI | p | Odds ratio | 95% CI | p |
| 15 – 24 | 1 | 1 | ||||
| 25 – 34 | 1.68 | (0.63, 4.47) | 0.29 | 3.34 | (0.81, 13.73) | 0.09 |
| 35 – 44 | 3.28 | (1.13, 9.49) | 0.02 | 5.54 | (1.41, 21.88) | 0.01 |
| 45 – 54 | 6.05 | (1.96, 18.65) | 0.002 | 7.12 | (1.80, 28.07) | 0.005 |
| 55 – 64 | 3.28 | (1.19, 9.02) | 0.02 | 13.51 | (3.35, 54.51) | < 0.001 |
During a ten-year period (1987–1997) the crude H pylori seroprevalence fell significantly by 10% (from 59.5% in group O-87, to 49.2% in group O-97, p = 0.039), and it was constantly lower in each age group (up to 55–64 years) by 15 – 24%.
Logistic regression analysis in all patients (group O-87 and group O-97, 401 patients) with independent variables such as age, gender and year of patients' selection (that is 1987 or 1997), showed that age over 35 years and the year 1987 were independent risk factors for H pylori infection (table 3).
Table 3.
Independent risk factors of H pylori seropositivity in O-87 and O-97 groups of patients (N:401) (multivariate logistic regression analysis).
| Variables Age(years) | Odds ratio | 95% CI | p |
| 15 – 24 | 1 | ||
| 25 – 34 | 2.02 | (0.94, 4.37) | 0.073 |
| 35 – 44 | 3.45 | (1.59, 7.49) | 0.002 |
| 45 – 54 | 5.07 | (2.31, 11.11) | < 0.001 |
| 55 – 64 | 5.60 | (2.57, 12.19) | < 0.001 |
| > 65 | 3.49 | (1.51, 8.10) | 0.004 |
| Period | |||
| 1997 | 1 | ||
| 1987 | 1.73 | (1.14, 2.65) | 0.010 |
The peak of H pylori seroprevalence differs by one decade in the two tested samples (fig 1). This may suggest that the risk of infection is associated with the year of patients' birth. Indeed, in both groups of patients the highest risk for H pylori seropositivity was found in patients who were born during the decade 1933–1942 in comparison with those born after 1953) (table 4).
Table 4.
Decade of birth as an independent risk factor of H pylori seropositivity (multivariate logistic regression analysis).
| 1987 | 1997 | |||||
| Year of patients' birth | Odds ratio | 95% CI | p | Odds ratio | 95% CI | p |
| > 1953 | 1 | 1 | ||||
| 1943–1952 | 2.49 | 1.01 – 6.14 | 0.047 | 2.08 | 1.00 – 4.34 | 0.050 |
| 1933–1942 | 4.54 | 1.71 – 12.03 | 0.002 | 3.57 | 1.67 – 7.62 | 0.001 |
| 1923–1932 | 2.47 | 1.07 – 5.73 | 0.035 | 2.87 | 0.96 – 8.56 | 0.058 |
| < 1923 | 1.69 | 0.70 – 4.06 | 0.24 | 2.08 | 0.10 – 28.38 | 0.704 |
The antigenic profile of H pylori infection (table 5)
Table 5.
Western-blot analysis in 62 H pylori (IgG antibodies) positive persons.
| Studied number | Rate (%) | ||||
| O-97 (N = 40) | BD-97 (N = 22) | O–97 | BD-97 | Total (N = 62) | |
| p120 (CagA) | 32 | 16 | 80 | 72.7 | 77.4 |
| p87 (VacA) | 25 | 12 | 62.5 | 54.5 | 59.7 |
| p63 (UreB) | 33 | 18 | 82.5 | 81.8 | 82.2 |
| p58 (HspA&B) | 32 | 12 | 77.5 | 54.5 | 71 |
| p54 (FlaA) | 17 | 4 | 42.5 | 18.2 | 33.9 |
| p29 (UreA) | 24 | 16 | 60 | 72.7 | 64.5 |
The seroprevalence of CagA and VacA antigens was 77.4% and 59.7%, respectively. These rates were constant across gender, age and group of patients (O-97 and BD-97). Type I (CagA+/VacA+) strains were significantly more common than type II (CagA-/VacA-) strains (59.7% vs. 22.6%, p < 0.001). The prevalence of the other antigens ranged from 33.9% (FlaA) to 82.2% (UreB).
Discussion
Our findings show that the prevalence of H pylori IgG antibodies increases with age in the three tested groups of patients (O-87, O-97 and BD-97). Provided that the ELISA used had 87% sensitivity and 94% specificity, the results reflect satisfactorily H pylori infection rate. H pylori seroprevalence did not differ between the group O-97 and the healthy controls (group BD-97)(49.2% vs 48.3%, respectively, p = 0.87). On the contrary, comparing the group O-87 with the group O-97, we found a significant decrease of the infection rate in 1997 (59.5% vs 49.2%, respectively, p = 0.039).
The dynamic of infection in 1987 seems to be similar with that seen in the developing countries. Ten years later, in 1997, the picture changed toward what was found in the developed countries. Thus, the crude seroprevalence was reduced about 10% units; multiple regression analysis showed that a person of the O-87 group had a significant higher risk of seropositivity than a comparable person of the O-97 group (OR:1.73, 95% CI: 1.14–2.65, p = 0.010).
As we did not found paired serum samples of the same patient in groups O-87 and O-97 we could not know the rate of serocorversion in our population. But, in both developing and developed countries, there are many studies suggesting that, during adulthood, the rate of seroconversion and seroreversion is almost the same[9,14,15]. Thus, though our data showed that increasing age does increase the risk of H pylori infection, the fact that patients from group O-87 had higher rates of infection than comparable patients from group O-97, supports the "birth cohort phenomenon" as the main cause for the increasing occurrence of the infection seen with age.
The highest risk of infection in both groups of patients, was found in those born around 1940, that is the period of war deprivation. This finding, in association with the reduction in the infection rate during the studied 10-year period is compatible with the hypothesis that better socioeconomic conditions and improved hygiene have reduced the risk of H pylori infection [4].
In both groups of patients (O-87 and O-97) we found a constant decrease of seropositivity in the oldest age group. This may be due to many factors like spontaneous seroreversion [14], increased antibiotics and NSAIDs consumption [21] and advanced gastric atrophy in association with the natural senility of the immunological system [22].
In this study the prevalence of CagA positive and VacA positive strains of H pylori were 77.4% and 59.7%, respectively; type I strains were significantly more common than type II strains (59.7% vs. 22.6%, p < 0.001). According to the Eurogast study group, Greece has low incidence of gastric cancer in comparison with other European countries [23]. It has been suggested that CagA positive infections are more likely to predispose to gastric cancer that the CagA negative ones [24]. Nevertheless, we found a high rate of CagA positive and type I strains of H pylori in our sample. In accordance with previous studies [20,25,26], these data suggest that CagA and/or VacA status are not the only factors that influence gastric cancer rates.
To conclude, a significant decrease of H pylori infections' rate was noticed in Greece during a ten-year period. Even in a country of south-east Europe, with relatively high rate of H pylori infection, our data support the birth cohort phenomenon as an explanation for the age-dependent increase of H pylori infection. The prevalence of CagA and/or VacA positive strains is relatively high for the reported incidence of gastric cancer in Greece.
Competing interests
None declared.
Authors' contributions
PA carried out the immunoassays, participated in its design and sequence alignment and drafted the manuscript. IV participated in its design and coordination. MT participated in the sequence alignment. DK performed the statistical analysis. NK participated in its design and coordination. AA conceived of the study and participated in its design and coordination. All authors read and approved the final manuscript.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Contributor Information
Periklis Apostolopoulos, Email: periklisapo@yahoo.com.
Irene Vafiadis-Zouboulis, Email: i.vafiadis@grmedia.gr.
Michael Tzivras, Email: pola@med.uoa.gr.
Dimitrios Kourtessas, Email: akorba@med.uoa.gr.
Nicolaos Katsilambros, Email: laennec@techlink.gr.
Athanasios Archimandritis, Email: aarchim@hotmail.com.
References
- Mitchell HM, Li YY, Hu PJ, Lin Q, Chen M, Du GG, et al. Epidemiology of Helicobacter pylori in Southern China: identification of early childhood as a critical period for acquisition. J Infect Dis. 1992;166:149–153. doi: 10.1093/infdis/166.1.149. [DOI] [PubMed] [Google Scholar]
- Mendall MA, Goggin PM, Molineaux N, Levy J, Toosy T, Strachan D, et al. Childhood living conditions and Helicobacter pylori seropositivity in adult life. Lancet. 1992;339:896–897. doi: 10.1016/0140-6736(92)90931-R. [DOI] [PubMed] [Google Scholar]
- Malaty HM, Graham DY. Importance of childhood socioeconomic status on the current prevalence of Helicobacter pylori infection. Gut. 1994;35:742–745. doi: 10.1136/gut.35.6.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talley NJ, Noack KB. The worldwide prevalence of Helicobacter pylori :asymptomatic infection and clinical states associated with infection in adults. In: Goodwin CS, Worsley BW, editor. Helicobacter pylori: Biology and clinical practice. Boca Raton, Florida:CRC Press;; 1993. pp. 63–83. [Google Scholar]
- Graham DY, Adam E, Reddy GT, Agarwal JP, Agarwal R, Evans DJ, et al. Seroepidemiology of Helicobacter pylori in India: Comparison of developing and developed countries. Dig Dis Sci. 1991;36:1084–1088. doi: 10.1007/BF01297451. [DOI] [PubMed] [Google Scholar]
- Buckley MJM, O'Shea J, Grace A, English I, Keane C, Hourihan D, et al. A community-based study of the epidemiology of Helicobacter pylori infection and associated asymptomatic gastroduodenal pathology. Eur J Gastroenterol Hepatol. 1998;10:375–379. doi: 10.1097/00042737-199805000-00004. [DOI] [PubMed] [Google Scholar]
- Milman N, Rosenstock S, Andersen L, Jorgensen T, Bonnevie O. Serum ferritin, hemoglobin, and Helicobacter pylori infection: A seroepidemiologic survey comprising 2794 Danish adults. Gastroenterology. 1998;115:268–274. doi: 10.1016/s0016-5085(98)70192-1. [DOI] [PubMed] [Google Scholar]
- Archimandritis A, Bitsikas J, Tzivras M, Fertakis A, Anastasakou E, Pitsouni E, et al. Helicobacter pylori infection in Greece in healthy people and in patients with ulcer and with dyspepsia without ulcer. J Clin Gastroenterol. 1993;16:257–258. doi: 10.1097/00004836-199304000-00020. [DOI] [PubMed] [Google Scholar]
- Parsonnet J, Blaser MJ, Perez-Perez GI, Hargrett-Bean N, Tauxe RV. Symptoms and Risk Factors of Helicobacter pylori Infection in a Cohort of Epidemiologists. Gastroenterology. 1992;102:41–46. doi: 10.1016/0016-5085(92)91782-y. [DOI] [PubMed] [Google Scholar]
- Banatalva N, Mayo K, Megraud F, Jennings R, Deeks JJ, Feldman RA. The cohort effect and Helicobacter pylori. J Infect Dis. 1993;168:219–221. doi: 10.1093/infdis/168.1.219. [DOI] [PubMed] [Google Scholar]
- Kosunen TU, Aromaa A, Knekt P, Salomaa A, Rautelin H, Lohi P, Heinonen OP. Helicobacter pylori antibodies in 1973 and 1994 in the adult population of Vammala, Finland. Epidemiol Infect. 1997;119:29–34. doi: 10.1017/S0950268897007565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gause-Nilsson I, Gnarpe H, Gnarpe J, Lundborg P, Steen B. Seroprevalence of Helicobacter pylori serology in elderly people: a 21-year cohort comparison in 70-year-olds and a 20-year longitudinal population study in 70–90 year-olds. Age Ageing. 1998;27:433–436. doi: 10.1093/ageing/27.4.433. [DOI] [PubMed] [Google Scholar]
- Fujisawa T, Kumagai T, Akamatsu T, Kiyosawa K, Matsunaga Y, et al. Changes in seroepidemiological pattern of Helicobacter pylori and hepatitis A virus over the last 20 years in Japan. Am J Gastroenterol. 1999;94:2094–2099. doi: 10.1016/S0002-9270(99)00339-1. [DOI] [PubMed] [Google Scholar]
- Kuipers EJ, Pena AS, van Kamp G, Uyterlinde AM, Pals G, Pels NF, et al. Seroconversion for Helicobacter pylori. Lancet. 1993;342:328–331. doi: 10.1016/0140-6736(93)91473-Y. [DOI] [PubMed] [Google Scholar]
- Kumagai T, Malaty HM, Graham DY, Hosogaya S, Misawa K, Furihata K, et al. Acquisition versus loss of Helicobacter pylori infection in Japan: Results from a 8-year birth cohort study. J Infect Dis. 1998;178:717–721. doi: 10.1086/515376. [DOI] [PubMed] [Google Scholar]
- Veldhuyzen van Zanten SJO, Pollak PT, Best LM, Bezanson GS, Marrie T. Increasing prevalence of Helicobacter pylori infection with age: continuous risk of infection in adults rather than cohort effect. J Infect Dis. 1994;169:434–437. doi: 10.1093/infdis/169.2.434. [DOI] [PubMed] [Google Scholar]
- Censini S, Lange C, Xiang Z, Crabtree JE, Chiara P, Borodovsky M, et al. CagA pathogenicity island of Helicobacter pylori encodes type I-specific and disease-associated virulence factors. Proc Natl Acad Sci USA. 1996;93:14648–14655. doi: 10.1073/pnas.93.25.14648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudi J, Rudy A, Maiwald M, Kuck D, Sieg A, Stremmel W, et al. Direct determination of Helicobacter pylori vacA genotypes and cagA gene in gastric biopsies and relationship to gastrointestinal diseases. Am J Gastroenterol. 1999;94:1525–1531. doi: 10.1016/S0002-9270(99)00194-X. [DOI] [PubMed] [Google Scholar]
- Peek RM, Miller G, Tham KT, Perez-Perez CI, Zhao X, Atherton JC, et al. Heightened inflammatory response and cytokine expression in vivo to cagA+ Helicobacter pylori strains. Lab Invest. 1995;73:760–770. [PubMed] [Google Scholar]
- Blaser MJ, Perez-Perez GI, Kleanthous H, Cover TL, Peek RM, Chyou PH, et al. Infection with Helicobacter pylori strains possessing cagA is associated with increased risk of developing adenocarcinoma of the stomach. Cancer Res. 1995;55:2111–2115. [PubMed] [Google Scholar]
- Taha AS, Reid J, Boothmann P, Gemmel CG, Lee FD, Sturrock RD, et al. Serological diagnosis of H pylori: evaluation of four tests in the presence or absence of non-steroid anti-inflammatory drugs. Gut. 1993;34:461–465. doi: 10.1136/gut.34.4.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond J, Kalach N, Bergeret M, Barbet JP, Benhamou PH, Gendrel D, et al. Evaluation of serological tests for diagnosis of H pylori infection in children. Eur J Clin Microbiol Infect Dis. 1996;15:415–417. doi: 10.1007/BF01690102. [DOI] [PubMed] [Google Scholar]
- An international association between Helicobacter pylori infection and gastric cancer. The EUROGAST study group. Lancet. 1993;341:1359–1362. doi: 10.1016/0140-6736(93)90938-D. [DOI] [PubMed] [Google Scholar]
- Rugge M, Bussato G, Cassaro M, Shiao YH, Russo V, Leandro G, et al. Cancer patients younger than 40 years with gastric carcinoma: Helicobacter pylori genotype and associated gastritis phenotype. Cancer. 1999;85:2506–2511. doi: 10.1002/(SICI)1097-0142(19990615)85:12<2506::AID-CNCR3>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Lin JT, Wang LY, Wang JT, Wang TH, Chen CJ. Ecological study of association between Helicobacter pylori infection and gastric cancer in Taiwan. Dig Dis Sci. 1995;40:385–388. doi: 10.1007/BF02065425. [DOI] [PubMed] [Google Scholar]
- Farinati F, Della Libera GD, Cardin R, Molari A, Plebani M, Rugge M, et al. Gastric antioxidant, nitrites and mucosal lipoperoxidation in chronic gastritis and Helicobacter pylori infection. J Clin Gastroenterol. 1996;22:275–281. doi: 10.1097/00004836-199606000-00007. [DOI] [PubMed] [Google Scholar]