Abstract
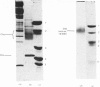
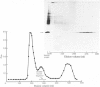
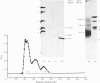
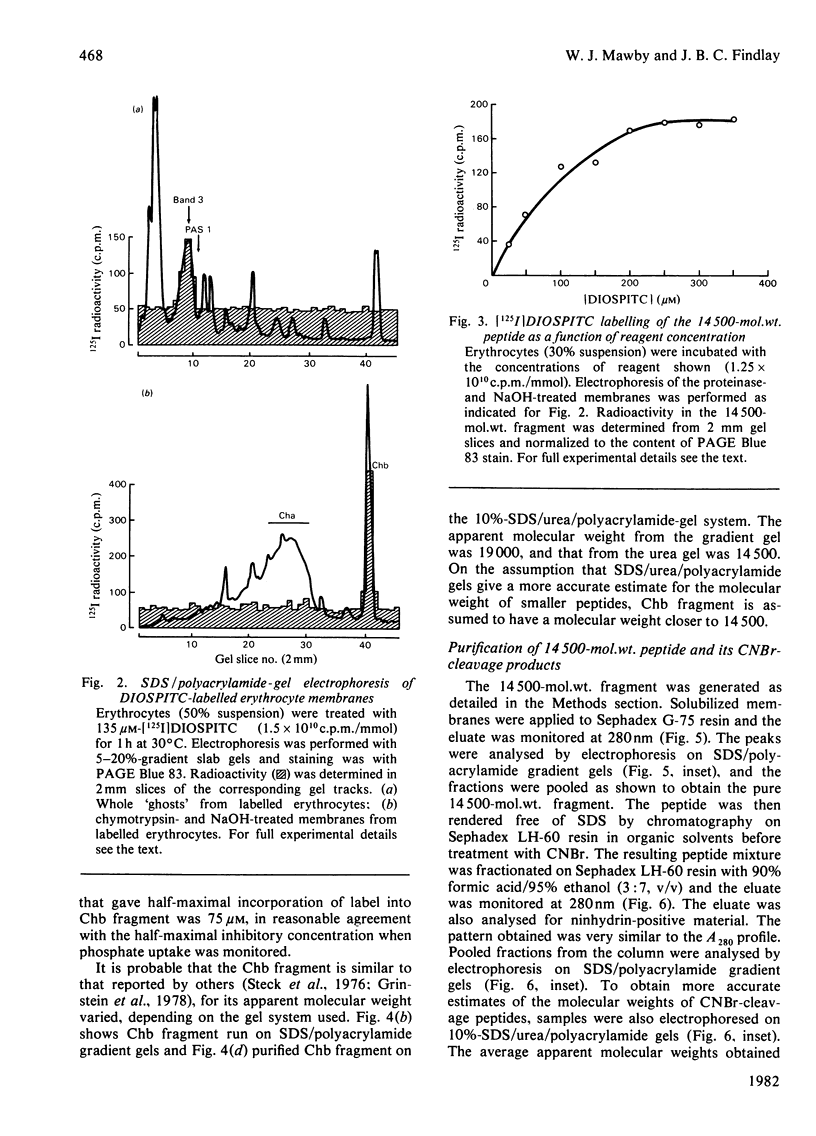
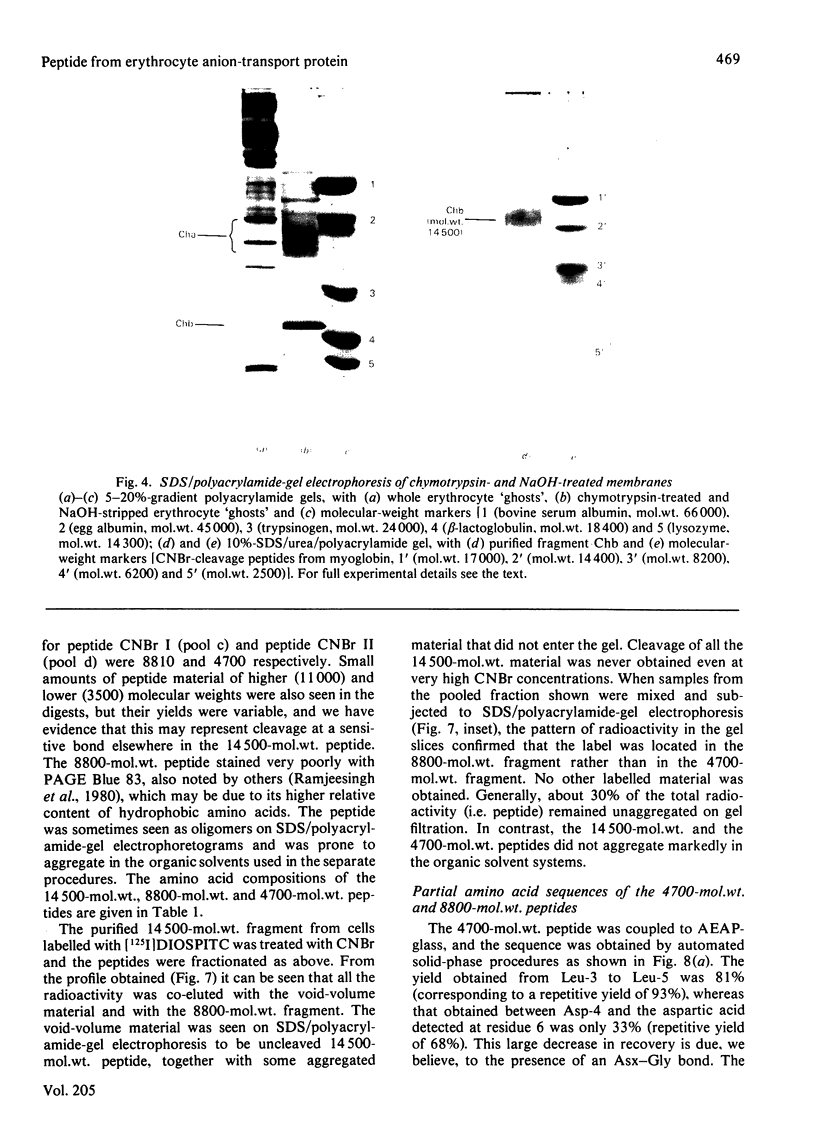
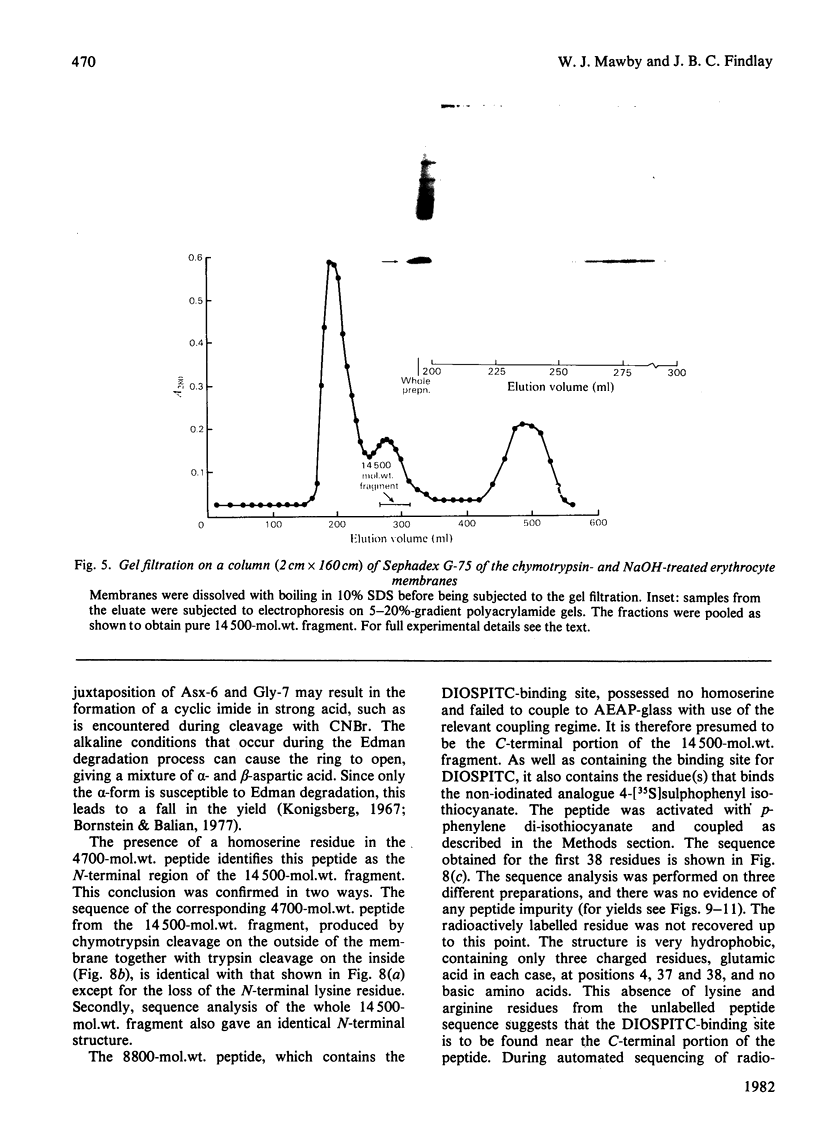
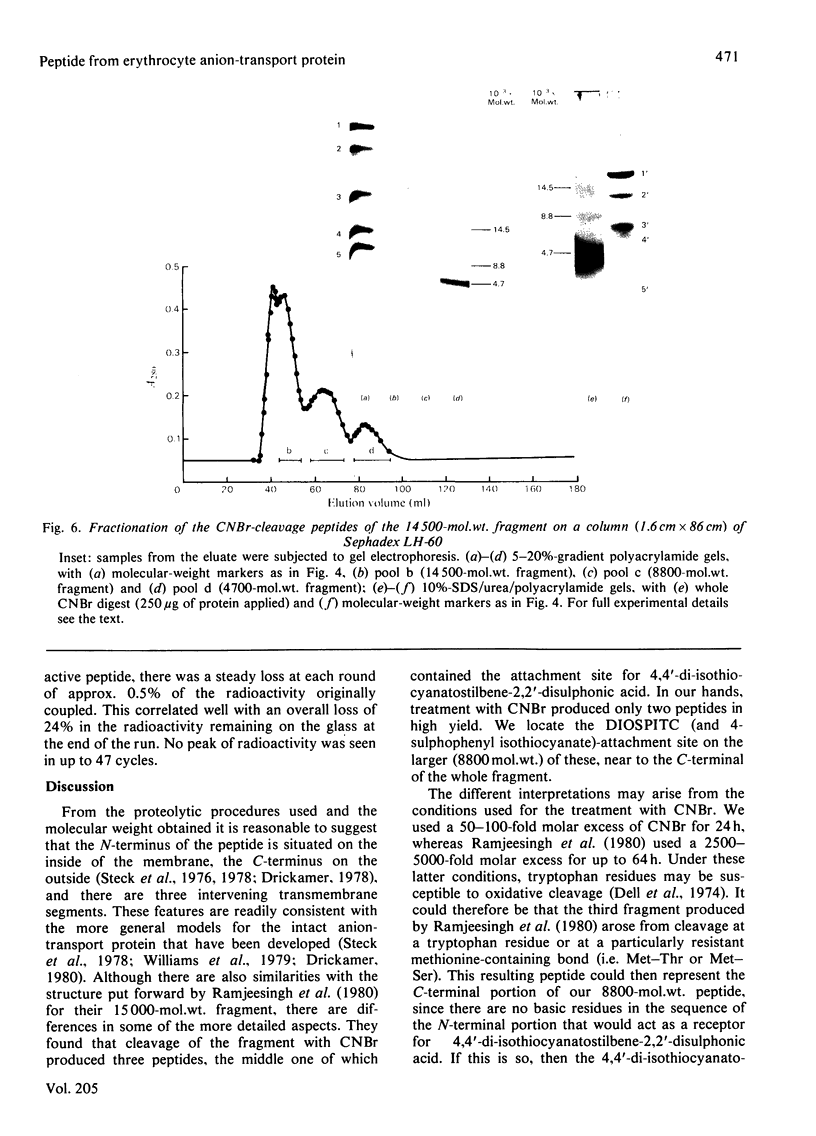
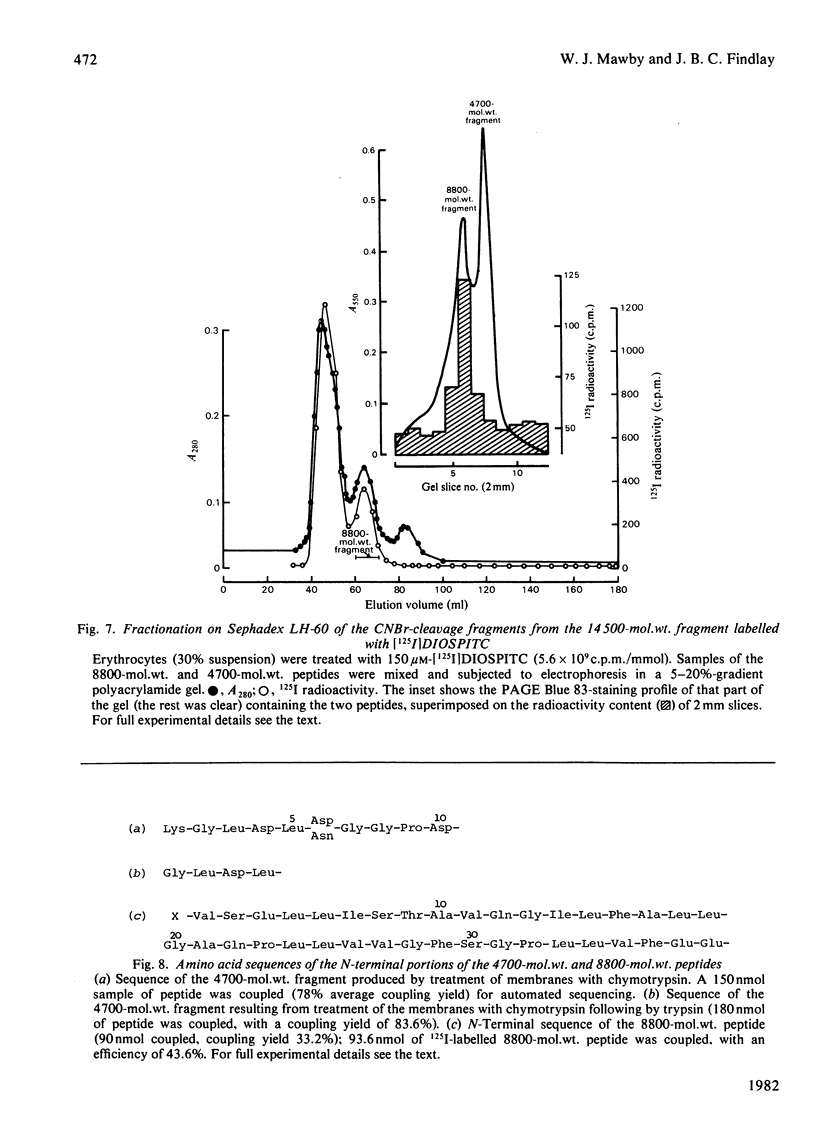
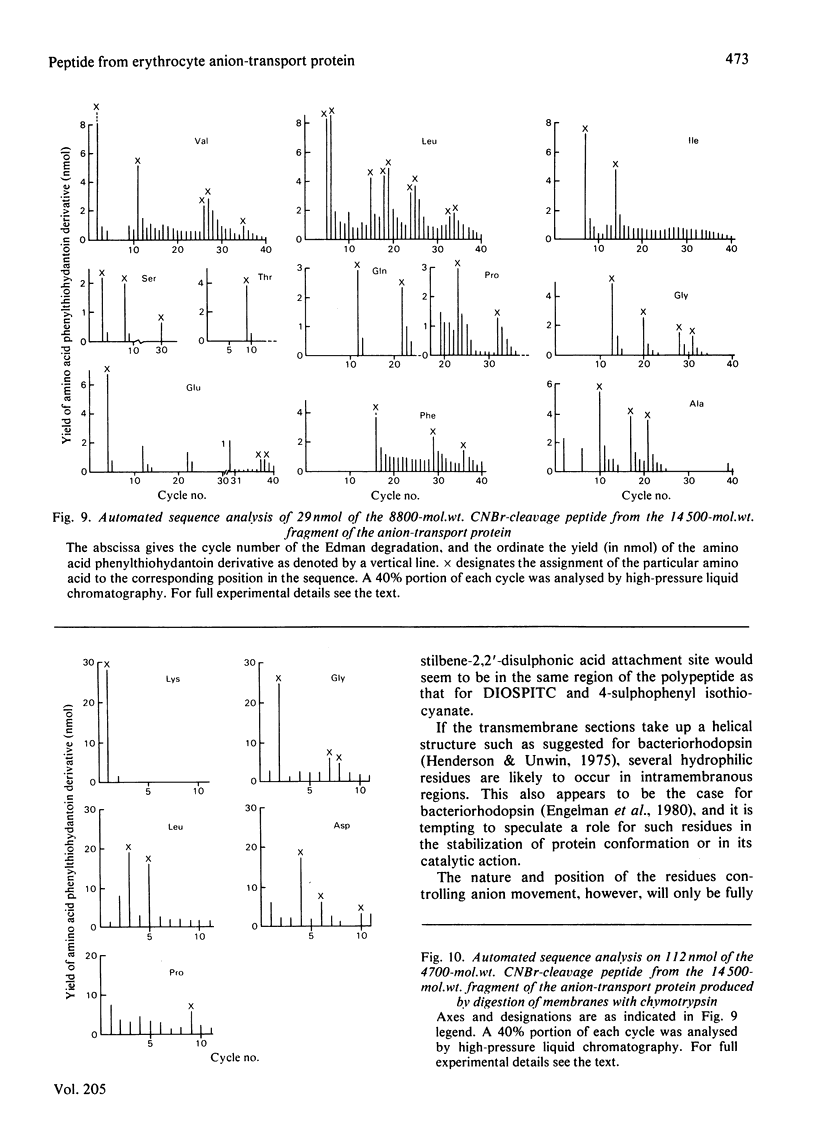
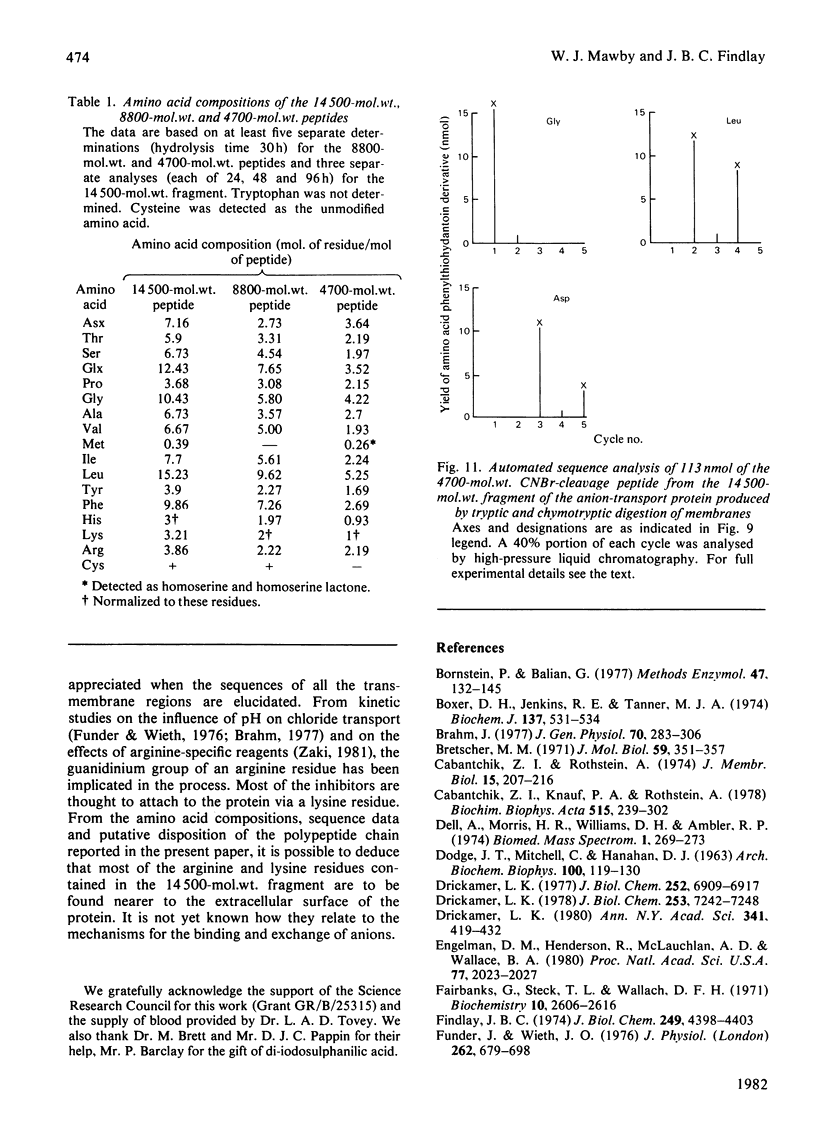
We investigated the presumed anion-binding domain of the anion-transport protein from human erythrocyte membranes, using 2,6-di-iodo-4-sulphophenyl isothiocyanate, an inhibitor of anion transport. The 125I-labelled reagent binds covalently to the protein with a half-maximal inhibitory concentration of 86 microM. Treatment of unsealed erythrocyte 'ghosts' with chymotrypsin yielded a membrane-bound fragment (mol.wt. 14 500 +/- 1000) that contained all the protein-bound radioactivity. The binding of the inhibitor to this peptide gave a pattern very similar to that obtained for the effect of the compound on phosphate transport into erythrocytes. The peptide is therefore presumed to be intimately involved in the mediation of anion exchange. Cleavage of the 14 500-mol.wt. transmembrane fragment with CNBr resulted in the production of two peptides with apparent molecular weights of 8800 and 4700. The 4700-mol.wt. peptide is the N-terminal portion of the 14 500-mol.wt. peptide. The attachment site for 2,6-di-iodo-4-sulphophenyl isothiocyanate is situated near the C-terminal of the 8800-mol.wt. peptide. This locates the inhibitor-binding site near the chymotrypsin cleavage point at the extracellular surface of the membrane. A partial sequence (residues 1--38) of the 8800-mol.wt. peptide was obtained.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bornstein P., Balian G. Cleavage at Asn-Gly bonds with hydroxylamine. Methods Enzymol. 1977;47:132–145. doi: 10.1016/0076-6879(77)47016-2. [DOI] [PubMed] [Google Scholar]
- Boxer D. H., Jenkins R. E., Tanner M. J. The organization of the major protein of the human erythrocyte membrane. Biochem J. 1974 Mar;137(3):531–534. doi: 10.1042/bj1370531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahm J. Temperature-dependent changes of chloride transport kinetics in human red cells. J Gen Physiol. 1977 Sep;70(3):283–306. doi: 10.1085/jgp.70.3.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabantchik Z. I., Knauf P. A., Rothstein A. The anion transport system of the red blood cell. The role of membrane protein evaluated by the use of 'probes'. Biochim Biophys Acta. 1978 Sep 29;515(3):239–302. doi: 10.1016/0304-4157(78)90016-3. [DOI] [PubMed] [Google Scholar]
- Cabantchik Z. I., Rothstein A. Membrane proteins related to anion permeability of human red blood cells. I. Localization of disulfonic stilbene binding sites in proteins involved in permeation. J Membr Biol. 1974;15(3):207–226. doi: 10.1007/BF01870088. [DOI] [PubMed] [Google Scholar]
- DODGE J. T., MITCHELL C., HANAHAN D. J. The preparation and chemical characteristics of hemoglobin-free ghosts of human erythrocytes. Arch Biochem Biophys. 1963 Jan;100:119–130. doi: 10.1016/0003-9861(63)90042-0. [DOI] [PubMed] [Google Scholar]
- Dell A., Morris H. R., Williams D. H., Ambler R. P. The determination of sequence information in homologously related proteins by mass spectrometry. Biomed Mass Spectrom. 1974 Aug;1(4):269–273. doi: 10.1002/bms.1200010411. [DOI] [PubMed] [Google Scholar]
- Drickamer K. Arrangement of the red cell anion transport protein in the red cell membrane: investigation by chemical labeling methods. Ann N Y Acad Sci. 1980;341:419–432. doi: 10.1111/j.1749-6632.1980.tb47187.x. [DOI] [PubMed] [Google Scholar]
- Drickamer L. K. Fragmentation of the band 3 polypeptide from human erythrocyte membranes. Identification of regions likely to interact with the lipid bilayer. J Biol Chem. 1977 Oct 10;252(19):6909–6917. [PubMed] [Google Scholar]
- Drickamer L. K. Orientation of the band 3 polypeptide from human erythrocyte membranes. Identification of NH2-terminal sequence and site of carbohydrate attachment. J Biol Chem. 1978 Oct 25;253(20):7242–7248. [PubMed] [Google Scholar]
- Engelman D. M., Henderson R., McLachlan A. D., Wallace B. A. Path of the polypeptide in bacteriorhodopsin. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2023–2027. doi: 10.1073/pnas.77.4.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Findlay J. B. The receptor proteins for concanavalin A and Lens culinaris phytohemagglutinin in the membrane of the human erythrocyte. J Biol Chem. 1974 Jul 25;249(14):4398–4403. [PubMed] [Google Scholar]
- Funder J., Wieth J. O. Chloride transport in human erythrocytes and ghosts: a quantitative comparison. J Physiol. 1976 Nov;262(3):679–698. doi: 10.1113/jphysiol.1976.sp011615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmkamp R. W., Sears D. A. A label for the red cell membrane: diazotized diiodosulfanilic acid. Int J Appl Radiat Isot. 1970 Nov;21(11):683–685. doi: 10.1016/0020-708x(70)90127-4. [DOI] [PubMed] [Google Scholar]
- Henderson R., Unwin P. N. Three-dimensional model of purple membrane obtained by electron microscopy. Nature. 1975 Sep 4;257(5521):28–32. doi: 10.1038/257028a0. [DOI] [PubMed] [Google Scholar]
- Ho M. K., Guidotti G. A membrane protein from human erythrocytes involved in anion exchange. J Biol Chem. 1975 Jan 25;250(2):675–683. [PubMed] [Google Scholar]
- Horn M. J., Laursen R. A. Solid-phase edman degradation: attachment of carboxyl-terminal homoserine peptides to an insoluble resin. FEBS Lett. 1973 Nov 1;36(3):285–288. doi: 10.1016/0014-5793(73)80392-8. [DOI] [PubMed] [Google Scholar]
- Jennings M. L., Passow H. Anion transport across the erythrocyte membrane, in situ proteolysis of band 3 protein, and cross-linking of proteolytic fragments by 4,4'-diisothiocyano dihydrostilbene-2,2'-disulfonate. Biochim Biophys Acta. 1979 Jul 5;554(2):498–519. doi: 10.1016/0005-2736(79)90387-0. [DOI] [PubMed] [Google Scholar]
- Johnstone A. P., Crumpton M. J. Comparison of diiodosulphophenylisothiocyanate with other reagents as surface labels for lymphocytes. FEBS Lett. 1979 Dec 1;108(1):119–123. doi: 10.1016/0014-5793(79)81191-6. [DOI] [PubMed] [Google Scholar]
- Kempf C., Brock C., Sigrist H., Tanner M. J., Zahler P. Interaction of phenylisothiocyanate with human erythrocyte band 3 protein. II. Topology of phenylisothiocyanate binding sites and influence of p-sulfophenylisothiocyanate on phenylisothiocyanate modification. Biochim Biophys Acta. 1981 Feb 20;641(1):88–98. doi: 10.1016/0005-2736(81)90571-x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laursen R. A., Horn M. J., Bonner A. G. Solid-phase Edman degradation. The use of p-phenyl diisothiocyanate to attach lysine- and arginine-containing peptides to insoluble resins. FEBS Lett. 1972 Mar;21(1):67–70. doi: 10.1016/0014-5793(72)80165-0. [DOI] [PubMed] [Google Scholar]
- Lepke S., Fasold H., Pring M., Passow H. A study of the relationship between inhibition of anion exchange and binding to the red blood cell membrane of 4,4'-diisothiocyano stilbene-2,2'-disulfonic acid (DIDS) and its dihydro derivative (H2DIDS). J Membr Biol. 1976 Oct 20;29(1-2):147–177. doi: 10.1007/BF01868957. [DOI] [PubMed] [Google Scholar]
- Lottspeich F. Identification of the phenylthiohydantoin derivatives of amino acids by high pressure liquid chromatography, using a ternary, isocratic solvent system. Hoppe Seylers Z Physiol Chem. 1980 Dec;361(12):1829–1834. doi: 10.1515/bchm2.1980.361.2.1829. [DOI] [PubMed] [Google Scholar]
- Mawby W. J., Findlay J. B. Some transport properties of resealed washed human erythrocyte membranes. Biochem J. 1978 Jun 15;172(3):605–611. doi: 10.1042/bj1720605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez E., Lai C. Y. Regeneration of amino acids from thiazolinones formed in the Edman degradation. Anal Biochem. 1975 Sep;68(1):47–53. doi: 10.1016/0003-2697(75)90677-6. [DOI] [PubMed] [Google Scholar]
- Ramjeesingh M., Gaarn A., Rothstein A. The location of a disulfonic stilbene binding site in band 3, the anion transport protein of the red blood cell membrane. Biochim Biophys Acta. 1980 Jun 20;599(1):127–139. doi: 10.1016/0005-2736(80)90062-0. [DOI] [PubMed] [Google Scholar]
- Ship S., Shami Y., Breuer W., Rothstein A. Synthesis of tritiated 4,4'-diisothiocyano-2,2'-stilbene disulfonic acid ([3H]DIDS) and its covalent reaction with sites related to anion transport in human red blood cells. J Membr Biol. 1977 May 12;33(3-4):311–323. doi: 10.1007/BF01869522. [DOI] [PubMed] [Google Scholar]
- Steck T. L., Koziarz J. J., Singh M. K., Reddy G., Köhler H. Preparation and analysis of seven major, topographically defined fragments of band 3, the predominant transmembrane polypeptide of human erythrocyte membranes. Biochemistry. 1978 Apr 4;17(7):1216–1222. doi: 10.1021/bi00600a013. [DOI] [PubMed] [Google Scholar]
- Steck T. L., Ramos B., Strapazon E. Proteolytic dissection of band 3, the predominant transmembrane polypeptide of the human erythrocyte membrane. Biochemistry. 1976 Mar 9;15(5):1153–1161. doi: 10.1021/bi00650a030. [DOI] [PubMed] [Google Scholar]
- Steck T. L. The organization of proteins in the human red blood cell membrane. A review. J Cell Biol. 1974 Jul;62(1):1–19. doi: 10.1083/jcb.62.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swank R. T., Munkres K. D. Molecular weight analysis of oligopeptides by electrophoresis in polyacrylamide gel with sodium dodecyl sulfate. Anal Biochem. 1971 Feb;39(2):462–477. doi: 10.1016/0003-2697(71)90436-2. [DOI] [PubMed] [Google Scholar]
- Tanner M. J., Jenkins R. E., Anstee D. J., Clamp J. R. Abnormal carbohydrate composition of the major penetrating membrane protein of En(a-) human erythrocytes. Biochem J. 1976 Jun 1;155(3):701–703. doi: 10.1042/bj1550701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams D. G., Jenkins R. E., Tanner M. J. Structure of the anion-transport protein of the human erythrocyte membrane. Further studies on the fragments produced by proteolytic digestion. Biochem J. 1979 Aug 1;181(2):477–493. doi: 10.1042/bj1810477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J., Steck T. L. Isolation and characterization of band 3, the predominant polypeptide of the human erythrocyte membrane. J Biol Chem. 1975 Dec 10;250(23):9170–9175. [PubMed] [Google Scholar]
- Zaki L. Inhibition of anion transport across red blood cells with 1,2-cyclohexanedione. Biochem Biophys Res Commun. 1981 Mar 16;99(1):243–251. doi: 10.1016/0006-291x(81)91738-1. [DOI] [PubMed] [Google Scholar]
- Zimmerman C. L., Appella E., Pisano J. J. Rapid analysis of amino acid phenylthiohydantoins by high-performance liquid chromatography. Anal Biochem. 1977 Feb;77(2):569–573. doi: 10.1016/0003-2697(77)90276-7. [DOI] [PubMed] [Google Scholar]