Abstract
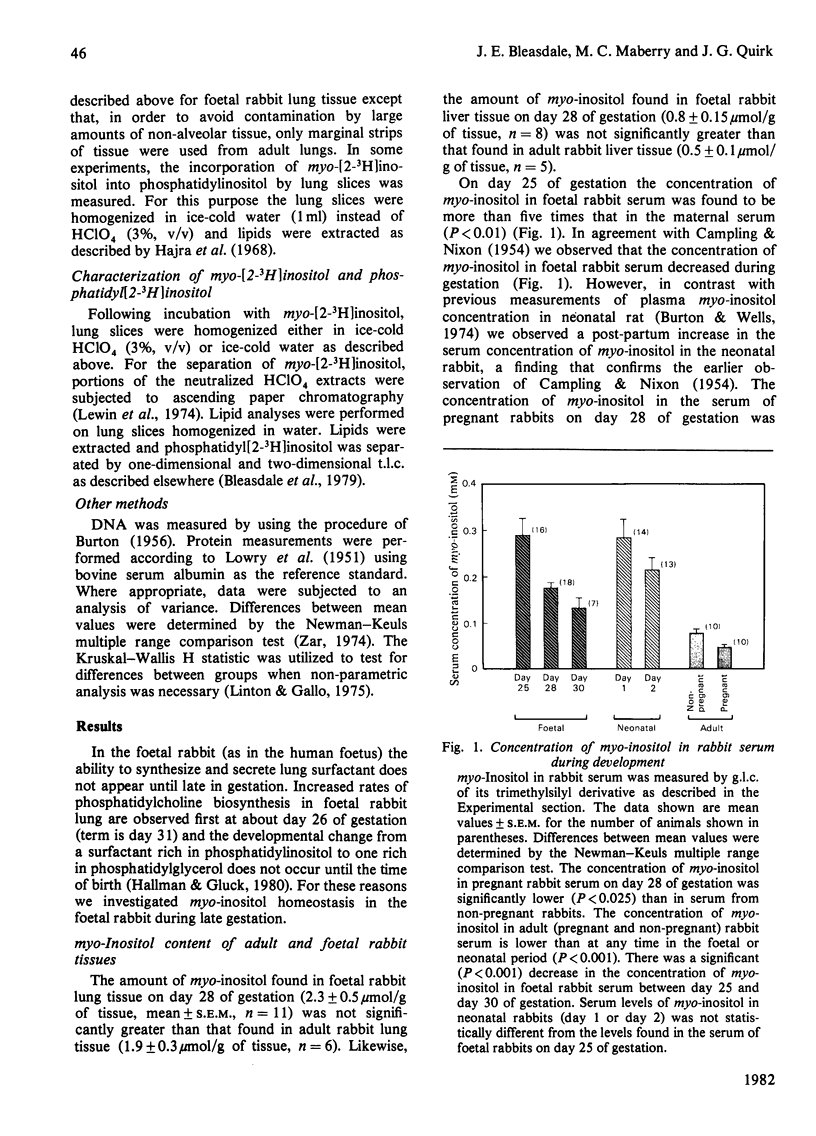
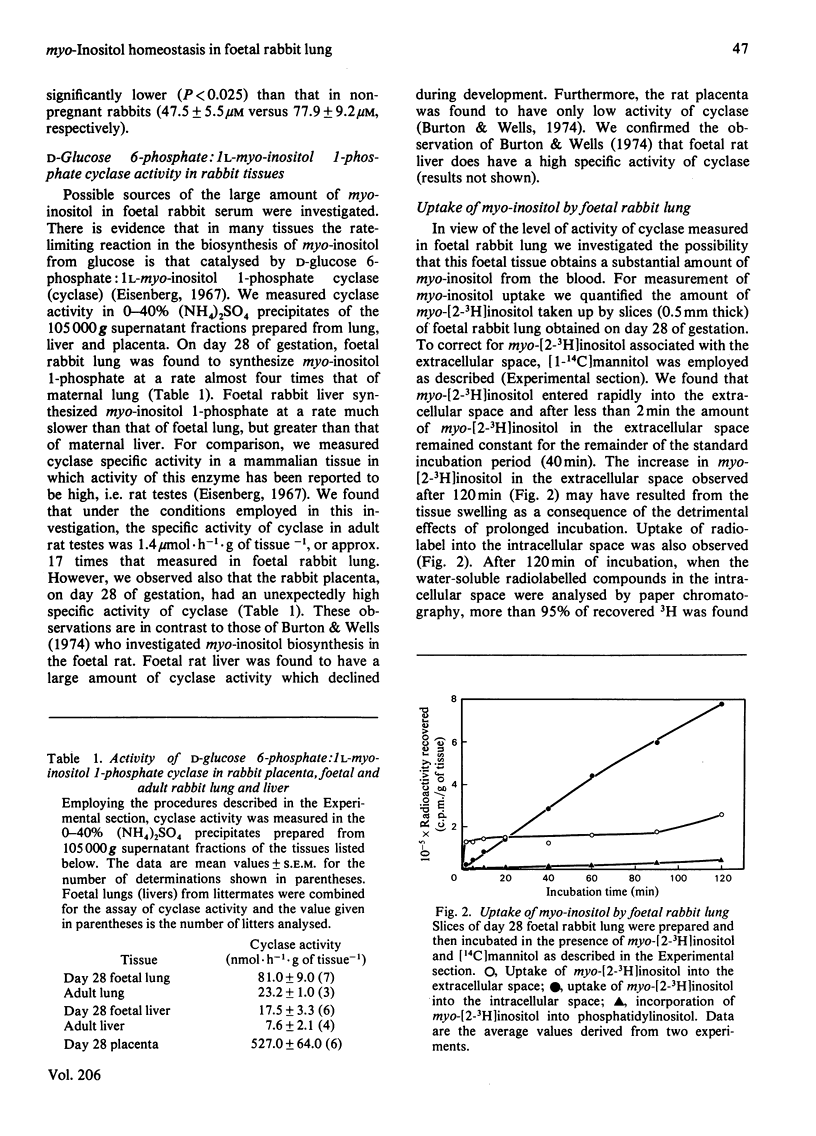
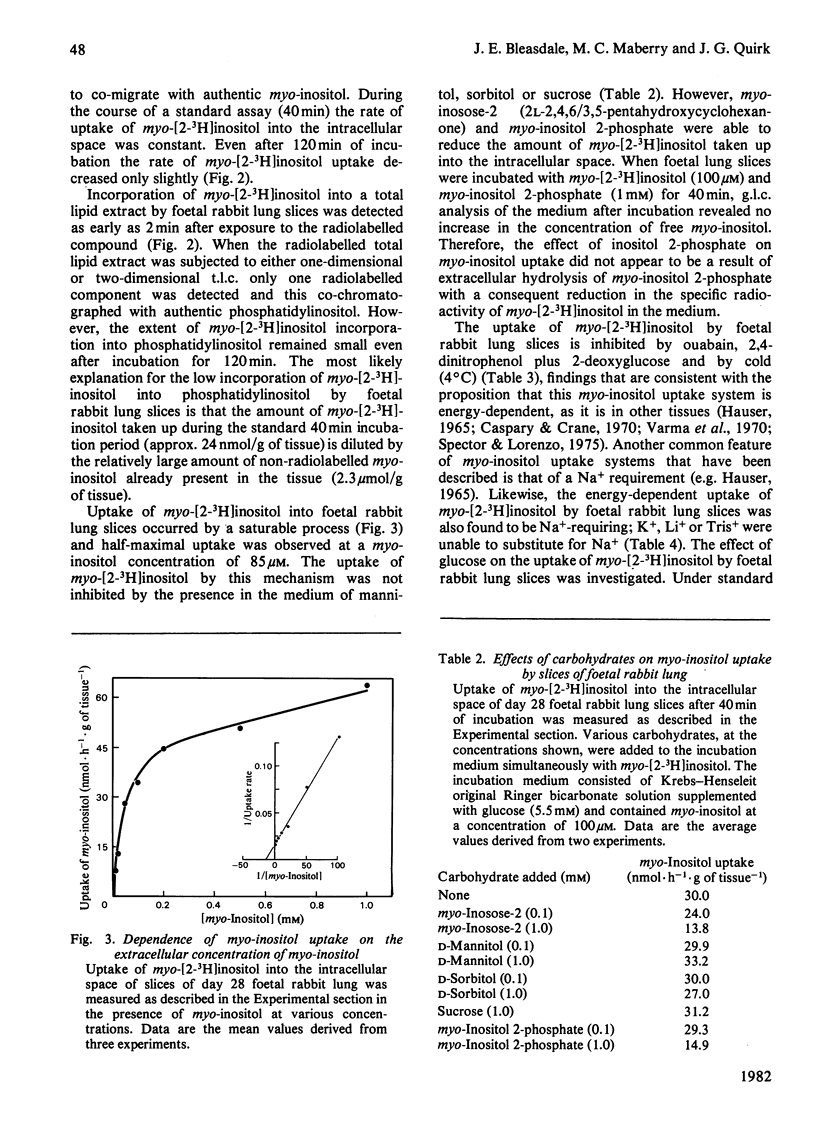
In several species, lung maturation is accompanied by a decline in the phosphatidylinositol content of lung surfactant and a concomitant increase in its phosphatidylglycerol content. To examine the possibility that this developmental change is influenced by the availability of myo-inositol, potential sources of myo-inositol for the developing rabbit lung were investigated. On day 28 of gestation the myo-inositol content of foetal rabbit lung tissue (2.3±0.5μmol/g of tissue) was not significantly different from that of adult lung tissue but the activity of d-glucose 6-phosphate:1l-myo-inositol 1-phosphate cyclase (cyclase) in foetal lung tissue (81.0±9.0nmol·h−1·g of tissue−1) was higher than that found in adult lung tissue (23.2±1.0nmol·h−1·g of tissue−1). Day 28 foetal rabbit lung tissue was found also to take up myo-inositol by a specific, energy-dependent, Na+-requiring mechanism. Half-maximal uptake of myo-inositol by foetal rabbit lung slices was observed when the concentration of myo-inositol in the incubation medium was 85μm. When the myo-inositol concentration was 1mm (but not 100μm) the addition of glucose (5.5mm) stimulated myo-inositol uptake. myo-Inositol uptake was observed also in adult rabbit lung and was found to be sub-maximal at the concentration of myo-inositol found in adult rabbit serum. The concentration of myo-inositol in the serum of pregnant adult rabbits (47.5±5.5μm) was significantly lower than that of non-pregnant adult female rabbits (77.9±9.2μm). On day 28 of gestation the concentration of myo-inositol in foetal serum (175.1±12.0μm) was much less than on day 25, but more than that found on day 30. A transient post-partum increase in the concentration of myo-inositol in serum was followed by a rapid decline. Much of the myo-inositol in foetal rabbit serum probably originates from the placenta, where on day 28 of gestation a high cyclase activity (527±64nmol·h−1·g of tissue−1) was measured. The gestational decline in serum myo-inositol concentration, together with the decreasing cyclase activity of the lungs, is consistent with the view that maturation of the lungs is accompanied by decreased availability of myo-inositol to this tissue.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett J. E., Brice R. E., Corina D. L. A colorimetric determination of inositol monophosphates as an assay for D-glucose 6-phosphate-1L-myoinositol 1-phosphate cyclase. Biochem J. 1970 Sep;119(2):183–186. doi: 10.1042/bj1190183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleasdale J. E., Wallis P., MacDonald P. C., Johnston J. M. Characterization of the forward and reverse reactions catalyzed by CDP-diacylglycerol:inositol transferase in rabbit lung tissue. Biochim Biophys Acta. 1979 Oct 26;575(1):135–147. doi: 10.1016/0005-2760(79)90139-5. [DOI] [PubMed] [Google Scholar]
- Burton L. E., Wells W. W. Studies on the developmental pattern of the enzymes converting glucose 6-phosphate to myo-inositol in the rat. Dev Biol. 1974 Mar;37(1):35–42. doi: 10.1016/0012-1606(74)90167-5. [DOI] [PubMed] [Google Scholar]
- CAMPLING J. D., NIXON D. A. The inositol content of foetal blood and foetal fluids. J Physiol. 1954 Oct 28;126(1):71–80. doi: 10.1113/jphysiol.1954.sp005192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspary W. F., Crane R. K. Active transport of myo-inositol and its relation to the sugar transport system in hamster small intestine. Biochim Biophys Acta. 1970 Apr 21;203(2):308–316. doi: 10.1016/0005-2736(70)90145-8. [DOI] [PubMed] [Google Scholar]
- Clements R. S., Jr, Reynertson R. Myoinositol metabolism in diabetes mellitus. Effect of insulin treatment. Diabetes. 1977 Mar;26(3):215–221. doi: 10.2337/diab.26.3.215. [DOI] [PubMed] [Google Scholar]
- Cunningham M. D., Desai N. S., Thompson S. A., Greene J. M. Amniotic fluid phosphatidylglycerol in diabetic pregnancies. Am J Obstet Gynecol. 1978 Aug 1;131(7):719–724. doi: 10.1016/0002-9378(78)90233-8. [DOI] [PubMed] [Google Scholar]
- Eichberg J., Gates J., Hauser G. The mechanism of modification by propranolol of the metabolism of phosphatidyl-CMP (CDP-diacylglycerol) and other lipids in the rat pineal gland. Biochim Biophys Acta. 1979 Apr 27;573(1):90–106. doi: 10.1016/0005-2760(79)90176-0. [DOI] [PubMed] [Google Scholar]
- Eisenberg F., Jr D-myoinositol 1-phosphate as product of cyclization of glucose 6-phosphate and substrate for a specific phosphatase in rat testis. J Biol Chem. 1967 Apr 10;242(7):1375–1382. [PubMed] [Google Scholar]
- Esko J. D., Raetz C. R. Mutants of Chinese hamster ovary cells with altered membrane phospholipid composition. Replacement of phosphatidylinositol by phosphatidylglycerol in a myo-inositol auxotroph. J Biol Chem. 1980 May 25;255(10):4474–4480. [PubMed] [Google Scholar]
- Freinkel N., El Younsi C., Dawson M. C. Inter-relations between the phospholipids of rat pancreatic islets during glucose stimulation, and their response to medium inositol and tetracaine. Eur J Biochem. 1975 Nov 1;59(1):245–252. doi: 10.1111/j.1432-1033.1975.tb02448.x. [DOI] [PubMed] [Google Scholar]
- Greene D. A., De Jesus P. V., Jr, Winegrad A. I. Effects of insulin and dietary myoinositol on impaired peripheral motor nerve conduction velocity in acute streptozotocin diabetes. J Clin Invest. 1975 Jun;55(6):1326–1336. doi: 10.1172/JCI108052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajra A. K., Seguin E. B., Agranoff B. W. Rapid labeling of mitochondrial lipids by labeled orthophosphate and adenosine triphosphate. J Biol Chem. 1968 Apr 10;243(7):1609–1616. [PubMed] [Google Scholar]
- Hallman M., Epstein B. L. Role of myo-inositol in the synthesis of phosphatidylglycerol and phosphatidylinositol in the lung. Biochem Biophys Res Commun. 1980 Feb 27;92(4):1151–1159. doi: 10.1016/0006-291x(80)90407-6. [DOI] [PubMed] [Google Scholar]
- Hallman M., Gluck L. Formation of acidic phospholipids in rabbit lung during perinatal development. Pediatr Res. 1980 Nov;14(11):1250–1259. doi: 10.1203/00006450-198011000-00020. [DOI] [PubMed] [Google Scholar]
- Hallman M., Kulovich M., Kirkpatrick E., Sugarman R. G., Gluck L. Phosphatidylinositol and phosphatidylglycerol in amniotic fluid: indices of lung maturity. Am J Obstet Gynecol. 1976 Jul 1;125(5):613–617. doi: 10.1016/0002-9378(76)90782-1. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lewin L. M., Melmed S., Bank H. Rapid screening test for detection of elevated myo-inositol levels in human blood serum. Clin Chim Acta. 1974 Aug 20;54(3):377–379. doi: 10.1016/0009-8981(74)90256-3. [DOI] [PubMed] [Google Scholar]
- Nixon D. A. The concentration of free meso-inositol in the plasma of perfused sheep foetuses. Biol Neonat. 1968;12(3):113–120. doi: 10.1159/000240098. [DOI] [PubMed] [Google Scholar]
- Spector R., Lorenzo A. V. Myo-inositol transport in the central nervous system. Am J Physiol. 1975 May;228(5):1510–1518. doi: 10.1152/ajplegacy.1975.228.5.1510. [DOI] [PubMed] [Google Scholar]
- Varma S. D., Chakrapani B., Reddy V. N. Intraocular transport of myoinositol. II. Accumulation in the rabbit lens in vitro. Invest Ophthalmol. 1970 Oct;9(10):794–800. [PubMed] [Google Scholar]
- Wang N. S., Kotas R. V., Avery M. E., Thurlbeck W. M. Accelerated appearance of osmiophilic bodies in fetal lungs following steroid injection. J Appl Physiol. 1971 Mar;30(3):362–365. doi: 10.1152/jappl.1971.30.3.362. [DOI] [PubMed] [Google Scholar]


