Abstract
Background
The etiological connection between intraocular pressure (IOP) and the risk of retinal vein occlusion (RVO) remains elusive, particularly regarding whether this risk emanates from the direct influence of elevated intraocular pressure (IOP), irrespective of the presence of primary open-angle glaucoma (POAG), or if it arises as a consequence of the sequelae of POAG. Therefore, we conducted a Mendelian Randomization (MR) mediation analysis to elucidate the mediating role of POAG in the association between IOP and RVO.
Methods
We identified 47 single-nucleotide polymorphisms (SNPs) associated with IOP (P-value < 5 × 10−8) leveraging data from a genome-wide association study (GWAS) (N = 97,653) obtained from the UK Biobank and 50 SNPs associated with POAG (P-value < 5 × 10−8) from a GWAS meta-analysis (16,677 cases and 199,580 controls). We related these SNPs with RVO using a GWAS of 775 RVO cases and 376,502 controls from FinnGen. By utilizing univariable and multivariable MR analyses we calculated the total effect of IOP on RVO and estimated the degree to which POAG mediates this association.
Results
MR analyses showed that higher IOP is associated with higher RVO risk (odds ratio of RVO per 1 mmHg increase in IOP: 1.53; 95% confidence interval: 1.04 to 2.26; p-value = 0.03). Moreover, our MR mediation analysis suggested that 91.6% of the total effect of IOP on RVO risk was mediated through POAG. The primary results were consistent with estimates of pleiotropy-robust MR methods.
Conclusion
Our findings suggest that higher IOP increases the risk of RVO and that the majority of this effect is mediated through POAG.
Subject terms: Risk factors, Retinal diseases
Introduction
Retinal vein occlusion (RVO) is the second most common retinal vascular disease following diabetic retinopathy and an important cause of vision loss in individuals older than 80 years with a prevalence of 4.6% [1]. Risk factors for RVO are mainly related to atherosclerosis, but also conditions altering the rheologic properties in the retinal veins, such as hypercoagulability and vasculitis [2].
One such condition is glaucoma, an ocular disorder characterized by optic nerve damage and visual field loss, has been implicated in the pathogenesis of RVO [3]. Specifically, numerous studies have investigated the association between RVO and primary open-angle glaucoma (POAG), revealing a significantly higher prevalence of RVO in patients with POAG compared to the general population [3, 4]. Different pathogenic mechanisms have been proposed, implicating intraocular pressure which is a well-established and modifiable risk factor for glaucoma. Increased IOP has been hypothesized to collapse the retinal vessel walls, resulting in occlusion [5, 6]. Furthermore, structural changes in the vessels at the glaucomatous optic discs might also make them more susceptible to thrombosis [7–10]. However, it remains undetermined whether RVO is caused by the effects of increased IOP independently from any glaucomatous optic disc abnormalities.
Mendelian randomization (MR) serves as a robust methodology utilizing genetic variants derived from genome-wide association studies (GWAS) as instrumental variables to elucidate the causal relationship between modifiable exposures and disease outcomes [11]. A GWAS scans the genome for genetic variants associated with specific traits or diseases by analysing large populations. This approach identifies single-nucleotide polymorphisms (SNPs) linked to the trait of interest, providing insights into the genetic basis of complex diseases and traits. A recent MR study has identified a positive association between POAG and RVO [12]. However, it was not assessed whether this observed effect is intrinsic to POAG itself or if it is a consequence of the elevated intraocular pressure that is mediated through POAG. To address this gap, we conducted an MR mediation analysis to discern the mediating role of POAG in the relationship between IOP and RVO and sought to enhance our understanding of the nuanced interplay between these factors.
Materials and Methods
Study design
MR employs genetic variants as instrumental variables to investigate the impact of risk factors on disease susceptibility [11]. This method mitigates susceptibility to confounding and reverse causation, as these genetic variants are randomly assigned at conception, creating a quasi-randomized exposure allocation analogous to a randomized trial [11]. In our investigation, a two-sample MR was executed using summary statistics of SNPs obtained from GWAS focusing on IOP [13] and RVO [14]. The aim was to evaluate the influence of IOP on the risk of RVO. Moreover, we tried to assess the proportion of the effect of IOP on RVO that is potentially mediated through POAG, by employing a two-step MR for mediation analysis [15]. The study protocol was not pre-registered and our research adhered to the STROBE-MR guidelines [16] and “Guidelines for performing Mendelian randomization investigations” [17]. The study protocol of FinnGen (number HUS/990/2017) has been approved by the Coordinating Ethics Committee of the Hospital District of Helsinki and Uusimaa and all participants of the study provided informed consent [14]. UK Biobank (UKBB) has approval from the North West Multi-centre Research Ethics Committee as a Research Tissue Bank approval. Written informed consent was obtained from all participants of the UKBB and details can be found at https://www.ukbiobank.ac.uk/learn-more-about-uk-biobank/about-us/ethics.
Data sources
Intraocular pressure
Summary data for corneal-compensated IOP were obtained from the UK Biobank (UKBB) cohort GWAS [13] (Supplementary Table 1). Participants with a history of laser or surgery for glaucoma, eye injury, corneal graft surgery, or refractive laser surgery were excluded (PMID: 29785010). In particular, a subset comprising 97,653 individuals within the UKBB underwent ophthalmic evaluations, encompassing the quantification of corneal-compensated IOP measured in millimetres of mercury (mmHg) through the application of a non-contact tonometer. The preference for corneal-compensated IOP as the selected exposure phenotype over regular IOP stems from its designed capability to accommodate corneal biomechanical properties. Moreover, this metric has been previously employed in GWAS for IOP [18].
Retinal vein occlusion
Summary data for RVO were obtained from the FinnGen consortium database’s R9 release, involving a cohort of 775 documented RVO cases and 376,502 controls [14]. The FinnGen GWAS participants were of European origin, and the identification of RVO cases adhered to diagnostic criteria established in either the International Classification of Diseases, Ninth Revision (ICD-9) or International Statistical Classification of Diseases, Tenth Revision (ICD-10) code.
Primary open-angle glaucoma
For the mediation MR analysis, GWAS summary statistics for POAG were also retrieved from a GWAS meta-analysis of 16,677 POAG cases and 199,580 controls of European ancestry [19] (Supplementary Table 1). POAG cases were selected based on the ICD-9/ICD-10 criteria [19]. The genotyping, quality control, and imputation methods applied to the GWAS data used in our study have been detailed elsewhere [14, 19, 20].
Selection of genetic variants as instrumental variables
We selected SNPs identified in the IOP GWAS that achieved genome-wide significance (P-value < 5*10-8), following clumping for linkage disequilibrium (LD) at r2 < 0.001 over a 10 mb window [17]. This stringent threshold was chosen to minimize the potential for correlated SNPs, which could bias the MR analysis by violating the assumption of instrument independence. Moreover, we included SNPs with a minor allele frequency greater than 1%. The MR-Steiger directionality test was employed to discern the directions of the causal relationship between IOP and RVO [21]. SNPs exhibiting a stronger correlation with the outcome than the exposure were systematically excluded, as were those demonstrating notable influence in the funnel plots and scatter plots. Ultimately, we identified 47 SNPs associated with IOP as instrumental variables. Furthermore, we quantified the proportion of variability in IOP that is explained by these 47 SNPs, through the summation of the coefficients of determination (R2) derived from the associations between the selected SNPs and IOP. Employing a similar approach, we chose 50 SNPs from the GWAS on POAG for utilization in our MR mediation analysis.
Statistical analysis
Data harmonization and calculation of Wald ratios
After the process of data harmonization based on HapMap3 [22], the removal of strand-ambiguous variants, and the alignment of association estimates, we proceeded to calculate Wald ratios. We calculated these ratios by dividing the logarithm of the odds ratio per allele for each SNP identified in the GWAS for RVO by its respective estimate obtained from the IOP GWAS. Subsequently, the total effect of IOP on RVO risk was assessed through a multiplicative random effects inverse variance weighted (IVW) meta-analysis of the Wald ratios [23]. Data harmonization is a crucial step to verify that the effect of an SNP on an outcome and exposure is relative to the same allele, since not all GWAS resources consistently report strand information accurately. Moreover, we tried to infer positive strand alleles, using allele frequencies for palindrome SNPs.
Two-sample Mendelian randomization methodology
We implemented a univariable two-sample MR methodology, utilizing summary data extracted from GWAS of IOP and RVO. Our two-sample MR analysis was based on three key assumptions. Firstly, the imperative that the genetic variants selected should be associated with the targeted risk factor, a principle known as the “relevance” assumption [24]. We adhered to this assumption by choosing SNPs as instrumental variables that reached the threshold of genome-wide significance (P-value < 5*10-8). Furthermore, we evaluated the strength of our instrumental variables by assessing the F-statistic of the selected SNPs, concurrently examining the proportion of variance in the exposure that they accounted for [25]. Secondly, the selected genetic instruments should not be correlated with factors that could potentially confound the relationship between the exposure and outcome, also known as the “exchangeability” assumption [24]. Thirdly, the “exclusion restriction” assumption [24], where the genetic instruments should not influence the outcome except through the risk factor of interest. While the “exchangeability” and “exclusion restriction” assumptions are inherently unverifiable, we executed sensitivity analyses to identify potential violations of these MR assumptions. PhenoScanner [26] was employed to assess whether any of our selected genetic instruments were associated with phenotypes that could serve as potential confounders in our analysis. In instances where pleiotropic pathways were identified, we applied multivariable MR to adjust for these effects [27]. Moreover, we examined heterogeneity among our selected SNPs using the Cochran Q heterogeneity test and IGX2 [28] to detect pleiotropy, utilized several MR methods proposed to enhance robustness in instances where genetic variants exhibit pleiotropy [29] (MR Egger regression, penalized weighted median, IVW radial regression, and MR-Pleiotropy Residual Sum and Outlier (MR-PRESSO)) and performed a leave-one-out analysis to ascertain if the IVW estimate was influenced by a singular SNP.
Mendelian randomization for mediation methodology
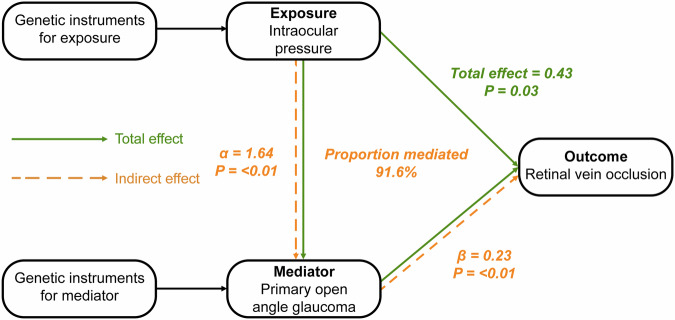
To further explore the mediating effect of IOP on RVO through POAG, we conducted a two-step MR for mediation analysis, as outlined in previous literature [15]. This process involves the calculation of two distinct MR estimates: firstly, calculating the effect of IOP on POAG through a univariable MR model (α, Fig. 1), and secondly, assessing the effect of POAG on RVO using a multivariable MR model that includes an adjustment for IOP (β, Fig. 1). The IOP estimate on POAG multiplied with the adjusted POAG estimate on RVO provided the indirect effect of IOP on RVO, mediated through POAG (indirect effect = α*β, Fig. 1). We also quantified the proportion of the total effect of IOP on RVO explained by the mediator (POAG) by dividing the calculated indirect effect of IOP on RVO by the total effect (proportion mediated = indirect effect/total effect). The delta method was employed to derive 95% confidence intervals (95%CI) for the indirect effect [30]. Furthermore, considering the inherent requirement for independence between the SNPs selected as instruments for the exposure (IOP) and mediator (POAG) in MR mediation analysis, we ensured that the selected SNPs from the IOP and POAG GWAS datasets were non-overlapping [15]. This precautionary step was essential to uphold the validity and integrity of the MR for mediation analysis, preventing potential bias arising from SNP overlap.
Fig. 1. Directed acyclic graphs of the mediation analysis with Mendelian randomization *.
* The indirect effect of intraocular pressure (IOP) on retinal vein occlusion (RVO) can be calculated by multiplying α times β, where α is the effect of IOP on primary open-angle glaucoma (POAG), and β the effect of POAG on RVO. The proportion mediated can be estimated by dividing the indirect effect by the total effect of IOP on RVO. All estimates are shown as the difference in the logarithm of odds of the outcome, per 1 unit increase of the exposure (continuous variables: IOP) or between the two exposure groups (binary variables: POAG).
We conducted all analyses with R version 4.2.1 [31] using the MVMR (0.3), TwoSampleMR (0.5.6), MendelianRandomization (0.5.1), MRPRESSO (1.0) and cause (1.2.0) packages.
Results
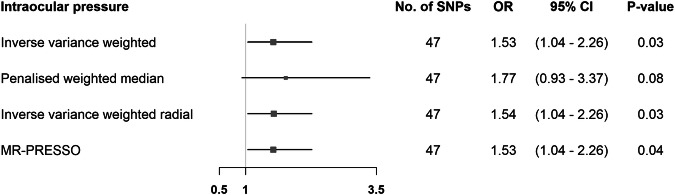
The 47 SNPs selected from the IOP GWAS (Supplementary Fig. 1) accounted for 2.64% of the variability in IOP, with all SNPs displaying F-statistics of ≥29.96 (Supplementary Table 2). Employing the IVW method, genetically predicted IOP was positively associated with RVO risk (OR = 1.53 per 1 mmHg increase in IOP; 95%CI = 1.04 to 2.26; P-value = 0.03) (Fig. 2 and Supplementary Fig. 2). Results from pleiotropy-robust MR methods aligned with the IVW analysis estimate (Fig. 2). We found no associations of our selected SNPs with RVO risk factors apart from POAG (Supplementary Table 3) and, thus, we refrained from conducting multivariable MR to correct for potential correlated horizontal pleiotropy.
Fig. 2. Mendelian randomization estimates for the effect of intraocular pressure on retinal vein occlusion.
Estimates are reported as changes in odds of retinal vein occlusion per 1 mmHg increase in intraocular pressure *. * SNP single nucleotide polymorphism, CI confidence interval, MR-PRESSO Mendelian randomization pleiotropy residual sum, and outlier.
The Wald ratios for IOP with RVO did not exhibit heterogeneity (Supplementary Table 4) and the Cochran’s Q heterogeneity test yielded a value of 37.89 (p-value = 0.797). Additionally, no evidence for directional pleiotropy was found, since the intercepts from the MR-Egger analyses did not deviate from zero (Supplementary Table 4). The leave-one-SNP-out analysis did not identify any SNPs exerting significant influence on the IVW estimate for the association between IOP and RVO risk (Supplementary Table 5).
An illustrative depiction of the MR mediation analysis can be seen in Fig. 1. The mediation analysis indicated that the majority of the effect of high IOP on increased RVO risk is mediated through POAG, suggesting that RVO may be primarily caused by glaucomatous optic disc abnormalities rather than the effects of increased IOP independently. We found that 91.6% of the total effect of IOP on RVO was mediated through POAG (Supplementary Table 6).
Discussion
In this MR mediation analysis, we utilized genetic data to disentangle the causal pathway between IOP and RVO, and to also explore the mediating role of POAG in this relationship. Our results support a positive causal effect of IOP on RVO which is mostly mediated through POAG.
Many studies have examined the relationship between glaucoma, specifically POAG, and risk for RVO [4]. Yin et al demonstrated in their meta-analysis that POAG is significantly associated with RVO risk (OR: 5.03; 95% CI: 3.97 to 6.37) [3]. Moreover, in their subgroup analysis, POAG and chronic open angle-glaucoma were also correlated with central RVO (OR: 13.30; 95% CI: 3.34 to 53.20) and branch RVO (OR: 2.14; 95% CI: 1.09 to 4.20), but conclusions are guarded due to the small number of studies included in the meta-analyses. Similarly, Na et al. determined that the RVO incidence rate for the open-angle glaucoma patients was 528.95 per 100,000 person-years (95% CI: 515.46 to 542.79), 3.27 times higher than in the general population [32]. Another Korean nationwide, population-based 11-year longitudinal study showed that patients with POAG were under higher risk for developing RVO (HR: 3.27, 95% CI: 2.55-4.19) [33], while in the Beaver Dam Eye Study, no significant difference was found in the age-adjusted 5-year incidence of RVO between participants with and without POAG [34].
Different potential mechanisms have been proposed to interpret the association between POAG and RVO, and IOP has been shown to be a major contributing factor affecting retinal haemodynamics. It has been suggested that increased IOP may compress blood vessels, leading to proliferation of the vein intima and subsequently to the collapse of retinal vessel walls [5]. The effects of this mechanical compression are more pronounced at the level of the lamina cribrosa where the venous pressure is lowest. In older individuals with impaired retinal circulation and compromised autoregulatory vascular mechanisms, even a slight increase in IOP could be significant enough to decrease ocular blood flow, leading to venous slowing or stasis [6]. Luntz et al. strongly support an etiological relationship between increased IOP and central RVO having shown that the incidence of RVO in ocular hypertension is similar to that of POAG [35]. However, the Ocular Hypertension Treatment study, which included only participants with ocular hypertension, did not find a significant difference in RVO incidence between the observation and medication group [36].
The effect of IOP on retinal vessels might be further accentuated by anatomical characteristics of the glaucomatous optic nerve head. The posterior bowing of the lamina cribrosa present in glaucomatous optic discs confers less glial support to the retinal vessels thus making them more vulnerable to changes in IOP [8]. Also, the higher cup-to-disc ratio has been correlated with the development of RVO, postulating that optic disc cupping induces structural abnormalities to the vessel at the disc, thus altogether making them susceptible to occlusion, venous stasis, and, consequently, to thrombosis, according to Virchow’s triad [7, 9, 10]. On top of that, according to the vascular theory of glaucoma pathogenesis, patients with POAG have narrower retinal arteries and veins compared to normal individuals, predisposing them to greater vessel wall compression following a rise in IOP [37]. Moreover, it has been hypothesized that RVO and POAG could potentially arise from the same vascular abnormality given their shared vascular risk factors. Optic disc haemorrhages frequently encountered in POAG and RVO indicate small vein occlusions, raising the question of a common underlying pathway [38].
The key strength of this study is that it stands out as the first study to utilize the approach of MR mediation analysis to disentangle the complex causal pathway between IOP, POAG and RVO. Another strength of it lies in the consistency of association estimates obtained from pleiotropy-robust methods, aligning with the IVW estimate and indicating an absence of model violations. Moreover, in our study the risk of weak instrument bias was low given the high value of F-statistics for our selected genetic instruments, thereby reducing the risk of bias towards the null of our association estimates. Nevertheless, certain limitations warrant consideration. Firstly, our investigation primarily focused on delineating the mediating role of POAG in the association between IOP and RVO, excluding exploration into other glaucoma subtypes such as primary angle-closure glaucoma. Secondly, our MR models assumed a linear relationship between the identified risk factors and the observed outcomes, whereas the true association among IOP, POAG, and RVO may manifest as non-linear. Thirdly, it is imperative to exercise caution when extrapolating the genetic associations derived from European populations to other ethnic groups due to potential population-specific variations. Fourthly, we did not assess the associations of IOP with the two main subtypes of RVO, namely branch RVO and central RVO, since no GWAS datasets are available for these phenotypes. Lastly, although by using GWAS summary statistics for our MR analyses analysis we were able to increase statistical power due to larger sample sizes, we did not have access to individual-level GWAS data, which are required for subgroup analyses.
In conclusion, our MR analyses infer an elevated risk of RVO associated with higher IOP. By utilizing MR mediation analysis, our findings additionally suggest that the majority of the effect of IOP on RVO risk is mediated through POAG. To advance our understanding of this intricate interplay, further investigations through population-based prospective studies, as well as experimental studies, are warranted to comprehensively explore the complex pathway involving IOP, POAG, and RVO.
Summary
What was known before
The etiological connection between intraocular pressure and the risk of retinal vein occlusion remains elusive, particularly regarding whether this risk emanates from the direct influence of elevated intraocular pressure, irrespective of the presence of primary open-angle glaucoma (POAG), or if it arises as a consequence of the sequelae of POAG.
What this study adds
Our findings suggest that higher intraocular pressure increases the risk of retinal vein occlusion and that the majority of this effect is mediated through POAG.
Supplementary information
Acknowledgements
We want to acknowledge the participants and investigators of the FinnGen and UK Biobank studies.
Author contributions
KA, BSE, and NM contributed to the study conception and design, drafted the manuscript, and analyzed the data. All authors critically revised the manuscript for important intellectual content, provided administrative, technical, or material support, and approved the final version.
Funding
Open Access funding enabled and organized by Projekt DEAL. We acknowledge support from the Open Access Publication Fund of the University of Münster.
Data availability
The summary statistics for the intraocular for the UKBB GWAS are available at https://pan.ukbb.broadinstitute.org (access date: 2023/10/12). The retinal vein occlusion summary statistics for the FinnGen GWAS are available at https://www.finngen.fi/en/access_results (access date: 2023/10/12). The primary open-angle glaucoma summary statistics are available at https://www.ebi.ac.uk/gwas/publications/33627673 (access date: 2023/10/12).
Competing interests
The author declares no competing interests.
Footnotes
The original online version of this article was revised: the first and last name of the author Hansjörg Baurecht were unfortunately interchanged.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
9/13/2024
A Correction to this paper has been published: 10.1038/s41433-024-03340-6
Contributor Information
Andreas Katsimpris, Email: a.katsimpris@gna-gennimatas.gr.
Michael Nolde, Email: nolde@uni-muenster.de.
Supplementary information
The online version contains supplementary material available at 10.1038/s41433-024-03303-x.
References
- 1.Mitchell P, Smith W, Chang A. Prevalence and associations of retinal vein occlusion in Australia: The Blue Mountains Eye Study. Arch Ophthalmol. 1996;114:1243–7. 10.1001/archopht.1996.01100140443012. [DOI] [PubMed] [Google Scholar]
- 2.Kolar P. Risk factors for central and branch retinal vein occlusion: a meta-analysis of published clinical data. J Ophthalmol. 2014;2014:724780. 10.1155/2014/724780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yin X, Li J, Zhang B, Lu P. Association of glaucoma with risk of retinal vein occlusion: A meta-analysis. Acta Ophthalmol. 2019;97:652–9. 10.1111/aos.14141. [DOI] [PubMed] [Google Scholar]
- 4.Jabbehdari S, Yazdanpanah G, Cantor LB, Hajrasouliha AR. A narrative review on the association of high intraocular pressure and glaucoma in patients with retinal vein occlusion. Ann Transl Med. 2022;10:1072. 10.21037/atm-22-2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Verhoeff FH. The effect of chronic glaucoma on the central retinal vessels. Arch Ophthalmol. 1913;42:145–52. [Google Scholar]
- 6.Frucht J, Shapiro A, Merin S. Intraocular pressure in retinal vein occlusion. Br J Ophthalmol. 1984;68:26–8. 10.1136/bjo.68.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beaumont PE, Kang HK. Cup-to-disc ratio, intraocular pressure, and primary open-angle glaucoma in retinal venous occlusion1. Ophthalmology. 2002;109:282–6. 10.1016/S0161-6420(01)00922-8. [DOI] [PubMed] [Google Scholar]
- 8.Behrman S. Retinal vein obstruction. Br J Ophthalmol. 1962;46:336–42. 10.1136/bjo.46.6.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dobree JH. Venous obstruction and neovascularization at the disc in chronic glaucoma. Trans Ophthalmol Soc U K. 1957;77:229–37. [PubMed] [Google Scholar]
- 10.Rubinstein K, Jones EB. Retinal vein occlusion: long-term prospects: 10 years’ follow-up of 143 patients. Br J Ophthalmol. 1976;60:148–50. 10.1136/bjo.60.2.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sanderson E, Glymour MM, Holmes MV, Kang H, Morrison J, Munafò MR, et al. Mendelian randomization. Nat Rev Methods Prim. 2022;2:6. 10.1038/s43586-021-00092-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Z, Li Z. Causal association between glaucoma and risk of retinal vascular occlusion: A Mendelian randomization study, 2023, PREPRINT (Version 1) available at Research Square. 10.21203/rs.3.rs-3223120/v1.
- 13.Pan-UKB team. https://pan.ukbb.broadinstitute.org. 2020.
- 14.Kurki MI, Karjalainen J, Palta P, Sipilä TP, Kristiansson K, Donner KM, et al. FinnGen provides genetic insights from a well-phenotyped isolated population. Nature. 2023;613:508–18. 10.1038/s41586-022-05473-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carter AR, Sanderson E, Hammerton G, Richmond RC, Davey Smith G, Heron J, et al. Mendelian randomisation for mediation analysis: current methods and challenges for implementation. Eur J Epidemiol. 2021;36:465–78. 10.1007/s10654-021-00757-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Skrivankova VW, Richmond RC, Woolf BAR, Davies NM, Swanson SA, VanderWeele TJ, et al. Strengthening the reporting of observational studies in epidemiology using mendelian randomisation (STROBE-MR): explanation and elaboration. BMJ. 2021;375:n2233. 10.1136/bmj.n2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burgess S, Davey Smith G, Davies NM, Dudbridge F, Gill D, Glymour MM, et al. Guidelines for performing Mendelian randomization investigations: update for summer 2023 [version 3; peer review: 2 approved]. Wellcome Open Research. 2023;4. 10.12688/wellcomeopenres.15555.3. [DOI] [PMC free article] [PubMed]
- 18.Simcoe MJ, Khawaja AP, Hysi PG, Hammond CJ. Genome-wide association study of corneal biomechanical properties identifies over 200 loci providing insight into the genetic etiology of ocular diseases. Hum Mol Genet. 2020;29:3154–64. 10.1093/hmg/ddaa155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gharahkhani P, Jorgenson E, Hysi P, Khawaja AP, Pendergrass S, Han X, et al. Genome-wide meta-analysis identifies 127 open-angle glaucoma loci with consistent effect across ancestries. Nat Commun. 2021;12:1258. 10.1038/s41467-020-20851-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bycroft C, Freeman C, Petkova D, Band G, Elliott LT, Sharp K, et al. The UK Biobank resource with deep phenotyping and genomic data. Nature. 2018;562:203–9. 10.1038/s41586-018-0579-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hemani G, Tilling K, Davey Smith G. Orienting the causal relationship between imprecisely measured traits using GWAS summary data. PLoS Genet. 2017;13:e1007081. 10.1371/journal.pgen.1007081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frazer KA, Ballinger DG, Cox DR, Hinds DA, Stuve LL, Gibbs RA, et al. A second generation human haplotype map of over 3.1 million SNPs. Nature. 2007;449:851–61. 10.1038/nature06258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burgess S, Dudbridge F, Thompson SG. Combining information on multiple instrumental variables in Mendelian randomization: comparison of allele score and summarized data methods. Stat Med. 2015;35:1880–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Labrecque J, Swanson SA. Understanding the assumptions underlying instrumental variable analyses: a brief review of falsification strategies and related tools. Curr Epidemiol Rep. 2018;5:214–20. 10.1007/s40471-018-0152-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lawlor DA, Harbord RM, Sterne JA, Timpson N, Davey Smith G. Mendelian randomization: using genes as instruments for making causal inferences in epidemiology. Stat Med. 2008;27:1133–63. 10.1002/sim.3034. [DOI] [PubMed] [Google Scholar]
- 26.Kamat MA, Blackshaw JA, Young R, Surendran P, Burgess S, Danesh J, et al. PhenoScanner V2: an expanded tool for searching human genotype-phenotype associations. Bioinformatics. 2019;35:4851–3. 10.1093/bioinformatics/btz469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sanderson E. Multivariable Mendelian randomization and mediation. Cold Spring Harb Perspect Med. 2021;11. 10.1101/cshperspect.a038984. [DOI] [PMC free article] [PubMed]
- 28.Hemani G, Bowden J, Davey Smith G. Evaluating the potential role of pleiotropy in Mendelian randomization studies. Hum Mol Genet. 2018;27:R195–208. 10.1093/hmg/ddy163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Slob EAW, Burgess S. A comparison of robust Mendelian randomization methods using summary data. Genet Epidemiol. 2020;44:313–29. 10.1002/gepi.22295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ogasawara H. Asymptotic standard errors of estimated standard errors in structural equation modelling. Br J Math Stat Psychol. 2002;55:213–29. 10.1348/000711002760554552. [DOI] [PubMed] [Google Scholar]
- 31.R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. 2022. https://www.R-project.org/.
- 32.Na KI, Jeoung JW, Kim YK, Lee WJ, Park KH. Incidence of retinal vein occlusion in open-angle glaucoma: a nationwide, population-based study using the Korean Health Insurance Review and Assessment Database. Clin Exp Ophthalmol. 2018;46:637–44. 10.1111/ceo.13157. [DOI] [PubMed] [Google Scholar]
- 33.Park HL, Jung Y, Han K, Lee MY, Park CK. Health care claims for primary open-angle glaucoma and retinal vein occlusion from an 11-year nationwide dataset. Sci Rep. 2017;7:8038. 10.1038/s41598-017-07890-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Klein R, Klein BE, Moss SE, Meuer SM. The epidemiology of retinal vein occlusion: the Beaver Dam Eye Study. Trans Am Ophthalmol Soc. 2000;98:133–41. [PMC free article] [PubMed] [Google Scholar]
- 35.Luntz MH, Schenker HI. Retinal vascular accidents in glaucoma and ocular hypertension. Surv Ophthalmol. 1980;25:163–7. 10.1016/0039-6257(80)90093-4. [DOI] [PubMed] [Google Scholar]
- 36.Barnett EM, Fantin A, Wilson BS, Kass MA, Gordon MO. The incidence of retinal vein occlusion in the ocular hypertension treatment study. Ophthalmology. 2010;117:484–8. 10.1016/j.ophtha.2009.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sonnsjö B, Krakau CE. Arguments for a vascular glaucoma etiology. Acta Ophthalmol. 1993;71:433–44. 10.1111/j.1755-3768.1993.tb04615.x. [DOI] [PubMed] [Google Scholar]
- 38.Hayreh SS. Progress in the understanding of the vascular etiology of glaucoma. Curr Opin Ophthalmol. 1994;5:26–35.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The summary statistics for the intraocular for the UKBB GWAS are available at https://pan.ukbb.broadinstitute.org (access date: 2023/10/12). The retinal vein occlusion summary statistics for the FinnGen GWAS are available at https://www.finngen.fi/en/access_results (access date: 2023/10/12). The primary open-angle glaucoma summary statistics are available at https://www.ebi.ac.uk/gwas/publications/33627673 (access date: 2023/10/12).