Abstract
Allergen Immunotherapy (AIT) is the only etiological therapeutic method available for allergic rhinitis (AR). Currently, several options for AIT in the market, such as subcutaneous immunotherapy (SCIT) and sublingual immunotherapy (SLIT), have different routes of administration. These traditional methods have achieved encouraging outcomes in clinic. However, the side effects associated with these methods have raised the need for innovative approaches for AIT that improve safety, shorten the course of treatment and increase local drug concentration. Nanoparticles (NPs) are particles ranging in size from 1 to 100 nm, which have been hired as potential adjuvants for AIT. NPs can be employed as agents for modulating immune responses in AR or/and carriers for loading proteins, peptides or DNA molecules. This review focuses on different kinds of nanoparticle delivery systems, including chitosan nanoparticles, exosomes, metal nanoparticles, and viral nanoparticles. We summarized the advantages and limitations of NPs for the treatment of allergic rhinitis. Overall, NPs are expected to be a therapeutic option for AR, which requires more in-depth studies and long-term therapeutic validation.
Keywords: allergen immunotherapy, nanoparticles, allergic disease, subcutaneous immunotherapy
Graphical Abstract
Introduction
Allergic rhinitis (AR) is an increasingly serious respiratory disease affecting about 600 million populations worldwide.1 Common symptoms of AR include rhinorrhea, nasal itching, pruritus and sneezing.2 These symptoms are not fatal, but the quality of the patients’ lives is seriously impaired with altered sleep quality, fluctuating emotion and poor daily performance in school or office.3,4 Effective treatments can greatly improve the life quality of those patients with AR.
As an allergic disease, the special immune responses caused by allergens contribute to the progress of AR. A variety of immune cells participate in these reactions including nasal epithelial cells, mast cells, eosinophils, basophils, T helper 2 cells (Th2 cells), group 2 innate lymphoid cells (ILC2s), antigen-presenting cells and B cells.5–7 Allergen immunotherapy (AIT) is the only etiological therapy for AR, which aims to reshape the immune system by repeatedly administrating pathogenic allergens to patients in a controlled manner.8 The mechanisms of AIT mainly include inducing a rapid decline in the degranulation of basophils and mast cells, triggering the transformation from Th2-type to Th1-type reaction, and resulting in the transformation of allergen-specific IgE to IgG4. AIT can induce immune tolerance, thus attenuating or even eliminating allergic reactions and subsequent immune events in patients who suffer from AR.9–13 At present, subcutaneous immunotherapy (SCIT) and sublingual immunotherapy (SLIT) are the most common AIT treatments, exhibiting excellent outcomes. However, long-term side effects and substandard local drug concentrations have raised the requirement for innovative approaches for AIT to address these pitfalls.
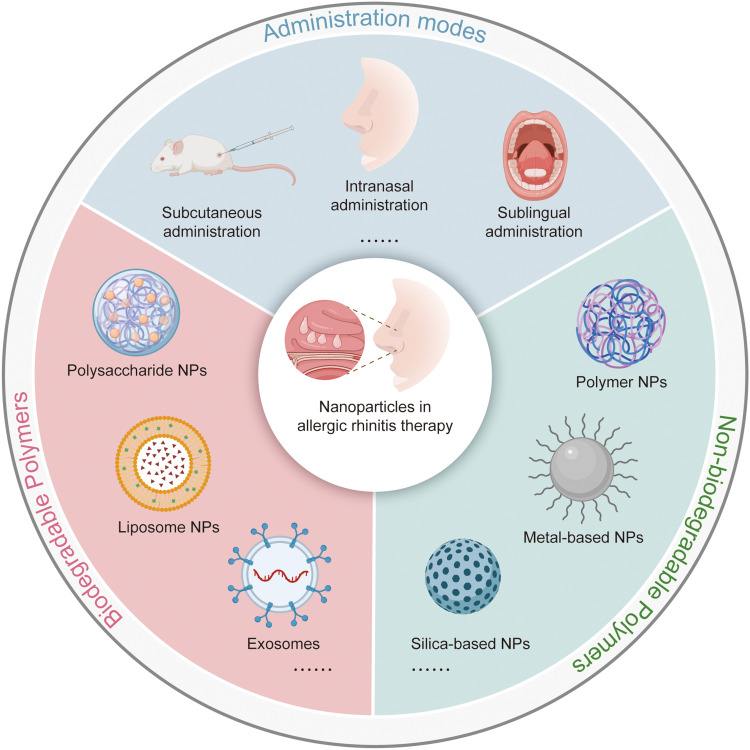
Currently, the application of nanoparticles (NPs) in the treatment of AR has been put forward and validated. NPs are particles of matter ranging from 1 to 100 nm in size, making them easily absorbed by cells.14 NPs can be employed as agents for modulating immune responses or/and carriers for loading allergens and protecting them from degradation.15–17 At present, there are various types of NPs available for use in AIT. According to whether they can be degraded in vivo, these particles are classified into biodegradable and non-biodegradable NPs. Biodegradable NPs mainly include natural polymers (protein, poly-γ-glutamine, polypeptide, polysaccharide and polyamide), synthetic polymers [poly(lactide-coglycolic acids), PLGA), poly(lactic acids), PLA), poly(ε-caprolactone), PCL), poly(methyl methacrylate), PMMA), poly(alkyl-cyanoacrylates), PACA), and copolymers], liposomes and virus-like particles. Meanwhile, non-biodegradable NPs are composed of many materials, including gold, silica, and polymers, etc. The availability of both biodegradable and non-biodegradable NPs has been explored, and their advantages in the treatment of AR compared with placebo have been confirmed. To enhance the administration of AIT for patients with AR, this review focuses on summarizing the immune responses associated with AR and the advantages and disadvantages of NP-based AIT.
Immune Responses in Allergic Rhinitis
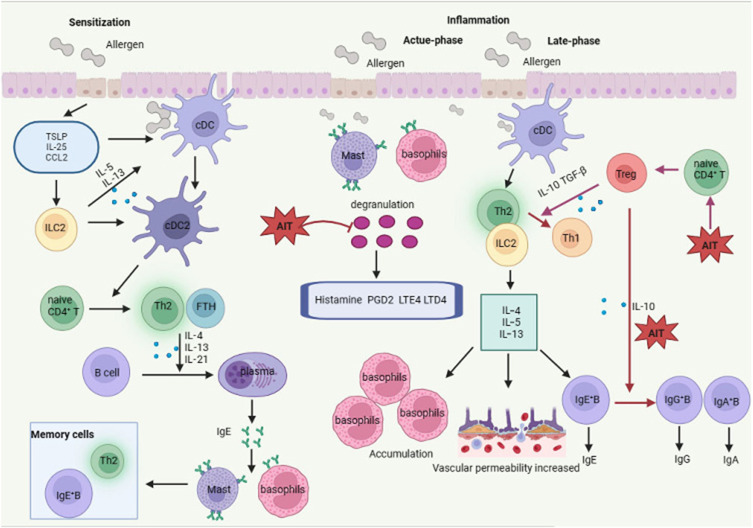
The immune response of AR is mainly induced by the inhalation of perennial or seasonal allergens. The general process of this response is antigen perception, antigen presentation and sensitization, and symptom generation and inflammation. During the whole process, nasal epithelial cells, mast cells, eosinophils, basophils, Th2 cells, group 2 innate lymphoid cells (ILC2s), antigen-presenting cells and B cells all play a role (Figure 1).6–8
Figure 1.
Pathophysiology and AIT of allergic rhinitis. During the sensitization phase, allergens are presented to and processed by dendritic cells and trigger a series of consequent immune events causing the transformation of plasma cells from B cells. Plasma cells produce allergen-specific IgE that binds to their receptor on the membrane of mast cells and basophils and subsequently induce the generation of memory allergen-specific Th2 cells and IgE-secreting B cells. Once these individuals were attacked by these allergens again, basophils and mast cells activates, and allergic mediators release and symptoms of allergic rhinitis happen within several minutes (acute-phase inflammation). Besides, the memory Th2 cells release IL-4, IL-5 and IL-13, resulting in the accumulation of inflammatory immune cells, increased vascular permeability and enhanced IgE generation by memory B cells (late-phase inflammation). Administration AIT to patients with allergic rhinitis improve the symptoms of inflammation via three means which are decreasing the degranulation of basophils and mast cells, inducing a shift from a Th2-type response to a Th1-type one, and leading to a switch from allergen-specific IgE to IgG4.
Abbreviations: cDC, conventional dendritic cells; ILC2, group 2 innate lymphoid cell; Th2, type 2 T helper cells; Th1, type 1 T helper cells; FTH, follicular helper T cells; Treg, regulatory T cells; IgE+B, IgE-secreting B cells; AIT, allergen immunotherapy; TSLP, thymic stromal lymphopoietin; IL-25, interleukin-25; CCL2, C-C motif chemokine ligand 2; PGD2, prostaglandin D2; LTD4, leukotriene D4; LTE4, leukotriene E4; TGFβ, transforming growth factor beta; IgE, immunoglobulin E.
Nasal mucosal epithelial cells are the first line of defense against allergens, which are responsible for sensing allergens.18–20 Once these cells are attacked by allergens, they adjust innate and acquired immunity, and promote the switch from innate immunity to acquired immunity. In detail, epithelial cells express almost all reported toll-like receptors (TLRs).21,22 Most of these TLRs are elevated during AR, such as TLR2 and TLR4, which can enhance Th1 response. Besides, epithelial cells can induce innate immune response by secreting thymic stromal lymphopoietin (TSLP), interleukin (IL-1α, IL-25, IL-33, etc.), chemokines (CCL-2, CCL-20, etc.), etc. As a consequence, conventional dendritic cells (cDCs) are stimulated and differentiated into cDC2, triggering the transformation from naive T cells to Th2 cells.22,23
When allergens enter the nasal mucosa, they are captured by DCs and processed into peptides, and the sensitization phase begins. Then, these antigen peptides are presented to naive CD4+ T cells in the draining lymph node. Upon receiving antigenic stimulation, the naive CD4+ T cells are activated, then differentiated into allergen-specific Th2 cells and follicular helper T cells (TFH).24–27 Th2 and TFH cells induce B cells to mature into plasma cells by secreting IL-4, IL-13 and IL-21, which in turn produce allergen-specific IgE.28 After IgE is released into the circulation, it binds to the high-affinity IgE receptor (FcεRI) on the surface of mast cells and basophils through its Cε3 domain, resulting in the formation of pools of memory allergen-specific Th2 and B cells.
When the allergen re-enters the nasal mucosa of allergic individuals, the symptom production and inflammation phase begins. This phase contains an immediate release of chemical mediators mainly derived from mast cells and basophils mediated by IgE (acute-phase response), and a late-phase response resulted from other effector cells mainly including eosinophils, neutrophils, basophils and Th2 cells.8 During the acute response, the allergens bind to IgE on mast cells and basophils in the nasal mucosa, leading to cross-linking between IgE and FcεRI.29 Subsequently, mast cells and basophils are activated and threshed, and pre-stored new synthetic mediators are released, such as histamine, sulfidopeptide leukotrienes (leukotriene C4 and D4) and prostaglandin D2.26 As a result, the acute AR symptoms appear within a few minutes.30–32 Besides the acute response, a late-phase response characterized by infiltration of eosinophils, neutrophils, basophils, Th2 cells, etc., in the nasal mucosa also contributes to this process.8 Among these cells, Th2 cells and ILC2s produce large amounts of classic type 2 cytokines, including IL-4, IL-5 and IL-13, which can be detected within several hours after exposure to allergens.31,33 These secreted cytokines result in recruitment and activation of inflammatory cells into the nasal mucosa and subsequently lead to increased mucus and IgE production and vascular leakage.34–36 Activated eosinophils release specific particles containing cationic proteins, cytokines, and chemokines, leading to tissue damage and remodeling.37,38 As a consequence of these complicated procedures, chronic inflammation develops in the nasal mucosa.
Allergen Immunotherapy for Allergic Rhinitis
Presently, the clinical therapeutic schemes for AR include avoiding irritation and allergens, pharmacotherapy and AIT.39,40 The drugs, including glucocorticoids, steroids, antihistamines, mast cell membrane stabilizers and leukotriene receptor antagonists, have exhibited excellent achievements in the treatment of AR.2 However, there still exist some patients who cannot tolerate drugs or their symptoms remain uncontrolled after pharmacotherapy. For those people, AIT, which is the only etiological treatment for AR, may be a good choice.9
AIT aims to reprogram the immune system by repeatedly giving patients pathogenic allergens in a controlled manner.41 AIT may lead to two possible payoffs: desensitization and tolerance. Desensitization is defined as short-term low reactivity, that is, the threshold of reactivity to causative allergens increases after continuous administration of the allergen. Tolerance refers to the ability to resist allergens even after the withdrawal of treatment.
The mechanisms of AIT in the treatment of AR are complicated (Figure 1). Generally speaking, its mechanism mainly involves three phases, namely, rapid desensitization, early tolerance and sustained tolerance.42 The first phase, rapid desensitization, includes a rapid decrease in the degranulation of basophils and mast cells. The second phase, early tolerance, is characterized by increased IL-10 secreting regulatory B cells (Breg) and regulatory T cells (Treg) and decreased IL-4 secreting Th2 cells, with a shift from a Th2-type response to a Th1-type one, which correlates with the improvement in clinical. The third and final phase, sustained tolerance, refers to a switch from allergen-specific IgE to IgG4 produced by B cells after stimulating by IL-10 secreted by Treg cells. IgG4 is a high affinity blocking antibody competing with IgE. Through this transformation, allergen-induced release of mediators by mast cells and basophils is greatly blocked. These sequential mechanisms result in immune tolerance, thereby attenuating or even eliminating allergic reactions and subsequent immune events.9–12,42
Various types of allergy vaccines have also brought some benefits, including recombinant allergen vaccine, T cell epitope peptide vaccine, B cell epitope peptide vaccine, modified allergen vaccine, allergen DNA/RNA vaccine and so on, we summarized the advantages and disadvantages of them (Table 1).
Table 1.
Vaccines Applied for AIT
| Type | Principle | Advantage | Disadvantage | References |
|---|---|---|---|---|
| Recombinant allergen vaccine | Use of recombinant DNA technology to enable bacteria, yeast, etc. to express antigenic proteins | Prolong the allergen half-life and reduce the combination with IgE. | Compared with standardized allergen extract, it has not shown additional benefits. | [43–46] |
| T cell epitope peptide vaccine | Synthetic allergen T-cell epitope polypeptides | Avoid unnecessary epitopes, will not induce IgE sensitization, and the preventive effect is lasting. | There are some difficulties in the synthesis of peptides, and there are limitations of histocompatibility complexes, which stimulate allergen-specific T cells in vivo and cause allergic inflammation. | [47–49] |
| B cell epitope peptide vaccine | Synthetic allergen B-cell epitope polypeptides | Reduce IgE reactivity and allergen-specific T cell activation without multiple injections. | Most of them are linear epitopes, and the prediction of conformational epitopes is complicated, so it is impossible to identify all epitopes accurately and comprehensively | [43,47,50] |
| Modified allergen vaccine | Modification of allergenic extracts by chemical modification, sonication and enzymatic digestion | Destroy IgE epitope and reduce allergy. | Processing conditions need further study. | [51–53] |
| Allergen DNA/RNA vaccine | Plasmid DNA/mRNA encoding allergens | Prevent the formation of IgE. | When the vaccine is integrated into the genome, there is a risk of cancer. Plasmid DNA induces autoimmunity, and the long-term expression of the encoded antigen causes systemic inflammation. | [54] |
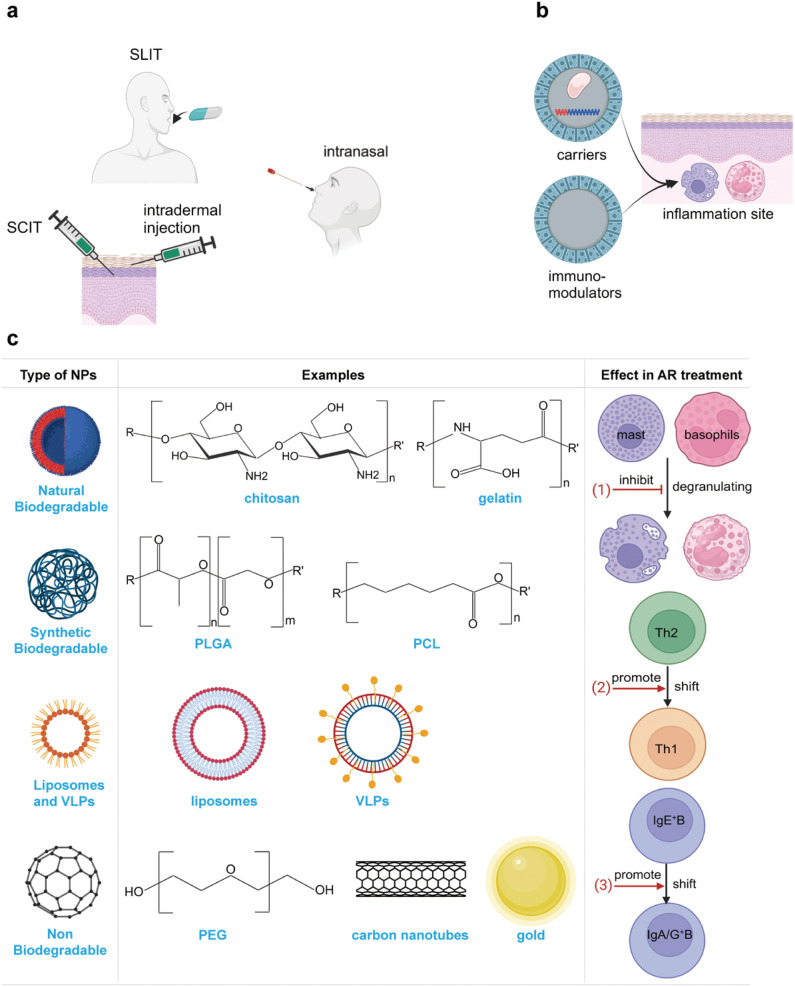
The traditional and most widely used AIT for AR is SCIT and SLIT (Figure 2a). The safety, effectiveness, and tolerance of both methods have been confirmed via several randomized controlled trials (RCTs) and large-scaled meta-analyses.26,55–58 Apart from SCIT and SLIT, new approaches such as intralymphatic, intradermal, epicutaneous, and intranasal routes have also been introduced into clinical practice, and satisfactory results have been achieved.40,59,60 However, the long-term safety and effectiveness of these novel approaches require validation through large-scaled clinical trials.60
Figure 2.
Schematic showing the nanoparticles. (a) main ways of administration of AIT. (b) The advantages of nanoparticles. (c) The classification of nanoparticles.
The above conventional AIT options have exhibited encouraging effects. However, several limitations have emerged over time.9,61 First, the quality and standardization of allergens containing microbial components or contaminated non-allergenic proteins are susceptible to induce side effects. Frequent treatment of allergens with T cell epitopes and IgE may trigger IgE- and T cell-mediated adverse events. Although the side effects of AIT are usually local reactions, rather than systemic reactions, some patients cannot tolerate these adverse effects because of the long duration (3 years).62 Also, the local drug concentration is sometimes substandard, resulting in undesirable therapeutic effects. Therefore, new methods to overcome these shortages are necessary.
Nanoparticle-Based Allergen Immunotherapy
To address the shortages of conventional AIT, adjuvants have been employed. An adjuvant refers to a compound or substance that is co-administered with the allergen extract and possesses the ability to enhance the immunogenicity of allergens and/or to regulate the immune response.42,63–65 Based on the function of the adjuvants, they can be classified into delivery systems and immunomodulators. The former category has the ability to modulate the presentation of antigens and the later one can be applied to affect the immune response directly.66,67 An ideal adjuvant need to promote stable and durable immune responses and should be equipped with non-toxic, economical and effective characteristics.68 Besides, they are required to provide optimal physiochemical properties (eg, absorption ability and particle morphology) and some biological activity properties (eg, avoiding the Th2-type immune response and increasing the IgG4 antibody titers).67 Till now, only four compounds have been employed as adjuvants in the market for AIT, which are aluminum hydroxide, microcrystalline tyrosine, calcium phosphate and monophosphoryl lipid A. Among the four adjuvants, the former three products are usually regarded as delivery systems with storage capacity and are first-generation adjuvants, while the last one refers to an immunostimulatory agent and is the only second-generation adjuvant used for AIT. Although the application of these products is encouraging, there exist some limitations which have been summarized in other reviews.69 For instance, aluminum can induce a Th2-type response and autoimmune/autoinflammatory syndrome. Calcium phosphate can cause local adverse events and exhibits inadequate adjuvant ability.70
In addition to these traditional agents, multiple potential adjuvants are under research, such as NPs, phosphatidylserine derivatives and immunostimulatory sequences (ISS). Recently, the application of NPs-based AIT in the treatment of AR has been proposed because of their properties and functions. NPs are particles of matter ranging from 1 to 100 nm in size, which can be up taken by cells via endocytosis. NPs can be hired as agents for modulating immune responses in AR or carriers for loading proteins, peptides or DNA molecules. Specifically, some NPs possess both the two functions concurrently. Five reasons may contribute to the potential application of NPs-based AIT for AR (Figure 2b). First, the size of NPs is extremely small, making it easily up-taken by cells, and NPs can be mass-produced making patients available for adequate drugs on time.14 Second, through encapsulating allergens into NPs, allergens can escape degradation by enzymes and get rid of the trouble of IgE recombination.17,64 Third, NPs can transport allergens to the site of action so that local allergens can reach a satisfactory concentration.63 Fourth, NP can deliver allergens and immune stimuli (eg, CPG motif and LPS derivative) synchronously.64 Finally, some NPs can modulate the immune responses themselves effectively and safely.15,17
According to whether NPs used for AIT are degradable in vivo, they are divided into biodegradable NPs and non-biodegradable NPs. We here summarize the applications, strengths and limitations of these NPs applied for AIT (Figure 2c).
Biodegradable Polymers
Biodegradable NPs are applied as one of the most exploited polymers in drug delivery system over the past years. Biodegradable NPs mainly include natural polymers (protein, poly-γ-glutamine, polypeptide, polysaccharide and polyamide), synthetic polymers [poly (lactide-coglycolic acids), PLGA), poly (lactic acids), PLA), poly (ε-caprolactone), PCL), poly (methyl methacrylate), PMMA), poly (alkyl- cyanoacrylates), PACA), and copolymers], liposomes and virus-like particles.
Natural Biodegradable Polymers
Polysaccharide
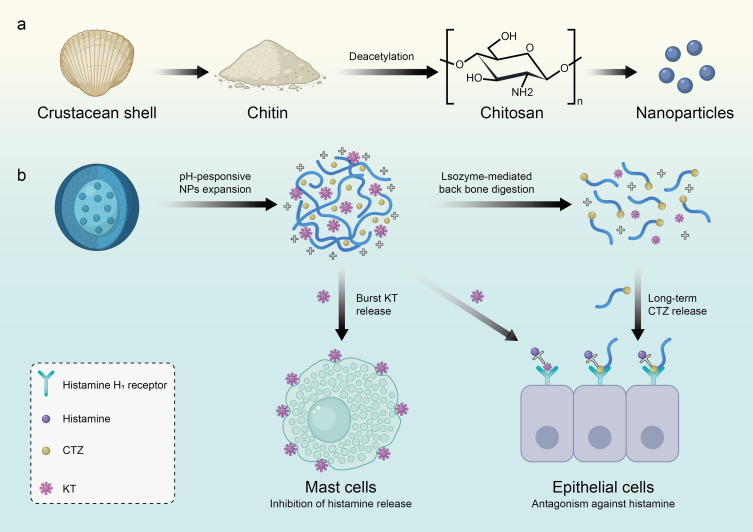
Poly(D-glucosamine) (also known as chitosan) is a very abundant linear amino-functional polysaccharide in nature derived from chitin through deacetylation. Chitin is mainly obtained from crustacean shells via a series of processes, including grinding shell waste, demineralization with acid, deproteinization with alkaline, and decolorization with ethanol (Figure 3a).71–73 Structurally, chitosan consists of randomly distributed n-acetyl-d-glucosamine (acetylation unit) and b- [1-4]- linked d-glucosamine (deacetylation unit). Chitosan-based NPs are regarded as excellent carriers because they can produce both carbon dioxide and water without causing toxic effects or immunogenicity. In fact, chitosan is the most widely explored natural biodegradable polymer in AIT. The glycosidic bond of chitosan is soluble in water and subsequently leads to the formation of positive charges, gelation, and membrane forming characteristics.74 The factor affecting the function of chitosan is its solubility which is determined by the pH value, the degree of deacetylation, molecular weight (MW) and ionic solution strength.75,76 When the degree of deacetylation is around 40%, chitosan can be dissolved to a pH of 9, but when that is about 80%, chitosan can only be dissolved to a maximum pH of 6.5, which is also the ideal pH for chitosan dissolution. The hydrolytic process of chitosan degradation consists of the reactivity of fragile polymer bonds with physiological fluids or water,77 and the rate of this process is determined by the accessibility of water into the polymer matrix.78 In this case, it is necessary to modify chitosan in order to expand the pH range of chitosan dissolution.79 Due to its physical and chemical properties, chitosan has been approved in the United States and Europe for its application in human hemostatic agents 61. As far as AR is concerned, chitosan has been developed as a carrier loading drugs, such as ketotifen (histamine release inhibitor),80 cetirizine (histamine receptor antagonist),80,81 astragalus polysaccharide82 and cromolyn.83 Through delivery by chitosan, the inherent defects, such as irritation to mucosa, lack of target-specific sequential release of drugs and short mucosal in situ retention, of administrating these traditional drugs by traditional methods can be greatly attenuated. Sun et al designed ketotifen-cetirizine loaded hydroxybutyl chitosan nanoparticles to prolong the drug retention in the nose and improve histamine-mediated AR (Figure 3b).80 In addition to these drugs, chitosan has been extensively studied as an agent to load allergens and maintain their immunogenicity.84 Functionally speaking, chitosan-based NPs encapsulated with allergens can result in altered abilities of DCs in up-taking, processing and presenting antigens, and a reduction of IFN-γ and IL-10 secretion.85,86 Also, chitosan-based NPs with or without loading allergens can induce Th1-type reaction or Treg generation to resist allergic responses.87–90 For example, Ou et al87 employed chitosan as a carrier loading a DNA vaccine co-expressing Der p 1 allergen and murine ubiquitin to treat AR in mouse model and confirmed its effect in controlling inflammation of nasal mucosa of AR. They also reported that the possible mechanism is that this complex can induce the transformation from IgE to IgG and the switch from Th2 reaction to Th1 response. In particular, chitosan-based IL-5 antisense oligodeoxynucleotide complex has been proved to be an efficient method to repair the disorder of IL-5 in AR.91
Figure 3.
Application of chitosan-based nanomaterials in allergic rhinitis immunotherapy. (a) Schematic showing the synthesis of Poly(D-glucosamine) (chitosan) based nanoparticles. (b) Therapeutic mechanism of chitosan nanoparticles loaded with ketotifen (KT) and cetirizine (CTZ) in allergic rhinitis rats.
Proteins
Gelatin is composed of denatured and hydrated collagen derived from animals, which has been approved by the Food and Drug Administration (FDA). When treating allergic horses, gelatin NP-based CpG exhibits an obvious effect on enhancing IL-10 level and reducing allergic symptoms (nasal discharge, respiratory effort, viscosity and tracheal secretion).92 Hathout et al reported a computer simulation system to predict the drug loading of gelatin nanospheres, which is helpful to save time and drug cost.93 Other than gelatin, protamine, a kind of arginine-rich protein derived from the sperm of fishes, has also shown its potential in AIT. When meeting DNA, nucleotides or peptides/proteins, protamine can bind to them and play a role. Treating mice with protamine-based NPs loaded with the major peanut allergen (Ara h2) from peanuts and CpG can weaken Th2-type allergic immune responses without causing obvious skin test reactivity. Besides, stimulation of DCs with this NPs complex carrying Ara h2 leads to enhanced CD11c, CD80 and IL-6 levels.94
Besides, proteins can be coupled to polysaccharides. Grass pollen or cat allergens connected to sepharose microbeads by cyanogen halides triggered an allergen-specific Th1 immune response without causing granulomatous tissue reactions in mice. Additionally, these particles can guard against or even diminish eosinophils in bronchoalveolar lavage fluid, as well as promote a switch from IgE to IgG2a generation. Besides, these particles can be internalized by human DCs in vitro, and give rise to increased levels of CD86, IL-8 and TNF-α.95,96 Apart from this type of particle, neoglycocomplexes of ovalbumin (OVA) or papain-conjugates with mannan induced elevated specific humoral immune responses and a shift from IgE towards IgG, especially IgG1 in mice. Finally, papain glycocomplexes can easily and efficiently combine with the DC targeting and masked B-cell epitopes.97
Polypeptide
Peptides with various lengths derived from allergens are also promising methods for AIT.98 At present, the most widely studied peptides are poly-gamma glutamic acid (PGA) and poly-hydroxyethylaspartamide (PHEA). Peptides possess the capacity to affect genes and pathways related to tolerogenic responses. A clinical trial concentrating on the application of peptides in skin revealed that the amount of both Th1 cells (CD4+/IFN-γ+) and CD25+ cells dramatically increase.99 Other clinical trials using a mixture of peptides obtained from bee venom allergen and the major cat allergen (Fel d 1) also found changes in both cellular and humoral immunity caused by the mixture.100,101 Importantly, besides to controlling tolerogenic responses, these peptides meet one of the most vital requirements for the creation of vaccines that are incapable of activating basophils, which makes them easily available for peptides-based AIT.102,103 The above evidence indicates that peptides can affect tolerogenic reactions and can be candidates to create vaccines. However, peptides have some inherent shortcomings that are weak immunogenicity and stability. Recently, researchers are attempting to readjust the composition of peptides-based NPs to solve their limitations. For instance, Ding et al104 designed and constructed smart peptide defense web technique to assemble peptides, thus enhancing their stability. It is found that this assembly can reduce symptoms of rhinitis and levels of inflammatory factors, even better than cetirizine (the first-line clinical drug for AR) in mice with AR. Another group of scholars designed four kinds of dendrimeric scaffolds containing an ester/ether with nine mannoses, an ester succinimidyl linker with nine N-acetyl-glucosamine or nine ethylene glycols, which are conjugated to T cell immunodominant epitope of the major pollen allergen Ole e 1 (OE109-130) in order to develop new vaccines against olive pollen allergy. They demonstrated that mannosylated dendrimers conjugated to OE109-130 peptide may be an appropriate option because they do not exhibit cytotoxicity and show low colocalization with a lysosomal marker and display significant effects on promoting Treg proliferation and IL-10 secretion.105 Zhuang et al106 developed the polymer-polypeptide nanomaterial with a CCR3 antagonistic peptide and a pH-responsive polyethylene and encapsulated ketotifen. The material played the role of “nasal in situ assembly” and was transformed into nanofibers in the nasal cavity, thus improving the retention of the drug in the nasal cavity. In a word, proper modification of the structure of peptides-based NPs is helpful to overcome their pitfalls and improve their efficiency.
Exosomes
Exosomes are nanosized lipid vesicles secreted by cells that are involved in intercellular communication and material transport.107 Exosomes are also present in nasal lavage fluids, and the contents of exosomes may interact with immune cells and be associated with the progression of allergic rhinitis108–110 Exosomes have also been developed as drug delivery vehicles for the treatment of allergic rhinitis. In mouse models, exosomes from different sources show inhibition of allergy (Table 2). However, translation of clinical studies still faces many challenges, such as low yields, low purity, and lack of standardized isolation and highly quantitative production methods, so in-depth studies are needed to better utilize exosomes.111,112
Table 2.
Exosomes Applied for AIT in Allergic Rhinitis
| Source of Exosomes | Cargo | Species | Route | Outcomes | References |
|---|---|---|---|---|---|
| Dendritic cell | OVA and tetramethylcurcumin | OVA and alum induced AR mice | Nasal instillation | Antigen-specific type 1 Tregs↑ | [113] |
| Mesenchymal stromal cells | miR-146-5p | Mice with allergic airway inflammation | Intravenous injection | Group 2 innate lymphoid cells ↓ | [114] |
| Human Embryonic Kidney 293 Cells | CpG DNA和OVA | OVA and aluminum hydroxide induced AR mice | Inject through the left and right nostril | Th1 response ↑ | [115] |
| Human umbilical cord mesenchymal stem cells (CP-CL11 cells) | P-D2 peptide | OVA induced AR mice | Intranasal | Th1 response ↑, Recruitment and activation of DC cells↓ | [116] |
Abbreviations: OVA, ovalbumin; DC, dendritic cells.
Synthetic Biodegradable Polymers
Poly Lactic-Co-Glycolic Acid Nanoparticles
Poly (lactic-co-glycolic acid) (PLGA) is the most extensively explored synthetic biodegradable polymers in AIT due to their safety, biocompatibility and biodegradability.17,61,64,67,84 Actually, PLGA has been approved by the European Medicine Agency and the US Food and Drug Administration (FDA) for drug delivery, vaccine preparation and tissue engineering.61 As for drug delivery, PLGA allows for controlled drug release, protection of the drug from biodegradation, and rapid clearance.48,91,117–119 The effects of PLGA-based NPs on both preventing mice against sensitization and improving the symptoms in allergic mice have been investigated and confirmed. For instance, administrating mice with allergens encapsulated by PLGA-based NPs can induce the switches toward Th1 immune response and allergen-specific IgG2a, and increase the level of IL-10, thus protecting mice from sensitization.118–120 Also, equipping allergens, such as the major birch allergen (Bet v 1), the Chenopodium allergen (rChe a 3), with PLGA-based NPs can trigger Th1 immune responses, elevate the number of Tregs, and reduce the number of eosinophils, and finally result in attenuated symptoms in AR and other allergic diseases in mice.121–123 In other cases, co-encapsulation of PLGA with CpG and peptides can enhance both the prophylactic and therapeutic effects of PLGA-based NPs.118,119,124,125 For example, co-encapsulation of PLGA with CpG-motif and Der p 2 (major house dust mite allergens) or the bee venom phospholipase A2 stimulate a strong Th1 response to protect mice from sensitization.
As a biodegradable NP, PLGA can be degraded by enzymatic hydrolysis. However, an extremely long degradation time of up to 2 years together with acidic degradation products that may negatively regulate the stability of allergens and lead to inflammation are the main drawbacks of PLGA. To address these limitations, copolymers (such as PEG) with basic features to neutralize acidity can be utilized.17,84
Poly ε-Caprolactone Nanoparticles
Poly (ε-caprolactone) (PCL) is another attractive synthetic biodegradable polymer that is biocompatible, biodegradable, and semicrystalline. PCL-based NPs can yield similar effects to PLGA. A previous study treated OVA-sensitized mice with OVA-PCL and found an increased IgG1 level and decreased levels of IgE and histamine compared to OVA-alum.126
In a word, PLGA and PCL are fascinating synthetic biodegradable NPs for AIT, which can attenuate the symptoms of AR and other allergic diseases.
Liposomes
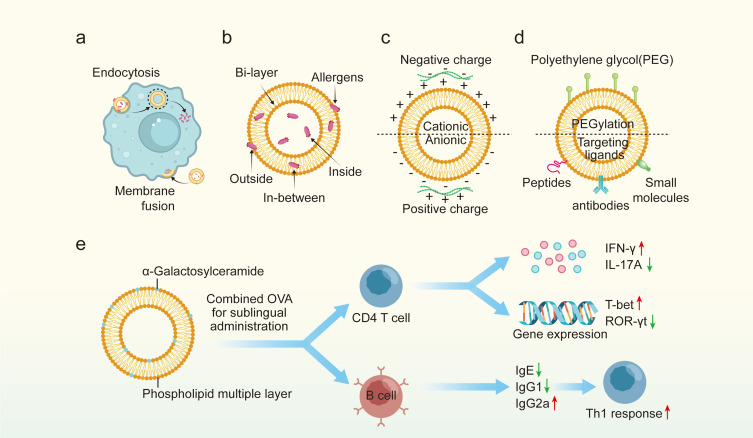
Liposomes are small spherical vesicles comprising at least one phospholipid bilayer that can separate hydrophilic drugs entrapped inside themselves from the aqueous environment.84,127 Because liposome and cell membrane are similar in composition and structure, they can be absorbed by cells in a fusion way. Also, as a type of nanoparticle, they can be ingested by cells and release cargoes (Figure 4a). In practice, allergens can be entrapped in-between or encapsulated within the lumen of the phospholipid bilayer or attached to the outer surface of liposomes via chemical conjugation (Figure 4b).71 Liposomes are similar to a cell-structure with high biocompatibility and biodegradability, and they can optimize specific targeting at the site of action without unnecessary side effects.61,84 Due to these special features, liposomes have been applied as both immunomodulators and drug carriers for decades.
Figure 4.
Application of liposomes in allergic rhinitis immunotherapy. (a) the approaches of liposomes absorbed by cells. (b) The composition of liposomes and the location of allergens. (c) The classification of liposomes based on charge. (d) The modification of liposomes. (e) Therapeutic mechanism of liposomes loaded with α-galactosylceramide in allergic rhinitis mice.
To maintain excellent electrostatic double-layer repulsion and dispersion and to interact with cells, a zeta potential no less than 30 mV (either positive or negative) is necessary.128,129 In light of this, liposomes are usually decorated with cationic or anionic ligands to obtain a proper zeta potential. Based on the ligands equipped, liposomes are divided into cationic, anionic and zwitterionic ones (Figure 4c). The advantages of cationic liposomes are that they have better affinity over anionic ones for cell surface and salivary pellicles which bear negative charge.130,131 But of course, anionic liposomes exhibit better ability in loading cargo with positive charge.132,133 It is mentioned to note that, the surface zeta potential of both cationic and anionic liposomes is dependent on the environment, especially the pH value.134 Given this, zwitterionic liposomes were designed, which show absolutely complete charge reversal between positive and negative zeta potential depending on the environmental PH value and their isoelectric points.135,136 Several experiments have been conducted to explore the application of liposomes as immunomodulators in allergic immune response. For instance, Nouri et al investigated the effect of cationic liposomes without allergens on mast cells, aiming to explore their value in rapid desensitization.137 They indicated that the application of liposomes consisting of cationic 1,2-dioleoyl-sn-glycero-3-phosphatidylethanolamine (DOPE) and cholesteryl-3bcarboxyamidoethylene-N-hydroxyethylamine (OH-Chol) can dramatically reduce the degranulation of mast cells and vascular protein leakage even after the cross-linking of IgE and FcεRI.
As mentioned above, liposomes can be applied as carried loading allergens via both surface-linking and encapsulating. The value of both these two methods in AIT has been explored and confirmed in animal models or even in humans (Table 3).137–142 For instance, Nouri et al143 found that liposome-based NPs with recombinant hybrid molecule (rHM) packing in them can result in a dramatic suppressive effect on the allergic immune reaction and a switch to a Th1 response. Similarly, treating allergic mice with liposomes containing Cry j1 (the major Japanese cedar pollen allergen) subcutaneously and L-Der p 1 and p 2 (the major dermatophagoides pteronyssinus allergen) intranasally can enhance a Th1 immune response with an increased IgG1 level and a decreased IgE level in allergic mice.138,139 As for AR, researches have also indicated similar effects of liposomes in modulating immune response. In detail, administrating mice suffering from AR with liposomes containing Fel d 1 (or α-galactosylceramide) and OVA attenuate nasal symptoms and enhance a shift from Th2 toward Th1 response and improve the production of IgG1 and reduce the level of IgE (Figure 4e).140,141 It is worth mentioning that the function of liposome-based NPs was also confirmed in patients with AR. In short, liposomes loading vitamin A and E can inhibit nasal symptoms and reduce levels of eosinophils, neutrophils and mast cells in AR patients without causing obvious adverse events compared with placebo, highlighting the safety of liposome-based NPs.142
Table 3.
Liposomes Applied for AIT in Allergic Rhinitis
| Cargo1 | Species2 | Route | Outcomes3 | References |
|---|---|---|---|---|
| rHM, protamine, DNA | CA induced allergic mice | Subcutaneous | Th2/Th1 switch↑, IgE/IgG2a switch↑ | [137] |
| Cry j 1 | Cry j1 induced allergic mice | Subcutaneous | Th2/Th1 switch↑, IgE/IgG2a switch↑ | [138] |
| L-Der p 1 and p 2 | DP induced allergic mice | Intranasal | Th2/Th1 switch↑ | [139] |
| Fel d 1 | Cat allergen induced AR mice | Intranasal | Nasal symptoms↓, Th2/Th1 switch↑, IgE/IgG1 switch↑ | [140] |
| α-GC, OVA | OVA induced AR mice | Sublingual | Nasal symptoms↓, Th2/Th1 switch↑, IgE/IgG1 switch↑ | [141] |
| Vitamin A and E | AR patients (n=106) | Intranasal | Nasal symptoms↓, eosinophils↓, neutrophils↓, mast cells↓ | [142] |
Abbreviations: rHM, recombinant hybrid molecule; Cry j 1, the major Japanese cedar pollen allergen; L-Der p 1, the major dermatophagoides pteronyssinus allergen; L-Der p 2, the major dermatophagoides pteronyssinus allergen; Fel d 1, the major cat allergen; α-GC, alpha-galactosylceramide; OVA, ovalbumin; CA, Chenopodium album; DP, dermatophagoides pteronyssinus; AR, allergic rhinitis; ↑, increase; ↓, decrease; Th2/Th1 switch, a switch from Th2 to Th1 response; IgE/IgG2a switch, a switch from IgE to IgG2a production; IgE/IgG1 switch, a switch from IgE to IgG1 production.
The above evidence confirms the significance of liposomes-based NPs in AIT. However, a vital issue that requires special attention is that the effect of liposomes is greatly determined by the membrane fluidity. In other words, creating liposome-based NPs with proper membrane fluidity via altering lipid components can affect the efficiency of these NPs. Previous research compared the efficiency of four liposomes containing different lipid components in controlling allergic symptoms and indicated that liposomes prepared by unsaturated lipids can cause the highest levels of IgG blocking antibodies against OVA, which may be attributed to the increased fluidity of the bilayer membrane and components within the membrane.144 Another study revealed that liposomes with abundant phosphatidylserine in the membranes are more likely to mimic apoptotic cells releasing “eat-me-signals” and result in enhanced secretion of IL-12 and interferon gamma (IFN-γ) and reduced OVA-specific IgE levels.145 In addition to modification of the membrane fluidity of liposomes, some other methods have also been applied to enhance the effect of liposomes, including PEGylation using polyethylene glycol (PEG) and decoration with targeted ligands (peptides, antibodies and small molecules) (Figure 4d). New liposomes are also worthy of further research. For example, modified liposomes with gelatinized core are suitable for encapsulating hydrophilic drugs to achieve the purpose of sustained and controlled release.146
In conclusion, liposomes possess clear-cut advantages that they can be composed of individual, chemical-grade, and high-purity components, making it easy to standardize production. However, the function of liposomes is largely determined by their size, zeta potential, membrane fluidity and the “decoration” on their surface. Thus, to ensure the liposomes can be absorbed and processed by APC cells and can ameliorate the allergic immune responses without causing undesired adverse effects, the formulations of them need to be carefully optimized.
Virus-Like Particles
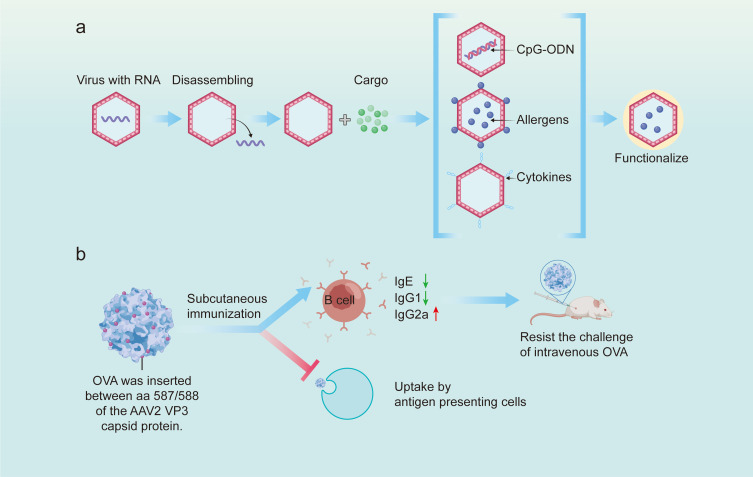
Virus-like particles (VLPs) consist numerous copies of a viral capsid protein that mimic a viral scaffold.84 VLPs are closely similar to viruses but are noninfectious because they are in the absence of viral genetic information.61,147 Generally speaking, VLPs can provide the following five advantages: (1) lack of genetic material with integrative or replicative ability, making them be safe products, (2) batch production of VLPs can be realized economically and quickly, (3) the shape and size enable them to stimulate strong and rapid immune responses, (4) antigens can be loaded by VLPs through covalent or non-covalent linkage to the surface of the VLPs or through genetic fusion into VLPs proteins, and (5) immunostimulators can be attached within the VLPs. Based on these advantages of VLPs, three approaches have been exploited to apply VLPs for the control of AR, which are VLPs embedding CpG motifs, VLPs loading allergens and VLPs carrying cytokines (Figure 5a). The effects of administrating VLPs through all the three approaches have been explored and confirmed148–161 (Table 4).
Figure 5.
Application of virus-like particles in allergic rhinitis immunotherapy. (a) Schematic showing the synthesis of virus-like particles. (b) Therapeutic mechanism of adeno-associated virus-like particle inserted with OVA peptide in systemic OVA-allergic mice.
Table 4.
Virus-Like Particles Applied for AIT in Allergic Rhinitis
| VLP Platform | Antigen | Size (nm) | Species | Route | Outcomes | References |
|---|---|---|---|---|---|---|
| (1) Loading allergen or allergen-derived T and B cell epitopes | ||||||
| AAV-2 | B epitope of OVA | N.A. | Mice | Subcutaneous | Suitable vaccine candidates | [148] |
| Ty | T epitope of Asp f 2 (aa60-71, aa235-249) | 60 | Mice | Subcutaneous | Antigen-specific immunoglobulin classes↑ | [149] |
| Ty | Der p 1 | 60 | Mice | Intraperitoneal | IL-5 production↓, allergen-specific cell proliferation↓ | [150] |
| eBP | Der p 2 | N.A. | Mice | Intraperitoneal | IgG2a↑, Th1 response↑ | [151] |
| Qβ-G10 | Fel d 1 | 30 | Mice | Subcutaneous | Treatment of Fel d 1-induced anaphylaxis | [152] |
| CuMV | T epitope of Fel d 1 (tt830-843) | 30–40 | Mice | Intramuscular | Neutralizing antibodies against Fel d 1↑, prevent allergies |
[153] |
| CuMV | Fel d 1 | 30–40 | Cat allergen induced AR patients (n=20) | Subcutaneous | Allergic symptoms↓ | [154] |
| Qβ-G10 | Der p 1 | 30 | Healthy people (n=24) | Intramuscular | IgG↑ | [155] |
| Qβ-G10 | HDM | 30 | HDM induced AR patients (n=20) | Subcutaneous | Nasal symptoms↓, IgE/IgG1 switch↑ | [156] |
| Qβ-G10 | CYT003 (TLR 9 agonist) |
30 | HDM induced AR patients (n=299) | Subcutaneous | Nasal symptoms↓, allergen tolerance↑ | [157] |
| Qβ-G10 | Amb a 1 | N.A. | Ragweed allergen induced AR patients (n=25) | N.A. | Nasal symptoms↓, IgE/IgG1 switch↑ | [158] |
| (2) Loading cytokines | ||||||
| HBcAg | Mouse IL-4 peptide | 25 | Mice | Subcutaneous | Prophylactic vaccine for OVA induced allergic response | [159] |
| HBcAg | Mouse IL-13 peptide | 25 | Mice | Subcutaneous | Prophylactic and therapeutic vaccine for OVA induced allergic response | [160] |
| HBcAg | Mouse IL-33 peptide | 25 | Mice | Subcutaneous | Prophylactic vaccine for OVA induced allergic response | [161] |
Abbreviations: VLP, virus-like particle; AAV-2, Adeno-associated viruses 2; Ty, Ty-transposon from yeast S. cerevisiae; eBP, enveloped bioparticle; Qβ, bacterial phage; G10, CpG motif 10; CuMV, cucumber mosaic virus; HBcAg, hepatitis B core antigen; OVA, ovalbumin; Asp f 2, the major fumigatus allergen; Der p 1, the major house dust mite allergen; Fel d 1, the major cat allergen; HDM, house dust mite; TLR 9, Toll-like receptor 9; Amb a 1, the major ragweed allergen; N.A., Not known; AR, allergic rhinitis; ↑, increase; ↓, decrease; IgE/IgG1 switch, a switch from IgE to IgG1 production.
Unmethylated CpG dinucleotide motif is a pathogen-associated molecular pattern within oligonucleotides (CpG-OND) or longer DNA molecules. CpG motifs can be recognized by TLR9 and then activate immune responses. As the receptor of CpG-OND, TLR9 is mainly detected in plasmacytoid DCs and B cells, which can stimulate the secretion of Th1-like cytokines and chemokines and induce the switch toward IgG antibodies.162 Besides, TLR9 mediated immune responses have also been found in mast cells, granulocytes, neutrophils, eosinophils, and basophils.162 Based on the pro-Th1 activity of CpG-OND, bacteriophage Qβ-based VLPs containing TLR9 A-type CpG motif G10 (Qβ-G10) were introduced in clinical trials for reprogramming Th2-biased immune response in AR. Data extracted from published researches concentrating on the application of this type of NPs in the treatment of AR were initially promising. The potential anti-allergy effects of Qβ-G10 were first explored in a Phase I/II trial containing 20 house dust mite (HDM)-allergic patients.156 Administration of Qβ-G10 boosted allergen-specific IgG1 and IgG4 levels and allergen tolerance (100-fold) and sharply attenuated the symptoms of AR without causing typical mild side effects. Another phase IIb study assessing the clinical efficacy of Qβ-G10 in 299 hDM-sensitized patients with AR symptoms also showed beneficial effects of Qβ-G10 in both modulating immune response and reducing symptoms.157 However, the authors also pointed out that the HDM applied in their research represents a ubiquitous allergen source and that they cannot definitely exclude the possibility that the enrolled patients were exposed to minimal amounts of HDM-allergen when receiving the treatment. As a result, an antigen-dependent component that affects the efficacy of the vaccine could not be entirely excluded. In short, the value of Qβ-G10-based NPs has been confirmed in AR, but large-scale prudent studies are still required.
The second VLP-based approach is to decorate VLPs with full-length allergens or T/B cell epitopes at their surface to induce blocking antibodies. Through experiments conducted in mice, full-length allergens have been identified as modulators improving allergic immune responses. These studied allergens mainly include the major HDM allergen (Der p 1 and Der p 2), Fel d 1, and the major mugwort pollen (Art v 1).150–152,163 Interestingly, Art v 1 attached to the surface of VLPs induced dramatically weaker degranulation of IgE-sensitized effector cells compared to their soluble types.163 It is worth mentioning that the effect of VLP-based NPs carrying full-length allergens has been estimated in clinical. The platforms of VLP used for clinical research mainly contain Qβ-G10 and CuMV. The positive effect of applying Qβ-G10 as a platform to treat patients with AR has been summarized in the above content, and employing CuMV as a platform yielded similar effects with Qβ-G10. The anti-allergy effects of CuMV-based NPs carrying Fel d 1 was first explored in a clinical trial containing 20 patients with AR caused by cat allergen.154 The results indicated that administration of this NP sharply attenuated the symptoms of AR. These animal and clinical trails confirmed the positive effect of full-length allergens loaded by VLPs. However, full-length allergens may lead to anaphylaxis in sensitized individuals because they may cause IgE cross-linking on effector cells. In light of this, T or B cell epitopes were explored as an alternative to full-length allergens. The first probative epitope was p1 protein, a protein derived from the yeast retrotransposon Ty, has been fused with T cell epitope of the major fumigatus allergen Asp f 2 (aa60-71, aa235-249) to prepare Ty-based VLP-Asp f 2 (Ty-Asp f 2).149 After stimulating mice with Ty-Asp f 2, dropped T cell responses and specific serum IgE level have been measured. However, the effect of this NP on T cell responses was not enduring and can be rescued by subsequent allergen contacting. Another attempt targeting T cell epitopes was cucumber mosaic virus (CuMV). CuMV carrying T cell epitope of Fel d 1 (tt830-843) can induce neutralizing antibodies against Fel d 1 and prevent allergies in mice.155 As for B cell epitopes, there also exist corresponding strategies. For instance, VP3 capsid protein obtained from adeno-associated virus 2 (AAV-2) was genetically conjugated with B cell epitope of OVA, generating chimeric VLP packing 60 copies of VP3-OVA (Figure 5b).148 Although the absorbability of AAV-2-OVA by mouse immune cells is impeded by OVA peptide insertion, administration of AAV-2-OVA results in a reduction in specific IgE level, contributing to the prevention of OVA challenge. In summary, the main strength of allergen-specific approaches is their accurate targeting of the aberrant immune responses induced by the allergens without affecting uncorrelated immune responses. The major disadvantage of this method is that full-length allergens may lead to anaphylaxis in sensitized individuals because they may cause IgE cross-linking on effector cells, which can be largely omitted by applying strict T cell or B cell epitopes of culprit allergens.
The third method exploiting VLPs is to pack them with effector cytokines inducing allergic immune responses and their corresponding receptors. These cytokines mainly include IL-4, IL-5, IL-13, Il-25 and IL-33.159–161,164 Although administration of these cytokines is not curative, their ability to improve the quality of allergic patients with AR has been validated by numerous clinical trials with severe symptom. Relative pre-clinical trials have been exclusively performed to evaluate their effects on AIT, and previous reviews have reviewed this content in detail.164 Therefore, we have not reviewed this here.
Two points of VLPs based AIT need special attention. On the one hand, translation of VLP-based AIT into human need massive clinical trials. On the other hand, batch production of VLP carrier is challenging in terms of yield, stability, or reproducibility. However, we believe that VLPs carrying CpG or allergens can meet the demand of modulating the immune responses in AR.
Non-Biodegradable Polymers
Non-biodegradable NPs are composed of various materials, including gold, silica, and polymers, etc. These NPs possess both physical and structural properties that can be modified according to the requirements,165 making them be interesting adjuvants for AIT. Up to now, polymers, carbon-based, silica-based NPs and metal-based NPs are the most studied non-biodegradable NPs for AIT, which can resist the allergic responses.
Polymer NPs
Dendrimers are branched polymeric NPs with numerous customizable end groups. Dendrimers have been applied for loading both allergens and immunostimulants (such as CpG, LPS, etc.).166–168 The allergens encapsulated are released over several weeks, retaining their immunoreactivity and integrity.117 A pre-clinical study administrated mice with OVA pDNA loaded in poly (ethyleneimine) based NPs and found balanced Th1/Th2 responses and reduced nasal symptoms of rhinitis. The synthetic water-soluble polymer poly (ethylene glycol) (PEG) is an aliphatic polyether, which is the most extensively studied dendrimer in AIT.169 Acid-labile PEG macromonomers have been exploited for controlled delivery of hydrophobic agents, which can break down at pH 5 and release the cargo inside.170 According to an in vitro assay, encapsulating allergens into these NPs can cause targeted administration, and therefore lead to T cells proliferation and to allergen shielding from being identified by IgE. In summary, employing polymer NPs as deliveries can separate the encapsulated cargo from the degrading biological environment and reduce IgE in AR.
Carbon-Based NPs
Carbon nanotubes are also widely explored NPs for drug delivery.171,172 Carbon-based NPs exhibit multi-immune modulatory effects, enhancing or inhibiting immune responses and acting as adjuvants.173 C60 fullerene is a carbon allotrope containing 60 atoms linked by single and double bonds. Previous studies have revealed that C60 fullerene can prevent the release of IgE from mast cells and basophil degranulation and subsequently inhibit anaphylaxis.174 Besides, administration of C60 fullerene to OVA-sensitized mice resulted in a switch from Th2 to Th1 responses, and a reduction in airway inflammation.175,176 It is also vital to indicate that carbon-based NPs can cause negative or even toxic effects and enhanced allergic inflammation, which has been verified in a mouse model of asthma.172 Taken together, carbon-based NPs may be a double-edged sword for AR, and the most important thing is to choose the best collocation scheme.
Silica-Based NPs
Silicon dioxide is a common nano-material for drug delivery, which has the characteristics of low cost, easy synthesis and simple surface modification.177 Compared with other nanoparticles, silica NPs have stronger drug loading capacity and lung biocompatibility.178 Mesoporous silica nanoparticles were considered as potential nanocarriers for nasal and pulmonary drug delivery.178,179 Shen et al developed a diselenide-bridged mesoporous silica nanostabilizer that specifically targeted mast cells by recognizing IgE aptamers.180 Moreover, the non-covalent physical interaction between allergen birch pollen and silica nanoparticles promotes lysosomes to process allergens, increases antigen presentation and reduces IgE antibody level, and finally promotes Th1 reaction.181 However, the clinical transformation of silica nano-materials is still slow, and the safety of materials must be evaluated. The organ toxicity, genetic toxicity and immunotoxicity caused by long-term exposure to silica nanomaterials need further verification.182
Metal-Based NPs
Gold NPs, dextran-coated magnetic NPs and nanodecoys are main types of metal-based NPs used for AIT. Previous studies have revealed that gold NPs can reduce the incidence of nasal symptoms, minimize IgE, IL-4, and IL-17a production, and enhance IL-10 generation. In other words, gold NPs can improve the state of AR by inducing Th1 response and inhibiting IgE production.183–185 However, as with the above non-biodegradable materials, the fate of metal-base NPs in vivo causes safety concerns because they may result in undesired adverse effects.
In summary, non-biodegradable NPs are attractive adjuvants for AIT, which can enhance Th1 response and improve the allergic responses. However, two major disadvantages need to be noted. First, these non-biodegradable NPs can remain in the body and cause undesired effects over time because they possess immunomodulatory activity themselves. Second, these NPs may form protein corona (PC), a more stable thermo-dynamic states of NPs. The composition of PC (abundance and types of proteins) and the conformation at the NP surface can affect the bio-distribution, cellular uptake, and intracellular localization of NPs. Also, PC is responsible for a variety of immune reactions, such as complement activation, which may attenuate the function of NPs. Therefore, to precisely predict the biological response to non-biodegradable NPs, a deeper understanding of the PC is required. In conclusion, the fate of non-biodegradable NPs requires to be thoroughly explored in vivo, especially in terms of their degradation and PC.
Conclusion
AIT is the only etiological treatment option for AR. Although the possible disadvantages of various AIT emerge over time, new options are under research to address the limitations. NPs possess the potential to be an outstanding choice as both immunomodulators and delivery systems for AIT due to their advantages mentioned above. Although NPs-based AIT has not yet been marketed, related clinical trials have confirmed their safety and efficacy. However, before employing NPs-based AIT in market, several problems should be addressed. First, the type, processing mode and chiral structure of NPs will affect its immunogenicity,186–188 which requires studying the mechanism of NPs affecting immune response, establishing evaluation criteria and making reasonable modifications. The drug loading of NPs is also worth considering, because it may be internalized by nonspecific cells during the delivery of NPs, resulting in the failure to reach the effective drug concentration.106 Second, the toxicity of NPs cannot be ignored from laboratory studies to translational clinical trials, including organ toxicity, immunotoxicity, genotoxicity, etc., which needs to be observed from the perspective of in vivo degradation rate of NPs, dose and route of administration, and so on. Acute or short-term animal experimental data cannot confirm the safety. Third, despite animal trails have confirmed the superiority of NPs-based AIT compared with conventional AIT and clinical trials have showed excellent performance of biodegradable NPs-based AIT, especially liposomes and VLP. However, the controls used in clinical studies are placebo instead of traditional AIT, and differences between animal models and humans and their responses to treatments are critical, indicating the need for comparative human trials of NPs- and non-NPs based AIT. Fourth, although the features of NPs make them easily been mass-produced, the cost of transferring nanotechnology advances to clinical practice is still an economic problem because the costs for both drug development and marketing can be too expensive for pharmaceutical industries. Fifth, there exist individual difference among patients, which may largely affect the efficiency of NPs-based AIT when treating different individuals. Thus, how to choose the most proper NPs for AIT for different persons need to be clarified. Finally, how to cleavage non-biodegradable NPs in vivo requires to be explored because they can cause unwanted side effects if they stay in body for a long time. It is noted that, from this perspective, biodegradable NPs seem to be a better option.
In conclusion, huge effort is necessary before applying NPs-based AIT in the treatment of AR. However, NPs, especially biodegradable NPs, -based AIT may be a safe and efficient method for patients with AR.
Acknowledgments
This work was financially supported by Zhejiang Medical and Health Science and Technology Project (2022KY529). The authors also acknowledged Biorender (www.biorender.com) for drawing figures.
Funding Statement
This work was supported by Zhejiang Medical and Health Science and Technology Project (2022KY529).
Abbreviations
AR, allergic rhinitis; AIT, allergen immunotherapy; Breg, regulatory B cells; cDCs, conventional dendritic cells; CpG-OND, CpG oligodeoxynucleotide; DOPE, cationic 1,2-dioleoyl-sn-glycero-3-phosphatidylethanolamine; FcεRI, the high-affinity IgE receptor; FDA, the Food and Drug Administration; IFN-γ, interferon gamma; ILC2s, group 2 innate lymphoid cells; ISS, immunostimulatory sequences; MW, molecular weight; OH-Chol, cholesteryl-3bcarboxyamidoethylene-N-hydroxyethylamine; OVA, ovalbumin; PACA, poly (alkyl-cyanoacrylates); PC, protein corona; PCL, poly (ε-caprolactone); PEG, polyethylene glycol; PGA, poly-gamma glutamic acid; PHEA, poly-hydroxyethylaspartamide; PLA, poly (lactic acids); PLGA, poly (lactide-coglycolic acids); PMMA, poly (methyl methacrylate); RCTs, randomized controlled trials; rHM, recombinant hybrid molecule; TFH, follicular helper T cells; TLRs, reported toll-like receptors; Treg, regulatory T cells; TSLP, thymic stromal lymphopoietin; VLPs, Virus-like particles.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Chhabra N, Houser SM. The surgical management of allergic rhinitis. Otolaryngol Clin North Am. 2011;44(3):779–795. doi: 10.1016/j.otc.2011.03.007 [DOI] [PubMed] [Google Scholar]
- 2.Meng Y, Wang C, Zhang L. Advances and novel developments in allergic rhinitis. Allergy. 2020;75(12):3069–3076. doi: 10.1111/all.14586 [DOI] [PubMed] [Google Scholar]
- 3.Schuler Iv CF, Montejo JM. Allergic rhinitis in children and adolescents. Immunol Allergy Clin North Am. 2021;41(4):613–625. doi: 10.1016/j.iac.2021.07.010 [DOI] [PubMed] [Google Scholar]
- 4.Costa DJ, Bousquetbh PJ, Ryan D, et al. Guidelines for allergic rhinitis need to be used in primary care. Prim Care Respir J. 2009;18(4):250–257. doi: 10.4104/pcrj.2009.00028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang Y, Li X, Zhu L, et al. Thrombin cleaves IL‐33 and modulates IL‐33‐activated allergic lung inflammation. Allergy. 2022;77(7):2104–2120. doi: 10.1111/all.15210 [DOI] [PubMed] [Google Scholar]
- 6.Zhang Y, Lan F, Zhang L. Update on pathomechanisms and treatments in allergic rhinitis. Allergy. 2022;77(11):3309–3319. doi: 10.1111/all.15454 [DOI] [PubMed] [Google Scholar]
- 7.Fernández‐Gallego N, Castillo‐González R, Méndez‐Barbero N, et al. The impact of type 2 immunity and allergic diseases in atherosclerosis. Allergy. 2022;77(11):3249–3266. doi: 10.1111/all.15426 [DOI] [PubMed] [Google Scholar]
- 8.Bousquet J, Anto JM, Bachert C, et al. Allergic rhinitis. Nat Rev Dis Primer. 2020;6(1):95. doi: 10.1038/s41572-020-00227-0 [DOI] [PubMed] [Google Scholar]
- 9.Bousquet J, Pfaar O, Togias A, et al. ARIA care pathways for allergen immunotherapy. Allergy. 2019;74(11):2087–2102. doi: 10.1111/all.13805 [DOI] [PubMed] [Google Scholar]
- 10.Bian S, Zhang P, Li L, et al. Anaphylaxis associated with allergen specific immunotherapy, omalizumab, and dupilumab: a real world study based on the US Food and Drug Administration adverse event reporting system. Front Pharmacol. 2021;12:767999. doi: 10.3389/fphar.2021.767999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Makatsori M, Calderon MA. Anaphylaxis: still a ghost behind allergen immunotherapy. Curr Opin Allergy Clin Immunol. 2014;14(4):316–322. doi: 10.1097/ACI.0000000000000075 [DOI] [PubMed] [Google Scholar]
- 12.Malipiero G, Melone G, Puggioni F, Pawankar R, Heffler E, Paoletti G. Allergen immunotherapy and biologics in respiratory allergy: friends or foes? Curr Opin Allergy Clin Immunol. 2021;21(1):16–23. doi: 10.1097/ACI.0000000000000707 [DOI] [PubMed] [Google Scholar]
- 13.Zhu H, Tang K, Chen G, Liu Z. Biomarkers in oral immunotherapy. J Zhejiang Univ-Sci B. 2022;23(9):705–731. doi: 10.1631/jzus.B2200047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson L, Duschl A, Himly M. Nanotechnology-based vaccines for allergen-specific immunotherapy: potentials and challenges of conventional and novel adjuvants under research. Vaccines. 2020;8(2):237. doi: 10.3390/vaccines8020237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Souza Rebouças J, Esparza I, Ferrer M, Sanz ML, Irache JM, Gamazo C. Nanoparticulate adjuvants and delivery systems for allergen immunotherapy. J Biomed Biotechnol. 2012;2012:1–13. doi: 10.1155/2012/474605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gamazo C, D’Amelio C, Gastaminza G, Ferrer M, Irache JM. Adjuvants for allergy immunotherapeutics. Hum Vaccines Immunother. 2017;13(10):2416–2427. doi: 10.1080/21645515.2017.1348447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pohlit H, Bellinghausen I, Frey H, Saloga J. Recent advances in the use of nanoparticles for allergen‐specific immunotherapy. Allergy. 2017;72(10):1461–1474. doi: 10.1111/all.13199 [DOI] [PubMed] [Google Scholar]
- 18.Singh N, Diebold Y, Sahu SK, Leonardi A. Epithelial barrier dysfunction in ocular allergy. Allergy. 2022;77(5):1360–1372. doi: 10.1111/all.15174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang C, Wang Y, Liao W, et al. MUC1 deficiency induces the nasal epithelial barrier dysfunction via RBFOX3 shortage augment ubiquitin–proteasomal degradation in allergic rhinitis pathogenesis. Allergy. 2022;77(5):1596–1599. doi: 10.1111/all.15235 [DOI] [PubMed] [Google Scholar]
- 20.Brusilovsky M, Rochman M, Rochman Y, et al. Environmental allergens trigger type 2 inflammation through ripoptosome activation. Nat Immunol. 2021;22(10):1316–1326. doi: 10.1038/s41590-021-01011-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li M, Wang ZN, Yang LF, et al. TLR4 antagonist suppresses airway remodeling in asthma by inhibiting the T-helper 2 response. Exp Ther Med. 2017;14(4):2911–2916. doi: 10.3892/etm.2017.4898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sakamoto M, Asahina R, Kamishina H, Maeda S. Transcription of thymic stromal lymphopoietin via Toll‐like receptor 2 in canine keratinocytes: a possible association of Staphylococcus spp. in the deterioration of allergic inflammation in canine atopic dermatitis. Vet Dermatol. 2016;27(3):184. doi: 10.1111/vde.12301 [DOI] [PubMed] [Google Scholar]
- 23.Kitajima M, Lee H, Nakayama T, Ziegler SF. TSLP enhances the function of helper type 2 cells. Eur J Immunol. 2011;41(7):1862–1871. doi: 10.1002/eji.201041195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Palomares O, Akdis M, Martín‐Fontecha M, Akdis CA. Mechanisms of immune regulation in allergic diseases: the role of regulatory T and B cells. Immunol Rev. 2017;278(1):219–236. doi: 10.1111/imr.12555 [DOI] [PubMed] [Google Scholar]
- 25.Ihara F, Sakurai D, Yonekura S, et al. Identification of specifically reduced Th2 cell subsets in allergic rhinitis patients after sublingual immunotherapy. Allergy. 2018;73(9):1823–1832. doi: 10.1111/all.13436 [DOI] [PubMed] [Google Scholar]
- 26.Nakayama T, Hirahara K, Onodera A, et al. Th2 cells in health and disease. Annu Rev Immunol. 2017;35(1):53–84. doi: 10.1146/annurev-immunol-051116-052350 [DOI] [PubMed] [Google Scholar]
- 27.Iinuma T, Okamoto Y, Morimoto Y, et al. Pathogenicity of memory Th2 cells is linked to stage of allergic rhinitis. Allergy. 2018;73(2):479–489. doi: 10.1111/all.13295 [DOI] [PubMed] [Google Scholar]
- 28.Colas L, Magnan A, Brouard S. Immunoglobulin E response in health and disease beyond allergic disorders. Allergy. 2022;77(6):1700–1718. doi: 10.1111/all.15230 [DOI] [PubMed] [Google Scholar]
- 29.Testera‐Montes A, Palomares F, Jurado‐Escobar R, et al. Sequential class switch recombination to IgE and allergen‐induced accumulation of IgE + plasmablasts occur in the nasal mucosa of local allergic rhinitis patients. Allergy. 2022;77(9):2712–2724. doi: 10.1111/all.15292 [DOI] [PubMed] [Google Scholar]
- 30.Wheatley LM, Togias A. Allergic Rhinitis. Solomon CG, ed. N Engl J Med. 2015;372(5):456–463. doi: 10.1056/NEJMcp1412282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eifan AO, Durham SR. Pathogenesis of rhinitis. Clin Exp Allergy. 2016;46(9):1139–1151. doi: 10.1111/cea.12780 [DOI] [PubMed] [Google Scholar]
- 32.Greiner AN, Hellings PW, Rotiroti G, Scadding GK. Allergic rhinitis. Lancet. 2011;378(9809):2112–2122. doi: 10.1016/S0140-6736(11)60130-X [DOI] [PubMed] [Google Scholar]
- 33.Renand A, Shamji MH, Harris KM, et al. Synchronous immune alterations mirror clinical response during allergen immunotherapy. J Allergy Clin Immunol. 2018;141(5):1750–1760.e1. doi: 10.1016/j.jaci.2017.09.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eifan AO, Orban NT, Jacobson MR, Durham SR. Severe persistent allergic rhinitis. inflammation but no histologic features of structural upper airway remodeling. Am J Respir Crit Care Med. 2015;192(12):1431–1439. doi: 10.1164/rccm.201502-0339OC [DOI] [PubMed] [Google Scholar]
- 35.Lambrecht BN, Hammad H. Allergens and the airway epithelium response: gateway to allergic sensitization. J Allergy Clin Immunol. 2014;134(3):499–507. doi: 10.1016/j.jaci.2014.06.036 [DOI] [PubMed] [Google Scholar]
- 36.Cameron L, Hamid Q, Wright E, et al. Local synthesis of ϵ germline gene transcripts, IL-4, and IL-13 in allergic nasal mucosa after ex vivo allergen exposure. J Allergy Clin Immunol. 2000;106(1):46–52. doi: 10.1067/mai.2000.107398 [DOI] [PubMed] [Google Scholar]
- 37.Tsuda T, Maeda Y, Nishide M, et al. Eosinophil-derived neurotoxin enhances airway remodeling in eosinophilic chronic rhinosinusitis and correlates with disease severity. Int Immunol. 2019;31(1):33–40. doi: 10.1093/intimm/dxy061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fettrelet T, Gigon L, Karaulov A, Yousefi S, Simon HU. The enigma of eosinophil degranulation. Int J Mol Sci. 2021;22(13):7091. doi: 10.3390/ijms22137091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sousa‐Pinto B, Sá‐Sousa A, Vieira RJ, et al. Behavioural patterns in allergic rhinitis medication in Europe: a study using MASK‐air ® real‐world data. Allergy. 2022;77(9):2699–2711. doi: 10.1111/all.15275 [DOI] [PubMed] [Google Scholar]
- 40.Meng Y, Wang C, Zhang L. Recent developments and highlights in allergic rhinitis. Allergy. 2019;74(12):2320–2328. doi: 10.1111/all.14067 [DOI] [PubMed] [Google Scholar]
- 41.Kiatiwat P, Sangasapaviliya A, Pradubpongsa P, et al. Switching from subcutaneous to sublingual immunotherapy during the maintenance phase in patients with house dust mite allergy. Allergy. 2023;78(7):2019–2021. doi: 10.1111/all.15669 [DOI] [PubMed] [Google Scholar]
- 42.Jutel M, Agache I, Bonini S, et al. International consensus on allergen immunotherapy II: mechanisms, standardization, and pharmacoeconomics. J Allergy Clin Immunol. 2016;137(2):358–368. doi: 10.1016/j.jaci.2015.12.1300 [DOI] [PubMed] [Google Scholar]
- 43.Asoudeh Moghanloo S, Forouzanfar M, Jafarinia M, Fazlollahi MR, Kardar GA. Allergen‐specific immunotherapy by recombinant Der P1 allergen‐derived peptide‐based vaccine in an allergic mouse model. Immun Inflamm Dis. 2023;11(6):e878. doi: 10.1002/iid3.878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Farjadian F, Moghoofei M, Mirkiani S, et al. Bacterial components as naturally inspired nano-carriers for drug/gene delivery and immunization: set the bugs to work? Biotechnol Adv. 2018;36(4):968–985. doi: 10.1016/j.biotechadv.2018.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fanuel S, Tabesh S, Rajani HF, Heidari S, Sadroddiny E, Kardar GA. Decorating and loading ghosts with allergens for allergen immunotherapy. Hum Vaccines Immunother. 2017;13(10):2428–2433. doi: 10.1080/21645515.2017.1365208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pauli G, Larsen TH, Rak S, et al. Efficacy of recombinant birch pollen vaccine for the treatment of birch-allergic rhinoconjunctivitis. J Allergy Clin Immunol. 2008;122(5):951–960. doi: 10.1016/j.jaci.2008.09.017 [DOI] [PubMed] [Google Scholar]
- 47.Moten D, Kolchakova D, Todorov K, Mladenova T, Dzhambazov B. Design of an epitope-based peptide vaccine against the major allergen amb a 11 using immunoinformatic approaches. Protein J. 2022;41(2):315–326. doi: 10.1007/s10930-022-10050-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Haselden BM, Barry Kay A, Larché M. Immunoglobulin E–independent major histocompatibility complex–restricted T cell peptide epitope–induced late asthmatic reactions. J Exp Med. 1999;189(12):1885–1894. doi: 10.1084/jem.189.12.1885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Frcpc AKE, Frankish CW, O’Hehir RE. Treatment with grass allergen peptides improves symptoms of grass pollen-induced allergic rhinoconjunctivitis. J Allergy Clin Immunol. 2017;140(2):486–496. doi: 10.1016/j.jaci.2016.11.043 [DOI] [PubMed] [Google Scholar]
- 50.Valenta R. Recombinant allergy vaccines based on allergen-derived B cell epitopes. Immunol Lett. 2017;189:19–26. doi: 10.1016/j.imlet.2017.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rauber MM, Wu HK, Adams B, et al. Birch pollen allergen‐specific immunotherapy with glutaraldehyde‐modified allergoid induces IL ‐10 secretion and protective antibody responses. Allergy. 2019;74(8):1575–1579. doi: 10.1111/all.13774 [DOI] [PubMed] [Google Scholar]
- 52.Unterhauser J, Ahammer L, Rainer T, et al. Covalent polyphenol modification of a reactive cysteine in the major apple allergen Mal d 1. Food Chem. 2023;410:135374. doi: 10.1016/j.foodchem.2022.135374 [DOI] [PubMed] [Google Scholar]
- 53.Dong X, Wang J, Raghavan V. Critical reviews and recent advances of novel non-thermal processing techniques on the modification of food allergens. Crit Rev Food Sci Nutr. 2021;61(2):196–210. doi: 10.1080/10408398.2020.1722942 [DOI] [PubMed] [Google Scholar]
- 54.Scheiblhofer S, Thalhamer J, Weiss R. DNA and mRNA vaccination against allergies. Pediatr Allergy Immunol. 2018;29(7):679–688. doi: 10.1111/pai.12964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rondón C, Campo P, Salas M, et al. Efficacy and safety of D. pteronyssinus immunotherapy in local allergic rhinitis: a double‐blind placebo‐controlled clinical trial. Allergy. 2016;71(7):1057–1061. doi: 10.1111/all.12889 [DOI] [PubMed] [Google Scholar]
- 56.Huang Y, Wang C, Wang X, Zhang L, Lou H. Efficacy and safety of subcutaneous immunotherapy with house dust mite for allergic rhinitis: a meta‐analysis of randomized controlled trials. Allergy. 2019;74(1):189–192. doi: 10.1111/all.13583 [DOI] [PubMed] [Google Scholar]
- 57.Maloney J, Durham S, Skoner D, et al. Safety of sublingual immunotherapy Timothy grass tablet in subjects with allergic rhinitis with or without conjunctivitis and history of asthma. Allergy. 2015;70(3):302–309. doi: 10.1111/all.12560 [DOI] [PubMed] [Google Scholar]
- 58.Masuyama K, Okamoto Y, Okamiya K, et al. Efficacy and safety of SQ house dust mite sublingual immunotherapy‐tablet in Japanese children. Allergy. 2018;73(12):2352–2363. doi: 10.1111/all.13544 [DOI] [PubMed] [Google Scholar]
- 59.Hoffmann HJ, Valovirta E, Pfaar O, et al. Novel approaches and perspectives in allergen immunotherapy. Allergy. 2017;72(7):1022–1034. doi: 10.1111/all.13135 [DOI] [PubMed] [Google Scholar]
- 60.Pfaar O, Lou H, Zhang Y, Klimek L, Zhang L. Recent developments and highlights in allergen immunotherapy. Allergy. 2018;73(12):2274–2289. doi: 10.1111/all.13652 [DOI] [PubMed] [Google Scholar]
- 61.Longo A, Longo V, Colombo P. Nanoparticles in allergen immunotherapy. Curr Opin Allergy Clin Immunol. 2021;21(6):576–582. doi: 10.1097/ACI.0000000000000782 [DOI] [PubMed] [Google Scholar]
- 62.Wang M, Zhao N, Wang C, Jin Z, Zhang L. Immunomodulatory properties of mesenchymal stem cells: a potential therapeutic strategy for allergic rhinitis. Allergy. 2023;78(6):1425–1440. doi: 10.1111/all.15729 [DOI] [PubMed] [Google Scholar]
- 63.Kristiansen M, Dhami S, Netuveli G, et al. Allergen immunotherapy for the prevention of allergy: a systematic review and meta‐analysis. Pediatr Allergy Immunol. 2017;28(1):18–29. doi: 10.1111/pai.12661 [DOI] [PubMed] [Google Scholar]
- 64.Zubeldia J, Ferrer M, Dávila I, Justicia J. Adjuvants in allergen-specific immunotherapy: modulating and enhancing the immune response. J Investig Allergol Clin Immunol. 2019;29(2):103–111. doi: 10.18176/jiaci.0349 [DOI] [PubMed] [Google Scholar]
- 65.Dhami S, Zaman H, Varga EM, et al. Allergen immunotherapy for insect venom allergy: a systematic review and meta-analysis. Allergy. 2017;72(3):342–365. doi: 10.1111/all.13077 [DOI] [PubMed] [Google Scholar]
- 66.O’Hagan DT, De Gregorio E. The path to a successful vaccine adjuvant – ‘The long and winding road. Drug Discov Today. 2009;14(11–12):541–551. doi: 10.1016/j.drudis.2009.02.009 [DOI] [PubMed] [Google Scholar]
- 67.Klimek L, Schmidt-Weber CB, Kramer MF, Skinner MA, Heath MD. Clinical use of adjuvants in allergen-immunotherapy. Expert Rev Clin Immunol. 2017;13(6):599–610. doi: 10.1080/1744666X.2017.1292133 [DOI] [PubMed] [Google Scholar]
- 68.Aguilar JC, Rodríguez EG. Vaccine adjuvants revisited. Vaccine. 2007;25(19):3752–3762. doi: 10.1016/j.vaccine.2007.01.111 [DOI] [PubMed] [Google Scholar]
- 69.Lambrecht BN, Kool M, Willart MA, Hammad H. Mechanism of action of clinically approved adjuvants. Curr Opin Immunol. 2009;21(1):23–29. doi: 10.1016/j.coi.2009.01.004 [DOI] [PubMed] [Google Scholar]
- 70.Goto N, Kato H, Maeyama J, et al. Local tissue irritating effects and adjuvant activities of calcium phosphate and aluminium hydroxide with different physical properties. Vaccine. 1997;15(12–13):1364–1371. doi: 10.1016/S0264-410X(97)00054-6 [DOI] [PubMed] [Google Scholar]
- 71.Kratzer B, Hofer S, Zabel M, Pickl WF. All the small things: how virus‐like particles and liposomes modulate allergic immune responses. Eur J Immunol. 2020;50(1):17–32. doi: 10.1002/eji.201847810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kean T, Thanou M. Biodegradation, biodistribution and toxicity of chitosan. Adv Drug Deliv Rev. 2010;62(1):3–11. doi: 10.1016/j.addr.2009.09.004 [DOI] [PubMed] [Google Scholar]
- 73.Chen MC, Mi FL, Liao ZX, et al. Recent advances in chitosan-based nanoparticles for oral delivery of macromolecules. Adv Drug Deliv Rev. 2013;65(6):865–879. doi: 10.1016/j.addr.2012.10.010 [DOI] [PubMed] [Google Scholar]
- 74.Zeng Z. Recent advances of chitosan nanoparticles as drug carriers. Int J Nanomed. 2011;765. doi: 10.2147/IJN.S17296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dong C, Chen W, Liu C. Flocculation of algal cells by amphoteric chitosan-based flocculant. Bioresour Technol. 2014;170:239–247. doi: 10.1016/j.biortech.2014.07.108 [DOI] [PubMed] [Google Scholar]
- 76.Choi C, Nam JP, Nah JW. Application of chitosan and chitosan derivatives as biomaterials. J Ind Eng Chem. 2016;33:1–10. doi: 10.1016/j.jiec.2015.10.028 [DOI] [Google Scholar]
- 77.Jose S, Prema MT, Chacko AJ, Thomas AC, Souto EB. Colon specific chitosan microspheres for chronotherapy of chronic stable angina. Colloids Surf B Biointerfaces. 2011;83(2):277–283. doi: 10.1016/j.colsurfb.2010.11.033 [DOI] [PubMed] [Google Scholar]
- 78.Laycock B, Nikolić M, Colwell JM, et al. Lifetime prediction of biodegradable polymers. Prog Polym Sci. 2017;71:144–189. doi: 10.1016/j.progpolymsci.2017.02.004 [DOI] [Google Scholar]
- 79.Pokhrel S, Yadav PN. Functionalization of chitosan polymer and their applications. J Macromol Sci Part A. 2019;56(5):450–475. doi: 10.1080/10601325.2019.1581576 [DOI] [Google Scholar]
- 80.Sun M, Qin D, Fan P, Chen X, Liu Y. Chitosan-centered nanosystems as sustained therapeutics for allergic rhinitis intervention: inhibition of histamine-induced cascades. J Control Release. 2021;335:422–436. doi: 10.1016/j.jconrel.2021.05.048 [DOI] [PubMed] [Google Scholar]
- 81.Sun M, Yu X, Wang T, Bi S, Liu Y, Chen X. Nasal adaptive chitosan-based nano-vehicles for anti-allergic drug delivery. Int J Biol Macromol. 2019;135:1182–1192. doi: 10.1016/j.ijbiomac.2019.05.188 [DOI] [PubMed] [Google Scholar]
- 82.Wang S, Sun Y, Zhang J, et al. Astragalus polysaccharides/chitosan microspheres for nasal delivery: preparation, optimization, characterization, and pharmacodynamics. Front Pharmacol. 2020;11:230. doi: 10.3389/fphar.2020.00230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Abruzzo A, Cerchiara T, Bigucci F, et al. Cromolyn-crosslinked chitosan nanoparticles for the treatment of allergic rhinitis. Eur J Pharm Sci. 2019;131:136–145. doi: 10.1016/j.ejps.2019.02.015 [DOI] [PubMed] [Google Scholar]
- 84.Krishna SS, Farhana SA, T.p. A, et al. Modulation of immune response by nanoparticle-based immunotherapy against food allergens. Front Immunol. 2023;14:1229667. doi: 10.3389/fimmu.2023.1229667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jirawutthiwongchai J, Iyanut K, Hobang N, et al. Chitosan-phenylalanine-mPEG nanoparticles: from a single step water-based conjugation to the potential allergen delivery system. Carbohydr Polym. 2016;141:41–53. doi: 10.1016/j.carbpol.2015.12.076 [DOI] [PubMed] [Google Scholar]
- 86.Saint‐Lu N, Tourdot S, Razafindratsita A, et al. Targeting the allergen to oral dendritic cells with mucoadhesive chitosan particles enhances tolerance induction. Allergy. 2009;64(7):1003–1013. doi: 10.1111/j.1398-9995.2009.01945.x [DOI] [PubMed] [Google Scholar]
- 87.Ou J, Shi W, Xu Y, Tao Z. Intranasal immunization with DNA vaccine coexpressing Der p 1 and ubiquitin in an allergic rhinitis mouse model. Ann Allergy Asthma Immunol. 2014;113(6):658–665.e1. doi: 10.1016/j.anai.2014.08.015 [DOI] [PubMed] [Google Scholar]
- 88.Chew JL, Wolfowicz CB, Mao HQ, Leong KW, Chua KY. Chitosan nanoparticles containing plasmid DNA encoding house dust mite allergen, Der p 1 for oral vaccination in mice. Vaccine. 2003;21(21–22):2720–2729. doi: 10.1016/S0264-410X(03)00228-7 [DOI] [PubMed] [Google Scholar]
- 89.Li G, Liu Z, Liao B, Zhong N. Induction of Th1-type immune response by chitosan nanoparticles containing plasmid DNA encoding house dust mite allergen der p 2 for oral vaccination in mice. Cell Mol Immunol. 2009;6(1):45–50. doi: 10.1038/cmi.2009.6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Li F, Wang L, Jin XM, Yan CH, Jiang S, Shen XM. The immunologic effect of TGF-beta1 chitosan nanoparticle plasmids on ovalbumin-induced allergic BALB/c mice. Immunobiology. 2009;214(2):87–99. doi: 10.1016/j.imbio.2008.07.007 [DOI] [PubMed] [Google Scholar]
- 91.Kim ST, Kim CK. Water-soluble chitosan-based antisense oligodeoxynucleotide of interleukin-5 for treatment of allergic rhinitis. Biomaterials. 2007;28(22):3360–3368. doi: 10.1016/j.biomaterials.2007.03.029 [DOI] [PubMed] [Google Scholar]
- 92.Klier J, Lehmann B, Fuchs S, et al. Nanoparticulate CpG immunotherapy in RAO ‐affected horses: Phase I and II a Study. J Vet Intern Med. 2015;29(1):286–293. doi: 10.1111/jvim.12524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hathout RM, Metwally AA, Woodman TJ, Hardy JG. Prediction of drug loading in the gelatin matrix using computational methods. ACS Omega. 2020;5(3):1549–1556. doi: 10.1021/acsomega.9b03487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pali-Schöll I, Szöllösi H, Starkl P, et al. Protamine nanoparticles with CpG-oligodeoxynucleotide prevent an allergen-induced Th2-response in BALB/c mice. Eur J Pharm Biopharm. 2013;85(3):656–664. doi: 10.1016/j.ejpb.2013.03.003 [DOI] [PubMed] [Google Scholar]
- 95.Thunberg S, Neimert‐Andersson T, Cheng Q, et al. Prolonged antigen‐exposure with carbohydrate particle based vaccination prevents allergic immune responses in sensitized mice. Allergy. 2009;64(6):919–926. doi: 10.1111/j.1398-9995.2008.01905.x [DOI] [PubMed] [Google Scholar]
- 96.Andersson TN, Ekman GJ, Grönlund H, et al. A novel adjuvant–allergen complex, CBP–rFel d 1, induces up‐regulation of CD86 expression and enhances cytokine release by human dendritic cells in vitro. Immunology. 2004;113(2):253–259. doi: 10.1111/j.1365-2567.2004.01943.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Weinberger EE, Himly M, Myschik J, et al. Generation of hypoallergenic neoglycoconjugates for dendritic cell targeted vaccination: a novel tool for specific immunotherapy. J Control Release. 2013;165(2):101–109. doi: 10.1016/j.jconrel.2012.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Calzada D, Baos S, Cremades L, Cardaba B. New treatments for allergy: advances in peptide immunotherapy. Curr Med Chem. 2018;25(19):2215–2232. doi: 10.2174/0929867325666171201114353 [DOI] [PubMed] [Google Scholar]
- 99.Alexander C, Ying SB, Kay A, Larché M. Fel d 1‐derived T cell peptide therapy induces recruitment of CD4 + CD25 +; CD4 + interferon‐γ + T helper type 1 cells to sites of allergen‐induced late‐phase skin reactions in cat‐allergic subjects. Clin Exp Allergy. 2005;35(1):52–58. doi: 10.1111/j.1365-2222.2005.02143.x [DOI] [PubMed] [Google Scholar]
- 100.Smith TRF, Alexander C, Kay AB, Larché M, Robinson DS. Cat allergen peptide immunotherapy reduces CD4 + T cell responses to cat allergen but does not alter suppression by CD4 + CD25 + T cells: a double‐blind placebo‐controlled study. Allergy. 2004;59(10):1097–1101. doi: 10.1111/j.1398-9995.2004.00601.x [DOI] [PubMed] [Google Scholar]
- 101.Jacquet A. Perspectives in allergen-specific immunotherapy: molecular evolution of peptide- and protein-based strategies. Curr Protein Pept Sci. 2020;21(2):203–223. doi: 10.2174/1389203720666190718152534 [DOI] [PubMed] [Google Scholar]
- 102.Calzada D, Cremades-Jimeno L, López-Ramos M, Cárdaba B. Peptide allergen immunotherapy: a new perspective in olive-pollen allergy. Pharmaceutics. 2021;13(7):1007. doi: 10.3390/pharmaceutics13071007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ding MR, Liang QL, Xu HG, et al. Smart peptide defense web in situ connects for continuous interception of IgE against allergic rhinitis. ACS Appl Mater Interfaces. 2022;14(26):29639–29649. doi: 10.1021/acsami.2c07092 [DOI] [PubMed] [Google Scholar]
- 104.Campbell JD, Buckland KF, McMillan SJ, et al. Peptide immunotherapy in allergic asthma generates IL-10–dependent immunological tolerance associated with linked epitope suppression. J Exp Med. 2009;206(7):1535–1547. doi: 10.1084/jem.20082901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Benedé S, Ramos-Soriano J, Palomares F, et al. Peptide glycodendrimers as potential vaccines for olive pollen allergy. Mol Pharm. 2020;17(3):827–836. doi: 10.1021/acs.molpharmaceut.9b01082 [DOI] [PubMed] [Google Scholar]
- 106.Teng Z, Yang J, Chen X, Liu Y. Intranasal morphology transformation nanomedicines for long-term intervention of allergic rhinitis. ACS Nano. 2023;17(24):25322–25334. doi: 10.1021/acsnano.3c08752 [DOI] [PubMed] [Google Scholar]
- 107.Liu Q, Li S, Dupuy A, et al. Exosomes as new biomarkers and drug delivery tools for the prevention and treatment of various diseases: current perspectives. Int J Mol Sci. 2021;22(15):7763. doi: 10.3390/ijms22157763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lässer C, O’Neil SE, Ekerljung L, Ekström K, Sjöstrand M, Lötvall J. RNA-containing exosomes in human nasal secretions. Am J Rhinol Allergy. 2011;25(2):89–93. doi: 10.2500/ajra.2011.25.3573 [DOI] [PubMed] [Google Scholar]
- 109.Wu G, Yang G, Zhang R, et al. Altered microRNA expression profiles of extracellular vesicles in nasal mucus from patients with allergic rhinitis. Allergy Asthma Immunol Res. 2015;7(5):449–457. doi: 10.4168/aair.2015.7.5.449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kocholata M, Prusova M, Auer Malinska H, Maly J, Janouskova O. Comparison of two isolation methods of tobacco-derived extracellular vesicles, their characterization and uptake by plant and rat cells. Sci Rep. 2022;12(1):19896. doi: 10.1038/s41598-022-23961-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kang C, He H, Liu P, et al. Role of dendritic cell‑derived exosomes in allergic rhinitis (Review). Int J Mol Med. 2023;52(6):117. doi: 10.3892/ijmm.2023.5320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Xia J, Miao Y, Wang X, Huang X, Dai J. Recent progress of dendritic cell-derived exosomes (Dex) as an anti-cancer nanovaccine. Biomed Pharmacother Biomedecine Pharmacother. 2022;152:113250. doi: 10.1016/j.biopha.2022.113250 [DOI] [PubMed] [Google Scholar]
- 113.Mo LH, Han HY, Jin QR, et al. T cell activator-carrying extracellular vesicles induce antigen-specific regulatory T cells. Clin Exp Immunol. 2021;206(2):129–140. doi: 10.1111/cei.13655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Fang S, Zhang H, Wang C, et al. Small extracellular vesicles derived from human mesenchymal stromal cells prevent group 2 innate lymphoid cell‐dominant allergic airway inflammation through delivery of miR‐146a‐5p. J Extracell Vesicles. 2020;9(1):1723260. doi: 10.1080/20013078.2020.1723260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Liu W, Ota M, Tabushi M, Takahashi Y, Takakura Y. Development of allergic rhinitis immunotherapy using antigen-loaded small extracellular vesicles. J Control Release. 2022;345:433–442. doi: 10.1016/j.jconrel.2022.03.016 [DOI] [PubMed] [Google Scholar]
- 116.Liu J. New insights into allergic rhinitis treatment: MSC nanovesicles targeting dendritic cells. Journal of Nanobiotechnology. 2024;22(1):575. doi: 10.1186/s12951-024-02748-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ding D, Zhu Q. Recent advances of PLGA micro/nanoparticles for the delivery of biomacromolecular therapeutics. Mater Sci Eng C. 2018;92:1041–1060. doi: 10.1016/j.msec.2017.12.036 [DOI] [PubMed] [Google Scholar]
- 118.Joshi VB, Adamcakova-Dodd A, Jing X, et al. Development of a poly (lactic-co-glycolic acid) particle vaccine to protect against house dust mite induced allergy. AAPS J. 2014;16(5):975–985. doi: 10.1208/s12248-014-9624-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Gómez JMM, Fischer S, Csaba N, et al. A protective allergy vaccine based on CpG- and protamine-containing PLGA microparticles. Pharm Res. 2007;24(10):1927–1935. doi: 10.1007/s11095-007-9318-0 [DOI] [PubMed] [Google Scholar]
- 120.Batanero E. Encapsulation of Ole e 1 in biodegradable microparticles induces Th1 response in mice: a potential vaccine for allergy. J Control Release. 2003;92(3):395–398. doi: 10.1016/S0168-3659(03)00337-7 [DOI] [PubMed] [Google Scholar]
- 121.Schöll I, Weissenböck A, Förster‐Waldl E, et al. Allergen‐loaded biodegradable poly(d, l ‐lactic‐co‐glycolic) acid nanoparticles down‐regulate an ongoing Th2 response in the BALB/c mouse model. Clin Exp Allergy. 2004;34(2):315–321. doi: 10.1111/j.1365-2222.2004.01884.x [DOI] [PubMed] [Google Scholar]
- 122.Salari F, Varasteh AR, Vahedi F, Hashemi M, Sankian M. Down-regulation of Th2 immune responses by sublingual administration of poly (lactic-co-glycolic) acid (PLGA)-encapsulated allergen in BALB/c mice. Int Immunopharmacol. 2015;29(2):672–678. doi: 10.1016/j.intimp.2015.09.011 [DOI] [PubMed] [Google Scholar]
- 123.Hajavi J, Hashemi M, Sankian M. Evaluation of size and dose effects of rChe a 3 allergen loaded PLGA nanoparticles on modulation of Th2 immune responses by sublingual immunotherapy in mouse model of rhinitis allergic. Int J Pharm. 2019;563:282–292. doi: 10.1016/j.ijpharm.2019.03.040 [DOI] [PubMed] [Google Scholar]
- 124.Marazuela EG, Prado N, Moro E, et al. Intranasal vaccination with poly(lactide‐ co ‐glycolide) microparticles containing a peptide T of Ole e 1 prevents mice against sensitization. Clin Exp Allergy. 2008;38(3):520–528. doi: 10.1111/j.1365-2222.2007.02922.x [DOI] [PubMed] [Google Scholar]
- 125.Kostadinova AI, Middelburg J, Ciulla M, et al. PLGA nanoparticles loaded with beta-lactoglobulin-derived peptides modulate mucosal immunity and may facilitate cow’s milk allergy prevention. Eur J Pharmacol. 2018;818:211–220. doi: 10.1016/j.ejphar.2017.10.051 [DOI] [PubMed] [Google Scholar]
- 126.Roman BS, Espuelas S, Gómez S, et al. Intradermal immunization with ovalbumin‐loaded poly‐ɛ‐caprolactone microparticles conferred protection in ovalbumin‐sensitized allergic mice. Clin Exp Allergy. 2007;37(2):287–295. doi: 10.1111/j.1365-2222.2007.02654.x [DOI] [PubMed] [Google Scholar]
- 127.Di Gioacchino M, Petrarca C, Gatta A, et al. Nanoparticle-based immunotherapy: state of the art and future perspectives. Expert Rev Clin Immunol. 2020;16(5):513–525. doi: 10.1080/1744666X.2020.1762572 [DOI] [PubMed] [Google Scholar]
- 128.Smith MC, Crist RM, Clogston JD, McNeil SE. Zeta potential: a case study of cationic, anionic, and neutral liposomes. Anal Bioanal Chem. 2017;409(24):5779–5787. doi: 10.1007/s00216-017-0527-z [DOI] [PubMed] [Google Scholar]
- 129.Wang Y. Liposome as a delivery system for the treatment of biofilm‐mediated infections. J Appl Microbiol. 2021;131(6):2626–2639. doi: 10.1111/jam.15053 [DOI] [PubMed] [Google Scholar]
- 130.Ng VWL, Ke X, Lee ALZ, Hedrick JL, Yang YY. Synergistic co‐delivery of membrane‐disrupting polymers with commercial antibiotics against highly opportunistic bacteria. Adv Mater. 2013;25(46):6730–6736. doi: 10.1002/adma.201302952 [DOI] [PubMed] [Google Scholar]
- 131.Nguyen S, Hiorth M, Rykke M, Smistad G. Polymer coated liposomes for dental drug delivery – interactions with parotid saliva and dental enamel. Eur J Pharm Sci. 2013;50(1):78–85. doi: 10.1016/j.ejps.2013.03.004 [DOI] [PubMed] [Google Scholar]
- 132.Derbali RM, Aoun V, Moussa G, et al. Tailored nanocarriers for the pulmonary delivery of levofloxacin against Pseudomonas aeruginosa: a comparative study. Mol Pharm. 2019;16(5):1906–1916. doi: 10.1021/acs.molpharmaceut.8b01256 [DOI] [PubMed] [Google Scholar]
- 133.Zhang M, Lemay SG. Interaction of anionic bulk nanobubbles with cationic liposomes: evidence for reentrant condensation. Langmuir. 2019;35(11):4146–4151. doi: 10.1021/acs.langmuir.8b03927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Makhathini SS, Kalhapure RS, Jadhav M, et al. Novel two-chain fatty acid-based lipids for development of vancomycin pH-responsive liposomes against Staphylococcus aureus and methicillin-resistant Staphylococcus aureus (MRSA). J Drug Target. 2019;27(10):1094–1107. doi: 10.1080/1061186X.2019.1599380 [DOI] [PubMed] [Google Scholar]
- 135.meng LM, Ge Y, Qiu J, et al. Redox/pH dual-controlled release of chlorhexidine and silver ions from biodegradable mesoporous silica nanoparticles against oral biofilms. Int J Nanomed. 2018;Volume 13:7697–7709. doi: 10.2147/IJN.S181168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Liu K, Li H, Williams GR, Wu J, Zhu LM. pH-responsive liposomes self-assembled from electrosprayed microparticles, and their drug release properties. Colloids Surf Physicochem Eng Asp. 2018;537:20–27. doi: 10.1016/j.colsurfa.2017.09.046 [DOI] [Google Scholar]
- 137.Nouri HR, Varasteh A, Jaafari MR, Davies JM, Sankian M. Induction of a Th1 immune response and suppression of IgE via immunotherapy with a recombinant hybrid molecule encapsulated in liposome–protamine–DNA nanoparticles in a model of experimental allergy. Immunol Res. 2015;62(3):280–291. doi: 10.1007/s12026-015-8659-8 [DOI] [PubMed] [Google Scholar]
- 138.Ishii M, Koyama A, Iseki H, Narumi H, Yokoyama N, Kojima N. Anti-allergic potential of oligomannose-coated liposome-entrapped Cry j 1 as immunotherapy for Japanese cedar pollinosis in mice. Int Immunopharmacol. 2010;10(9):1041–1046. doi: 10.1016/j.intimp.2010.06.003 [DOI] [PubMed] [Google Scholar]
- 139.Chaisri U, Tungtrongchitr A, Indrawattana N, et al. Immunotherapeutic efficacy of liposome-encapsulated refined allergen vaccines against Dermatophagoides pteronyssinus allergy. PLoS One. 2017;12(11):e0188627. doi: 10.1371/journal.pone.0188627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Tasaniyananda N, Chaisri U, Tungtrongchitr A, Chaicumpa W, Sookrung N. Mouse model of cat allergic rhinitis and intranasal liposome-adjuvanted refined fel d 1 vaccine. Lai HC, ed. PLoS One. 2016;11(3):e0150463. doi: 10.1371/journal.pone.0150463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Suzuki S, Sakurai D, Sakurai T, et al. Sublingual administration of liposomes enclosing alpha-galactosylceramide as an effective adjuvant of allergen immunotherapy in a murine model of allergic rhinitis. Allergol Int. 2019;68(3):352–362. doi: 10.1016/j.alit.2019.02.003 [DOI] [PubMed] [Google Scholar]
- 142.Lauriello M, Di Marco GP, Necozione S, et al. Effects of liposomal nasal spray with vitamins A and E on allergic rhinitis. Acta Otorhinolaryngol Ital. 2020;40(3):217–223. doi: 10.14639/0392-100X-N0357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Inoh Y, Tadokoro S, Tanabe H, et al. Inhibitory effects of a cationic liposome on allergic reaction mediated by mast cell activation. Biochem Pharmacol. 2013;86(12):1731–1738. doi: 10.1016/j.bcp.2013.09.023 [DOI] [PubMed] [Google Scholar]
- 144.Nakano Y, Mori M, Nishinohara S, et al. Surface-linked liposomal antigen induces ige-selective unresponsiveness regardless of the lipid components of liposomes. Bioconjug Chem. 2001;12(3):391–395. doi: 10.1021/bc0001185 [DOI] [PubMed] [Google Scholar]
- 145.Yotsumoto S, Kakiuchi T, Aramaki Y. Enhancement of IFN-γ production for Th1-cell therapy using negatively charged liposomes containing phosphatidylserine. Vaccine. 2007;25(29):5256–5262. doi: 10.1016/j.vaccine.2007.05.037 [DOI] [PubMed] [Google Scholar]
- 146.Hathout RM, Gad HA, Metwally AA. Gelatinized‐core liposomes: toward a more robust carrier for hydrophilic molecules. J Biomed Mater Res A. 2017;105(11):3086–3092. doi: 10.1002/jbm.a.36175 [DOI] [PubMed] [Google Scholar]
- 147.Zeltins A. Construction and characterization of virus-like particles: a review. Mol Biotechnol. 2013;53(1):92–107. doi: 10.1007/s12033-012-9598-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Manzano-Szalai K, Thell K, Willensdorfer A, et al. Adeno-associated virus-like particles as new carriers for B-cell vaccines: testing immunogenicity and safety in BALB/c mice. Viral Immunol. 2014;27(9):438–448. doi: 10.1089/vim.2014.0059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Svirshchevskaya EV, Alekseeva L, Marchenko A, et al. Immune response modulation by recombinant peptides expressed in virus-like particles. Clin Exp Immunol. 2002;127(2):199–205. doi: 10.1046/j.1365-2249.2002.01776.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Hirschberg S, Layton GT, Harris SJ, Savage N, Dallman MJ, Lamb JR. CD4+ T cells induced by virus-like particles expressing a major T cell epitope down-regulate IL-5 production in an ongoing immune response to Der p 1 independently of IFN-γ production. Int Immunol. 1999;11(12):1927–1934. doi: 10.1093/intimm/11.12.1927 [DOI] [PubMed] [Google Scholar]
- 151.Gomord V, Stordeur V, Fitchette AC, et al. Design, production and immunomodulatory potency of a novel allergen bioparticle. Chapoval SP, ed. PLoS One. 2020;15(12):e0242867. doi: 10.1371/journal.pone.0242867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Schmitz N, Dietmeier K, Bauer M, et al. Displaying Fel d1 on virus-like particles prevents reactogenicity despite greatly enhanced immunogenicity: a novel therapy for cat allergy. J Exp Med. 2009;206(9):1941–1955. doi: 10.1084/jem.20090199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Thoms F, Jennings GT, Maudrich M, et al. Immunization of cats to induce neutralizing antibodies against Fel d 1, the major feline allergen in human subjects. J Allergy Clin Immunol. 2019;144(1):193–203. doi: 10.1016/j.jaci.2019.01.050 [DOI] [PubMed] [Google Scholar]
- 154.Thoms F, Haas S, Erhart A, et al. Immunization of cats against Fel d 1 results in reduced allergic symptoms of owners. Viruses. 2020;12(3):288. doi: 10.3390/v12030288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kundig T, Senti G, Schnetzler G, et al. Der p 1 peptide on virus-like particles is safe and highly immunogenic in healthy adults. J Allergy Clin Immunol. 2006;117(6):1470–1476. doi: 10.1016/j.jaci.2006.01.040 [DOI] [PubMed] [Google Scholar]
- 156.Senti G, Johansen P, Haug S, et al. Use of A‐type CpG oligodeoxynucleotides as an adjuvant in allergen‐specific immunotherapy in humans: a phase I/IIa clinical trial. Clin Exp Allergy. 2009;39(4):562–570. doi: 10.1111/j.1365-2222.2008.03191.x [DOI] [PubMed] [Google Scholar]
- 157.Klimek L, Willers J, Hammann‐Haenni A, et al. Assessment of clinical efficacy of CYT003‐QbG10 in patients with allergic rhinoconjunctivitis: a phase IIb study. Clin Exp Allergy. 2011;41(9):1305–1312. doi: 10.1111/j.1365-2222.2011.03783.x [DOI] [PubMed] [Google Scholar]
- 158.Creticos PS, Schroeder JT, Hamilton RG, et al. Immunotherapy with a ragweed–toll-like receptor 9 agonist vaccine for allergic rhinitis. N Engl J Med. 2006;355(14):1445–1455. doi: 10.1056/NEJMoa052916 [DOI] [PubMed] [Google Scholar]
- 159.Ma Y, Hayglass KT, Becker AB, et al. Novel cytokine peptide‐based vaccines: an interleukin‐4 vaccine suppresses airway allergic responses in mice. Allergy. 2007;62(6):675–682. doi: 10.1111/j.1398-9995.2007.01384.x [DOI] [PubMed] [Google Scholar]
- 160.Ma Y, Halayko AJ, Basu S, et al. Sustained suppression of IL-13 by a vaccine attenuates airway inflammation and remodeling in mice. Am J Respir Cell Mol Biol. 2013;48(5):540–549. doi: 10.1165/rcmb.2012-0060OC [DOI] [PubMed] [Google Scholar]
- 161.Long Q, Huang W, Yao Y, et al. Virus-like particles presenting interleukin-33 molecules: immunization characteristics and potentials of blocking IL-33/ST2 pathway in allergic airway inflammation. Hum Vaccines Immunother. 2014;10(8):2303–2311. doi: 10.4161/hv.29425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Klimek L, Schendzielorz P, Mueller P, Saudan P, Willers J. Immunotherapy of Allergic Rhinitis: new therapeutic opportunities with virus-like particles filled with Cpg Motifs. Am J Rhinol Allergy. 2013;27(3):206–212. doi: 10.2500/ajra.2013.27.3875 [DOI] [PubMed] [Google Scholar]
- 163.Kratzer B, Köhler C, Hofer S, et al. Prevention of allergy by virus‐like nanoparticles (VNP) delivering shielded versions of major allergens in a humanized murine allergy model. Allergy. 2019;74(2):246–260. doi: 10.1111/all.13573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Van De Veen W, Akdis M. The use of biologics for immune modulation in allergic disease. J Clin Invest. 2019;129(4):1452–1462. doi: 10.1172/JCI124607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Kim CS, Tonga GY, Solfiell D, Rotello VM. Inorganic nanosystems for therapeutic delivery: status and prospects. Adv Drug Deliv Rev. 2013;65(1):93–99. doi: 10.1016/j.addr.2012.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Gómez S, Gamazo C, San Roman B, et al. Allergen immunotherapy with nanoparticles containing lipopolysaccharide from Brucella ovis. Eur J Pharm Biopharm. 2008;70(3):711–717. doi: 10.1016/j.ejpb.2008.05.016 [DOI] [PubMed] [Google Scholar]
- 167.Ballester M, Jeanbart L, De Titta A, et al. Nanoparticle conjugation enhances the immunomodulatory effects of intranasally delivered CpG in house dust mite-allergic mice. Sci Rep. 2015;5(1):14274. doi: 10.1038/srep14274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Gómez S, Gamazo C, San Roman B, et al. A novel nanoparticulate adjuvant for immunotherapy with Lolium perenne. J Immunol Methods. 2009;348(1–2):1–8. doi: 10.1016/j.jim.2009.06.005 [DOI] [PubMed] [Google Scholar]
- 169.Herzberger J, Niederer K, Pohlit H, et al. Polymerization of ethylene oxide, propylene oxide, and other alkylene oxides: synthesis, novel polymer architectures, and bioconjugation. Chem Rev. 2016;116(4):2170–2243. doi: 10.1021/acs.chemrev.5b00441 [DOI] [PubMed] [Google Scholar]
- 170.Pohlit H, Bellinghausen I, Schömer M, Heydenreich B, Saloga J, Frey H. Biodegradable pH-sensitive poly(ethylene glycol) nanocarriers for allergen encapsulation and controlled release. Biomacromolecules. 2015;16(10):3103–3111. doi: 10.1021/acs.biomac.5b00458 [DOI] [PubMed] [Google Scholar]
- 171.Dumortier H. When carbon nanotubes encounter the immune system: desirable and undesirable effects. Adv Drug Deliv Rev. 2013;65(15):2120–2126. doi: 10.1016/j.addr.2013.09.005 [DOI] [PubMed] [Google Scholar]
- 172.Ronzani C, Casset A, Pons F. Exposure to multi-walled carbon nanotubes results in aggravation of airway inflammation and remodeling and in increased production of epithelium-derived innate cytokines in a mouse model of asthma. Arch Toxicol. 2014;88(2):489–499. doi: 10.1007/s00204-013-1116-3 [DOI] [PubMed] [Google Scholar]
- 173.Laverny G, Casset A, Purohit A, et al. Immunomodulatory properties of multi-walled carbon nanotubes in peripheral blood mononuclear cells from healthy subjects and allergic patients. Toxicol Lett. 2013;217(2):91–101. doi: 10.1016/j.toxlet.2012.12.008 [DOI] [PubMed] [Google Scholar]
- 174.Ryan JJ, Bateman HR, Stover A, et al. Fullerene nanomaterials inhibit the allergic response. J Immunol. 2007;179(1):665–672. doi: 10.4049/jimmunol.179.1.665 [DOI] [PubMed] [Google Scholar]
- 175.Popescu R, Grumezescu A. Metal based frameworks for drug delivery systems. Curr Top Med Chem. 2015;15(15):1532–1542. doi: 10.2174/1568026615666150414145323 [DOI] [PubMed] [Google Scholar]
- 176.Shershakova N, Baraboshkina E, Andreev S, et al. Anti-inflammatory effect of fullerene C60 in a mice model of atopic dermatitis. J Nanobiotechnology. 2016;14(1):8. doi: 10.1186/s12951-016-0159-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Hussein HA, Nazir MS, Azra N, et al. Novel drug and gene delivery system and imaging agent based on marine diatom biosilica nanoparticles. Mar Drugs. 2022;20(8):480. doi: 10.3390/md20080480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.García-Fernández A, Sancenón F, Martínez-Máñez R. Mesoporous silica nanoparticles for pulmonary drug delivery. Adv Drug Deliv Rev. 2021;177:113953. doi: 10.1016/j.addr.2021.113953 [DOI] [PubMed] [Google Scholar]
- 179.Mehmood Y, Shahid H, Rashid MA, Alhamhoom Y, Kazi M. Developing of SiO2 nanoshells loaded with fluticasone propionate for potential nasal drug delivery: determination of pro-inflammatory cytokines through mRNA expression. J Funct Biomater. 2022;13(4):229. doi: 10.3390/jfb13040229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Shen Q, Cao M, Yu C, et al. Biodegradable mesoporous organosilica-based nanostabilizer targeting mast cells for long-term treatment of allergic diseases. ACS Nano. 2024;18(26):16934–16946. doi: 10.1021/acsnano.4c03069 [DOI] [PubMed] [Google Scholar]
- 181.Johnson L, Aglas L, Punz B, et al. Mechanistic insights into silica nanoparticle–allergen interactions on antigen presenting cell function in the context of allergic reactions. Nanoscale. 2023;15(5):2262–2275. doi: 10.1039/D2NR05181H [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Tan Y, Yu D, Feng J. Toxicity evaluation of silica nanoparticles for delivery applications. Drug Deliv Transl Res. 2023;13(9):2213–2238. doi: 10.1007/s13346-023-01312-z [DOI] [PubMed] [Google Scholar]
- 183.Pordel S, Haghnavaz N, Rezaee M, et al. An epicutaneous therapeutic pollen-allergen extract delivery system in an allergic rhinitis mouse model: based on allergen loading on DC-specific aptamers conjugated nanogolds. Immunol Res. 2023;72(3):460–475. doi: 10.1007/s12026-023-09445-6 [DOI] [PubMed] [Google Scholar]
- 184.Koushki K, Varasteh AR, Shahbaz SK, et al. Dc-specific aptamer decorated gold nanoparticles: a new attractive insight into the nanocarriers for allergy epicutaneous immunotherapy. Int J Pharm. 2020;584:119403. doi: 10.1016/j.ijpharm.2020.119403 [DOI] [PubMed] [Google Scholar]
- 185.Peng Q, Chen R. Biosynthesis of gold nanoparticles using Caffeoylxanthiazonoside, chemical isolated from Xanthium strumarium L. fruit and their Anti-allergic rhinitis effect- a traditional Chinese medicine. J Photochem Photobiol B. 2019;192:13–18. doi: 10.1016/j.jphotobiol.2018.12.015 [DOI] [PubMed] [Google Scholar]
- 186.Xu L, Wang X, Wang W, et al. Enantiomer-dependent immunological response to chiral nanoparticles. Nature. 2022;601(7893):366–373. doi: 10.1038/s41586-021-04243-2 [DOI] [PubMed] [Google Scholar]
- 187.De S, Rebouças J, Irache JM, et al. Immunogenicity of peanut proteins containing poly(anhydride) nanoparticles. Litwin VM, ed. Clin Vaccine Immunol. 2014;21(8):1106–1112. doi: 10.1128/CVI.00359-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Roach KA, Stefaniak AB, Roberts JR. Metal nanomaterials: immune effects and implications of physicochemical properties on sensitization, elicitation, and exacerbation of allergic disease. J Immunotoxicology. 2019;16(1):87–124. doi: 10.1080/1547691X.2019.1605553 [DOI] [PMC free article] [PubMed] [Google Scholar]