Abstract
Photobiomodulation (PBM) is a safe and effective neurotherapy that modulates cellular pathways by altering cell membrane potentials, leading to beneficial biological effects such as anti-inflammatory and neuroregenerative responses. This review compiles studies from PubMed up to March 2024, investigating the impact of light at wavelengths ranging from 620 to 1270 nm on ion channels. Out of 330 articles screened, 19 met the inclusion criteria. Research indicates that PBM can directly affect various ion channels by influencing neurotransmitter synthesis in neighboring cells, impacting receptors like glutamate and acetylcholine, as well as potassium, sodium channels, and transient receptor potential channels. The diversity of studies hampers a comprehensive meta-analysis for evaluating treatment strategies effectively. This systematic review aims to explore the potential role of optoelectronic signal transduction in PBM, studying the neurobiological mechanisms and therapeutic significance of PBM on ion channels. However, the lack of uniformity in current treatment methods underscores the necessity of establishing standardized and reliable therapeutic approaches.
Graphical Abstract
PBM is a promising neurotherapeutic approach with anti-inflammatory and neuroregenerative potential through its effects on cell membrane potential and ion channels. This systematic review analyzes 19 studies, revealing its influence on channel activity and emphasizing the need for standardized treatment protocols.
Keywords: Photobiomodulation, Ion channels, Nervous system, Neural plasticity, Non-invasive nerve stimulation
Introduction
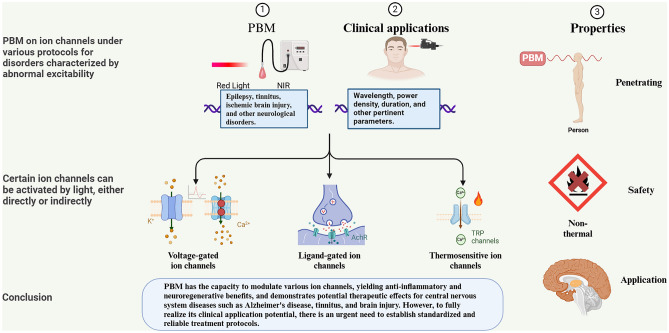
Photobiomodulation (PBM), a non-invasive and precise neuromodulation technique, utilizes light in the red or near-infrared wavelengths to achieve its therapeutic effects with minimal side effects (Hamblin 2024). This method operates by leveraging the interaction of light particles with cellular structures to enhance ATP synthesis, modulate gene expression, control levels of reactive oxygen species (ROS), and modify ion channel activity (Huang et al. 2012; Khuman et al. 2012). In recent years, PBM has emerged as a promising therapy for neurological diseases. Utilizing laser technology, PBM penetrates deep tissues and activates intracellular photosensitive molecules, demonstrating potential for various neurological conditions. Research has also explored PBM’s role in modulating the cerebral–gut axis (Blivet et al. 2024) and the use of home portable LED devices (Razzaghi et al. 2024). The U.S. Food and Drug Administration (FDA) has approved several laser devices for medical use, showing positive results in treating Alzheimer’s disease (Berman et al. 2019), tinnitus (Choi et al. 2024), ischemic brain injury (Chan et al. 2024), and other disorders. As research advances, PBM may increasingly contribute to the treatment and rehabilitation of these challenging conditions. The efficacy of PBM in influencing organisms can be attributed to three key properties—Penetration, Safety, and Application, which form the basis for our investigation into the mechanism of action of PBM on ion channels.
The Penetration of PBM
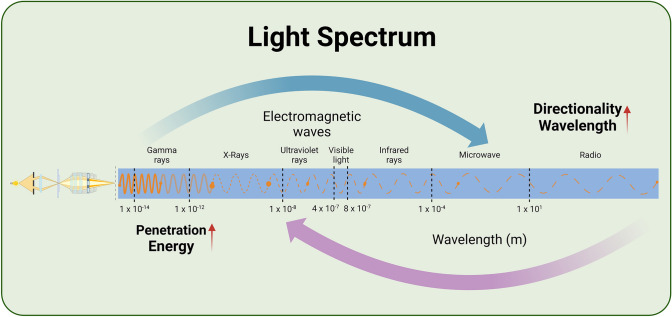
Light, with its wave properties manifested through photons at a macroscopic level, exhibits characteristics of penetrability that are essential to clarify the effects of PBM. The relationship between the frequency and wavelength of light is crucial, as longer wavelengths result in better directionality, reduced scattering, energy attenuation, and minimal diffraction (Castaño-Castaño et al. 2024; Escalé et al. 2017; Souto-Neto et al. 2024). This concrete interplay is highlighted in Fig. 1, aiding in comprehending how different wavelengths impact PBM. Selecting the appropriate light wavelength is a complex task for researchers and medical professionals, as it necessitates a delicate balance between penetration and directionality to optimize bioregulation outcomes (Bullock-Saxton et al. 2021; Hamblin 2016). Tissue penetration of light is influenced by various optical parameters like wavelength, power density, and coherence, while tissue structure at the irradiation site can alter light energy absorption (Henderson et al. 2015). Alterations in these parameters significantly affect PBM processes, impacting ion channel activity.
Fig. 1.
Impact of varying light wavelengths on PBM. Shorter wavelengths of light reflect mainly the wave properties in the figure with a solid line; longer wavelengths reflect mainly the particle nature in the figure with a dotted line, and the stronger the particles, the wider the distance between the dotted line
Channelrhodopsins (ChR) are light-gated ion channels first discovered in Chlamydomonas reinhardtii (Sineshchekov et al. 2002). These channels respond to light by opening or closing, resulting in various photobiological effects. The primary photosensitive ion channel in humans is Kalium channelrhodopsin (KCR) found in the eye (Oppermann et al. 2024). Light-activated retinal undergoes chemical transformations through distinct intermediates, converting light signals into electrical signals that excite optic neurons (Govorunova et al. 2022). Additionally, the TRP channel protein family plays a crucial role in light signal transduction (do Nascimento et al. 2024). Wei Wu et al. (2023) identified TRPA1 as a potential therapeutic target for ultraviolet radiation b-related (UVB-related) skin pigmentation diseases. The TRPA1 antagonist HC-030031, applied topically, reduced UVB-induced pigmentation. Furthermore, the ability of light to penetrate tissues opens the door for bioregulation without relying on optogenetics. Though PBM may not match optogenetics in precision and efficacy, its lack of gene editing requirements and ease of application position it as a promising strategy for treating CNS disorders.
The Safety of PBM
PBM is a safe and effective therapeutic approach that harnesses non-photothermal effects to induce beneficial biological responses (Tang et al. 2023). The wavelength of light plays a crucial role in determining the photothermal effect, with far-infrared light, ranging from 1.4 to 3 µm, being widely utilized in photothermal therapy within fields such as rehabilitation physiotherapy and dermatology (Aggarwal et al. 2023; Mineroff et al. 2024). Uozumi et al. (2010) have shown that at 808 nm and a high intensity of 1.1 W/cm2, the temperature of laser-irradiated tissues remains stable, with minimal increase in temperature. The safety of PBM is further underscored by the bidirectional dose response (Barolet 2021), as outlined in the Arndt–Schulz law, indicating that both too low and too high doses of light can be counterproductive or even harmful (Huang et al. 2009). Optimal biological effects are achieved within a safe range of light doses, emphasizing the significance of power density over total dose in determining the therapeutic outcomes of PBM (Lanzafame et al. 2007; Oron et al. 2001; Vasilenko et al. 2010).
The Application of PBM
As a technique harnessing the power of photons to activate or inhibit ion channels, PBM is a field with broad applicability and potential. Cells rich in mitochondria and with high metabolic activity, such as neuronal and muscle cells, are particularly responsive to the effects of PBM (Hamblin 2018). Presently, this technology finds application in diverse medical areas, including the treatment of epilepsy (Ma et al. 2024), ischemic encephalopathy (Cardoso et al. 2022), Alzheimer’s disease (Gaggi et al. 2024), autism (Ceranoglu et al. 2024), anxiety (Chen et al. 2024a, b), injury recovery (Behroozi et al. 2024; Dehghanpour et al. 2023), arthritis (Du et al. 2024), tumors (da Silva et al. 2023), and various other disorders. The effectiveness of PBM in these diseases needs confirmation through further clinical trials. Individual differences significantly impact PBM. Physiological and psychological traits, such as gender (Gutiérrez-Menéndez et al. 2022), age (Rodríguez-Fernández et al. 2024), and mental state (Chamkouri et al. 2024), can affect how PBM influences biorhythms, mood regulation, and other functions. Candela Zorzo et al. (2024) found that PBM notably increased cerebral metabolic activity in males more than in females, potentially due to higher CCO activity in males. Therefore, understanding these individual differences is crucial for optimizing light interventions and enhancing their effectiveness in clinical and daily applications, allowing for more effective promotion of physical and mental health through tailored light programs.
Ion channels, encompassing ligand-gated, voltage-gated, and stress-activated variants, are pivotal in cell signaling by managing sodium, potassium, and calcium ion concentrations within and outside cells, thus governing cellular function regulation (Liu et al. 2024). Recent studies have made significant advancements in the understanding of various ion channels, including voltage-gated, ligand-gated, TRPs, acid-sensitive ion channels (ASICs), chloride channels (e.g., CFTRs), ATP-gated channels (e.g., P2X7 receptor), and mechanosensitive channels (e.g., Piezo). These ion channels are crucial in regulating cellular signaling, neurotransmission, and a wide range of physiological processes. Their detailed characterization offers valuable molecular targets for elucidating the pathogenesis of associated diseases and for the development of innovative therapeutic strategies. PBM has garnered attention for its applications in the central nervous system due to its penetration, safety, and application. Although the mechanisms of action remain not fully understood, existing research has revealed the influence of PBM on voltage-gated ion channels, ligand-gated ion channels, and TRPs, which play critical roles in PBM. However, the heterogeneity and reproducibility issues of PBM protocols in animal experiments and clinical trials underscore the urgent need for standardized parameters to facilitate further research and clinical application of PBM. Additionally, the biological effects of PBM and its potential in the treatment and prevention of neurological disorders require deeper exploration. Future research should focus on elucidating the cellular mechanisms of PBM, optimizing treatment protocols, and overcoming the limitations of existing studies to advance innovative therapeutic strategies in the field of neuroscience.
Methods
Search of the Literature
We conducted an extensive literature search in the PubMed database to explore the impact of PBM on ion channel function and its potential applications in the nervous system. Our search, which encompassed studies up to 6 March 2024, utilized MeSH terms such as “photobiomodulation,” “low-intensity laser therapy,” “ion channel,” and “receptor.” The detailed search strategy is shown as follows: ((Low-intensity laser therapy) OR (photobiomodulation)) AND ((channel) OR (receptor) OR (ion channel)).
Study Selection
The selection of literature was predicated on the alignment of titles and abstracts with our inclusion criteria. Subsequently, full texts were scrutinized to ascertain adherence to these criteria. This process was conducted manually, with no reliance on software or AI assistance.
Inclusion Criteria
In adhering to the established inclusion criteria, our literature selection process focused on in vitro and in vivo studies investigating the impact of light on ion channels. Wavelengths outside the range of 620–1270 nm were omitted due to their dissimilarity to PBM light and limited effect on the nervous system (Barbora et al. 2021). Exclusions comprised studies from unrelated fields like materials’ science, those unrelated to nervous system, non-English publications, and non-original articles such as reviews, case report, and commentary papers.
Extraction of Qualitative Data
Data extraction involved a meticulous cross-review process by two independent reviewers to ensure thoroughness, with discrepancies resolved through consultation with a third reviewer. The final selection encompassed literature pertinent to the role of PBM in ion channel modulation within the nervous system, with extracted information including authors, publication year, study population, PBM parameters, targeted ion channels, and key findings.
Results
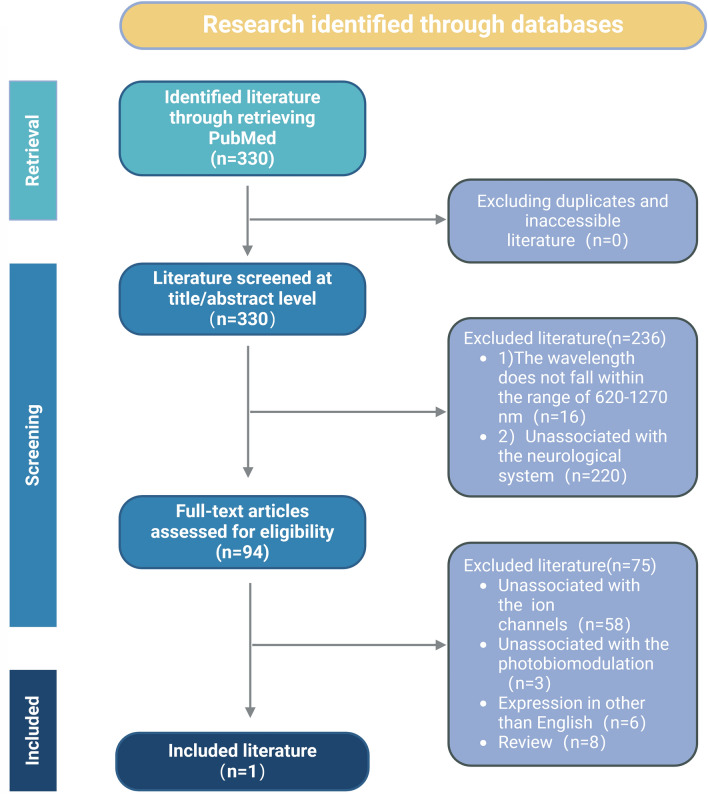
In conducting a literature search on PubMed utilizing the specified search strategy, a total of 330 articles were identified, devoid of duplicates or unavailable literature. Upon review of titles and abstracts, 94 papers were selected for eligibility assessment. Following a thorough screening process, 19 articles met the inclusion criteria and pertained to the study population under review. All 19 studies underwent a comprehensive qualitative analysis. The study’s inclusion criteria and literature flow are visually represented in Fig. 2.
Fig. 2.
Flowchart of studies selected for PBM on ion channels
The primary objective of this paper is to delve into the mechanism of action of PBM in modulating ion channels within the nervous system. The overarching goal is to harness the potential of light for non-invasive neurostimulation. The study also aims to evaluate various PBM protocols, weighing their respective advantages and disadvantages in terms of their impact on ion channels. Furthermore, the research seeks to elucidate the intricate interplay between light and cellular signals and their effects on ion channels. Various discussions on animal models, research goals, and treatment protocols have led to experiments with significant heterogeneity. This diversity presents challenges in accurately assessing the conclusions through Meta-analysis. In Table 1, we have summarized the different treatment protocols used in these experiments as a reference for future research. A detailed synopsis of the included studies is provided in Table 2.
Table 1.
Protocol and major findings of studies included in the review
| Authors | Year | Experimental model | Laser sources | Wavelength (nm) | Mode | Power density (mW/cm2) | Radiant exposure (J/cm2) | Duration | Spot area (cm2) | Location | Distance (cm) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cervetto et al. (2023) | 2023 | Mice (males, C57BL/6 J; 8–12 weeks old). Purified nerve terminals (synaptosomes) were made of mouse cerebral cortex | Diode laser (GaAlAs) | 810 | CW | 1000; 100 | 60; 6 | 60 s, once daily for 1 day | 1 | Cell surface | _ |
| Marques et al. (2023) | 2023 | Rats (males, Wistar, 200–220 g). They had surgical injury of the infraorbital nerve | Diode laser (GaAs) | 904 | PW, 9500 Hz, pulse time of 65 ns | – | 6.23 | 18 s per point, 10 sessions, performed every other day | 0.13*5 | Orbital region | 1 |
| Correia Rocha et al. (2021) | 2021 | Rats (males, Wistar, 250-300 g). They were induced with type 1 DM and peripheral nerve injured | Diode laser (GaAs) | 904 | PW, 9500 Hz, pulse time of 65 ns, duty cycle (DC) 0.0617% | – | 6.23 | 18 s per point, 10 sessions, performed every other day | 0.13*9 | Right leg (peripheral nerve injured area) | 0 or superficial skin contact |
| Zhang et al. (2021) | 2021 | Mice (male, C57BL/6 J, 5 weeks old). They were induced with depression | Semiconductor laser | 635 | CW | Mice: 10.05 Cell: 3.3 | Mice:6 Cell: 1/2/4 | Mice: 600 s, once daily for 30 days. Cell: 75 s/150 s/300 s | 7.06 | Mice: above the brain Cell: Cell surface | Mice: 12 Cell: 20 |
| Shen et al. (2021) | 2021 | Transgenic mice (APP/PS1) | Semiconductor laser | 635 | CW | 117.2 | 6 | Mice: 600 s, once daily for 30 days. Cell: 75 s/150 s/300 s | 0.875 | The upper part of the exposed brain to the interior of the hippocampus | 0 or superficial skin contact |
| Golovynska et al. (2021) | 2021 | Primary rat hippocampus neurons, human neuroblastoma (SH-SY5Y), and human cervical cancer cells (HeLa) | Diode laser | 650/808/1064 | – | 100 | – | 300 s/600 s/1500 s, once daily for 1 day | – | Cell surface | – |
| de Oliveira et al. (2021) | 2021 | Rats (males, Wistar, 200–220 g, 2 months old). They had surgical injury of the sciatic nerve | Diode laser (GaAs) | 904 | PW, 9500 Hz, pulse time of 60 ns | 700 | 6 | 18 s per point, 10 sessions, performed every other day | 0.1*9 | The skin over the chronic constriction injury (CCI) area | 0 or superficial skin contact |
| Zupin et al. (2021) | 2021 | 50B11 cell line | Diode laser | 800/970 | PW, 5 Hz, DC 50% | 300 | – | 20 s/40 s/80 s/120 s, once daily for 1 day | 1 | Cell surface | – |
| Zupin et al. (2019) | 2019 | Dorsal root ganglia (DRG) were dissected from cluster of differentiation 1 (CD1) male mice at 6–8 weeks of age | Diode laser | 800/970 | PW, 5 Hz, DC 50% | 100 | 6 | 120 s, once daily for 1 day | – | Cell surface | – |
| Han et al. (2019) | 2019 | Mice (C57/BL6 8–12 weeks old), knockout mice (Tac1−/−). A mouse model of acid-induced chronic muscle pain, or the Sluka model was in the hind paws | – | 685 | – | – | 4/8 | – | – | The surface of the left legs of mice | 0 or superficial skin contact |
| Golovynska et al. (2019) | 2019 | HeLa cells | Semiconductor laser | 650/808 | CW | 1/10/100/300 | 6/12/30 | 720 s/720 s/60 s, 120 s, 300 s/–, once daily for 1 day | – | Cell surface | – |
| de Sousa et al. (2018) | 2018 | Mice (male, BALB/c, adult, weight 20–24 g) | Semiconductor laser | 810 | CW | 300/50/50 | 36/0, 1.2, 6, 30/6 | 120 s/0 s, 24 s, 120 s, 600 s/120 s, once daily for 7 day | 1/6/6 | Head, neck, DRG, tail, abdomen, ipsilateral right hind-paw, contralateral left hind-paw | – |
| Neves et al. (2018) | 2018 | Mice (male, Swiss-albino, 25-40 g). They were induced as hind paw edema | Semiconductor laser | 660 | CW | – | 1/10/50/100/150/200 | 2 s/20 s/100 s/200 s/300 s/400 s, once daily for 1 day | 0.06 | Hind paws | 0 or superficial skin contact |
| Wang et al. (2017b) | 2017 | Human adipose-derived stem cells (hASCs) | Diode laser (GaAlAs) | 660/810 | CW | 16 | 3 | 188 s, once daily for 1 day | 4 | Cell surface | – |
| Pissulin et al. (2017) | 2017 | Rats (males, Wistar, about 300 g). They had induction of degeneration–regeneration of muscle fibers by local anesthetics | Diode laser (GaAs) | 904 | CW | 1428.6 | 68.6 | 48 s, once daily for 5 days | 0.035 | The ventral surface of the neck | 0 or superficial skin contact |
| Pires de Sousa et al. (2016) | 2016 | Mice (male, BALB/c, adult, weight 20–24 g). The nociceptive tests were conducted, in the following order: cold plate, radiant heat, and formalin injection | Diode laser | 810 | CW | 300 | 7.2/36 | 24 s/120 s, once daily for 1 day | 1 | The head | 0 or superficial skin contact |
| Huang et al. (2014) | 2014 | Mice (female, CD1, 8–10 weeks old). They were used to prepare primary mouse cortical neurons | Diode laser | 810 | CW | 25 | 3 | 120 s, once daily for 1 day | 78.5 | Cell surface | – |
| Rochkind et al. (2013) | 2013 | Rats (males, Wistar, about 250 g, 12 weeks old). They had removed of the sciatic nerve segment resulted in complete denervation of the muscles | Diode laser | 632.8 | CW | – | – | 1800s, once daily for 14 days | – | Right leg (peripheral nerve injured area) | – |
| Ignatov et al. (2005) | 2005 | Neurons of the periglottal ring of neural ganglia of the pond snail | Diode laser | 632.8 | – | 15 | – | 60 s/600 s, once daily for 1 day | 0.0144 | Cell surface | 0.1 |
The parameters outlined above have been sourced from the literature provided. In cases where information is not explicitly stated or could not be determined from the data calculations, the symbol “–” has been used to indicate this
Table 2.
Major findings of studies included in the review
| Authors | Title | Ion channels | Major findings |
|---|---|---|---|
| Cervetto et al. | Photons Induce Vesicular Exocytotic Release of Glutamate in a Power-Dependent Way | Voltage-dependent Na+ channels | PBM facilitates the vesicular glutamate release in a power-dependent way. This process can be facilitated by the activation of voltage-dependent Na+ and voltage-dependent Ca2+ channels |
| Marques et al. | Photobiomodulation and vitamin B treatment alleviate both thermal and mechanical orofacial pain in rats | TRPV1 | CCI-IoN leads to the upregulation of TRPV1, Substance P, and CGRP in the trigeminal ganglion of rats, which plays a vital role in the neuronal hyperexcitability in response to inflammation or nerve injury. PBM can modulated the expression of nociceptive mediators after CCI-IoN, such as TRPV1 |
| Correia Rocha et al. | Modulatory effects of photobiomodulation in the anterior cingulate cortex of diabetic rats | Glutamate Receptor 1 (GluR1) | PBM decreased even further GluR1 and μ-Opioid Receptor (MOR) protein levels in the ACC of diabetic rats that were treated with PBM |
| Zhang et al. | Photobiomodulation Therapy Ameliorates Glutamatergic Dysfunction in Mice with Chronic Unpredictable Mild Stress-Induced Depression | GluA1 | PBM was likely to reduce glutamate levels by upregulating GLT-1 expression to exert neuroprotective effects. PBM promotes AMPA receptor insertion through activation of PKA in corticosterone-treated primary neurons |
| Shen et al. | Photobiomodulation suppresses JNK3 by activation of ERK/MKP7 to attenuate AMPA receptor endocytosis in Alzheimer's disease | AMPA receptor | The inhibition of JNK3 phosphorylation by PBM treatment can effectively rescue AMPA receptor endocytosis and dramatically reduce amyloid load, neuroinflammation, and synaptic loss in APP/PS1 transgenic mice |
| Golovynska et al. | Red and near-infrared light evokes Ca2+ influx, endoplasmic reticulum release and membrane depolarization in neurons and cancer cells | NMDA receptor | 650 and 808 nm light promotes elevation of intracellular Ca2+ level. The Ca2+ influx occurs primarily via NMDARs, which become open due to the plasma membrane depolarization |
| de Oliveira et al. | Effects of photobiomodulation therapy on neuropathic pain in rats: evaluation of nociceptive mediators and infrared thermography | TRPV1 | PBM reduces the expression of TRPV1. PBM is an effective technique for neuropathic pain relief and may modulate important nociceptive mediators involved in thermoregulation and thermal sensitivity |
| Zupin et al. | In vitro effects of photobiomodulation therapy on 50B11 sensory neurons: evaluation of cell metabolism, oxidative stress, mitochondrial membrane potential (MMP), and capsaicin-induced calcium flow | TRPV1 | Although the mechanism by which PBM could modulate TRPV1 and calcium flow remains elusive, laser light could directly and specifically act on TRPV1 functionality |
| Zupin et al. | Analgesic effect of Photobiomodulation Therapy: An in vitro and in vivo study | TRPV1 | 970 nm markedly reduces Ca2+ peaks induced by capsaicin stimulation. 970 nm is effective in reducing calcium flow and pain sensation in vivo |
| Han et al. | Involvement of substance P in the analgesic effect of low-level laser therapy in a mouse model of chronic widespread muscle pain | TRPV1 | Low-level laser at a wavelength of 685 nm showed potent analgesic effect, while applied to muscles with a dose of 8 J/cm2. Although the analgesic effect was transient and only lasted < 24 h, the LLLT analgesia was reproducible after repeated treatment. The LLLT analgesia was impaired when substance/NK1R or TRPV1 signaling was blocked |
| Golovynska et al. | Red and near-infrared light induces intracellular Ca2+ flux via the activation of glutamate N-methyl-d-aspartate receptors | NMDA receptor | The irradiation of 650 and 808 nm lasers elevated intracellular Ca2+ influx in HeLa cells without altering NMDAR function. Light partially relieved NMDAR pharmacological blockade, thereby enhancing Ca2+ influx. Specifically, 650 nm light stimulated cell proliferation, leading to apoptosis and necrosis with increasing dosage, while 808 nm light solely promoted cell proliferation |
| de Sousa et al. | Pain management using photobiomodulation: Mechanisms, location, and repeatability quantified by pain threshold and neural biomarkers in mice | GluR1 | 810 nm laser irradiation to the lower back of mice reversibly increased the pain threshold in the hind paw up to threefold, with a peak at 2–3 h post-PBM. Irradiation of the head, neck and ipsilateral paw was also effective, but not irradiation of the abdomen, tail, or contralateral paw. The treatment was equally effective when repeated daily for one week. mGluR1 was reduced and PAP and tubulin-positive varicosities were increased at 3 h post-PBM |
| Neves et al. | Photobiomodulation Therapy Improves Acute Inflammatory Response in Mice: the Role of Cannabinoid Receptors/ATP-Sensitive K+ Channel/p38-MAPK Signalling Pathway | ATP-Sensitive K+ Channel | PBM anti-inflammatory property seems likely related with its ability to activate CB1 and CB2 cannabinoid receptors, as well as signaling pathway downstream of CB receptors activation, such as ATP-dependent K+ channels and p38-MAPK |
| Wang et al. | Red (660 nm) or near-infrared (810 nm) photobiomodulation stimulates, while blue (415 nm), green (540 nm) light inhibits proliferation in human adipose-derived stem cells | TRPV1 | The effects of red and near-infrared (NIR) light are consistent with a mitochondrial chromophore (giving more ATP, increasing MMP, and producing only a low level of ROS). Red and NIR (810 nm) light stimulate proliferation of hASCs |
| Pissulin et al. | GaAs laser therapy reestablishes the morphology of the NMJ and nAChRs after injury due to bupivacaine | AChRs | LLLT effectively mitigates bupivacaine-induced structural modifications in the neuromuscular junction (NMJ) and molecular alterations in the nicotinic acetylcholine receptor (nAChR), thereby serving as a potential therapeutic approach for the treatment of the degeneration–regeneration of muscle fibers by local anesthetics |
| Pires de Sousa et al. | Transcranial low-level laser therapy (810 nm) temporarily inhibits peripheral nociception: photoneuromodulation of glutamate receptors, prostatic acid phophatase, and adenosine triphosphate | GluR1 | PBM decreases glutamate receptor expression, and increases ATP and prostatic acid phosphatase |
| Huang et al. | Low-level laser therapy (810 nm) protects primary cortical neurons against excitotoxicity in vitro | GluR | PBM enhances intracellular ATP and MMP biosynthesis, while suppressing glutamate receptor-mediated calcium influx in excitotoxic cells, yet promoting it in healthy cells |
| Rochkind et al. | Protective effect of laser phototherapy on acetylcholine receptors and creatine kinase activity in denervated muscle | AChRs | During the initial stages of muscle degeneration, PBM temporarily maintained AChR and CK levels in denervated muscle near pre-injury thresholds, and subsequently sustained CK activity and AChR quantity throughout subsequent stages of muscle degeneration |
| Ignatov et al. | Effects of helium–neon laser irradiation and local anesthetics on potassium channels in pond snail neurons | Voltage-dependent slow potassium channels | Laser irradiation modulates the magnitude of transmembrane potassium ion current, reflecting alterations in the total number of functionally active channels, with the specific effect of radiation varying according to its dose |
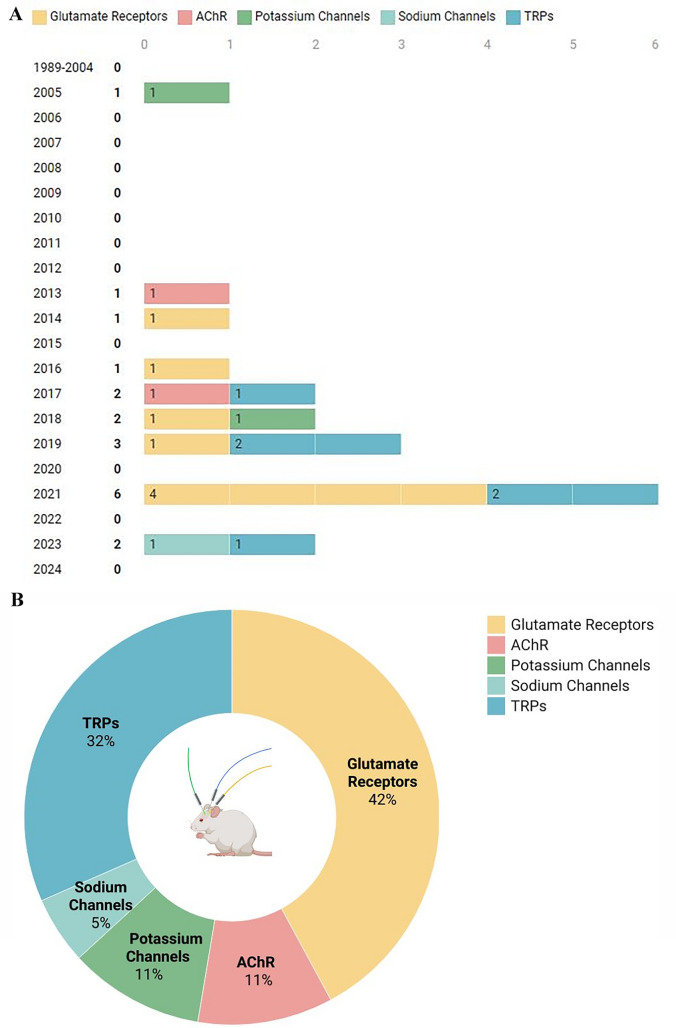
The analysis presented in Fig. 3A underscores the escalating research interest in utilizing PBM for modulating ion channels within the nervous system. Our investigation, guided by specific search parameters, revealed that one of the pioneering studies exploring PBM’s impact on ion channel modulation in the nervous system dates back to 2005 (Ignatov et al. 2005). Since 2016, there has been a gradual uptick in the number of related publications, reaching a pinnacle in 2021 with the publication of six original papers. As of 2024, although ongoing research persists, no new papers have been released to date. The graphical representation vividly illustrates the varying degrees of research attention dedicated to distinct ion channels, each denoted by a distinct color. Figure 3B delves into the specific ion channels that have garnered significant interest in recent years within the realm of PBM application in the nervous system. Notably, glutamate receptors emerge as the most extensively studied group, with a total of 8 papers, closely followed by TRPs (6 papers), AChR (2 papers), potassium channels (2 papers), and sodium channels (1 paper).
Fig. 3.
Visualization of the included literature. Selected article statistics on the mechanism of PBM in ion channel regulation in the nervous system. A Publication schedule of papers on PBM in ion channel regulation in the nervous system. B Relative number of papers on PBM effects on various ion channels
Discussion
PBM, a technique gaining increasing attention in the realm of neurological disorders, holds promise for modulating ion channels to influence cellular excitability. Various conditions, like epilepsy, tinnitus, and Alzheimer’s disease, are known to stem from disruptions in cellular excitability, often linked to ion channel activities (Maiorov et al. 2024; Martin et al. 2023; Negandhi et al. 2014; Ruiz-Clavijo et al. 2023). While existing studies have shed light on the anti-inflammatory and stem cell differentiating effects of PBM through CCO photostimulation, which enhances ATP synthesis and nitric oxide release, investigations into how this modality impacts ion channels and cellular excitability are still limited (Moos et al. 2023; Powner et al. 2024).
PBM induces neuromodulatory effects by interacting with various ion channels, altering their activity. (1) PBM harnesses light energy absorbed by photosensitive or thermosensitive ion channels in cell membranes, leading to energy transfer that modifies ion channel function and intracellular signaling. Caiyun Meng et al. (Meng et al. 2020) found that 630 nm LED inhibits the growth of human synovial cell MH7A, likely by regulating the TRPV4/PI3K/AKT/mTOR signaling pathway, resulting in decreased inflammatory factors (IL-6, IL-1β, IL-8, and MMP-3) and increased anti-inflammatory factor IL-10. (2) PBM may also directly impact calcium channels, decreasing Ca2 + influx, which activates downstream transcription factors, alters gene expression, and decreases protein synthesis (Navarro et al. 2024). Iuliia Golovynska et al. (2022) found that PBM significantly reduced pro-inflammatory cytokine expression and increased Ca2+ influx in macrophages. These insights not only elucidate the therapeutic potential of PBM in inflammatory conditions but also highlight the intricate interplay between light exposure and cellular ion homeostasis, offering avenues for further research and clinical applications in the management of inflammatory diseases.
PBM modulates crucial intracellular signaling pathways like MAPK/ERK and AKT/PI3K, which are vital for neuronal growth, differentiation, and synaptic plasticity (Bathini et al. 2022). Its mechanism for promoting neural regeneration involves altering ion channel activity and enhancing the synthesis of neurotrophic factors. Yun-Hee Rhee et al. (2019) demonstrated that PBM reversed cellular morphology disruption and improved cell viability in neurons inhibited by ouabain, indicating that PBM treatment alleviates Na/K imbalance and aids neural repair. Xiaodong Yan et al. (2017) highlighted the Ca2+-ERK-CREB cascade as a key pathway in PBM-induced BDNF synthesis, underscoring the role of calcium ions in enhancing neurotrophic factor production. Collectively, these effects contribute significantly to the repair and regeneration of neuronal cells. By promoting cellular resilience and enhancing the intrinsic regenerative mechanisms, these interventions facilitate the restoration of neural function. Such positive outcomes are crucial for advancing treatments for neurodegenerative diseases and injuries, ultimately improving patient recovery and quality of life.
Ion Channels
Ion channels are particularly vital for the nervous system due to their involvement in transmembrane ion flow, affecting cellular electrophysiology (Kariev et al. 2024). Neuronal communication, essential for transmitting sensory, cognitive, and motor signals in the brain, relies on electrical signaling through synapses (González-Cota et al. 2024). When we receive external stimuli, neurons generate electrical signals and transmit these signals to other neurons through synapses. This process is fundamental to the normal functioning of the nervous system and is an important mechanism that enables us to perceive and respond. PBM influences the action potentials of neurons through various pathways, including enhancing mitochondrial function, regulating the activity of ion channels, providing neuroprotection, and increasing synaptic plasticity (Ishibashi et al. 2024; Yoo et al. 2013). The ability of PBM to modulate ion channels offers a non-invasive means of neurostimulation, crucial not only for disease treatment but also for applications in optical communication and brain–computer interfaces (Ghaderi et al. 2021; Savić et al. 2023). Our research has identified several key ion channels influenced by PBM, such as glutamate receptors, AChR, potassium channels, sodium channels, and TRPs. Further exploration into the modulation of these channels by PBM is anticipated to deepen our understanding of its therapeutic mechanisms and potential applications in the future.
Glutamate Receptors
Glutamate receptors are ubiquitous in the nervous system, serving as key regulators of neuronal excitability, synaptic transmission, learning, memory, and various physiological processes (Gurung et al. 2016; Ladagu et al. 2023). These receptors are involved in excitatory neurotransmission, being the primary excitatory neurotransmitter in the central nervous system (CNS) (Chakraborty et al. 2023). Glutamate receptors can be broadly classified into two major categories: ionotropic and metabotropic (McElroy et al. 2024; Parent et al. 2024). Ionotropic glutamate receptors (iGluRs) are ligand-gated ion channels that directly gate the flow of ions across the cell membrane, while metabotropic glutamate receptors (mGluRs) are G-protein-coupled receptors that regulate ion channel activities indirectly through second-messenger systems (Paoletti et al. 2013).
Within the ionotropic glutamate receptors, there are three major subclasses: N-methyl-d-aspartame receptors (NMDARs), α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPARs), and erythrocyanine receptors, of which the first two types have been studied the most (Goldsmith 2019). NMDARs play a crucial role in synaptic plasticity and learning and memory processes (Kim et al. 2024). When the cell membrane remains unpolarized, voltage-gated Mg2+ channels prevent NMDAR activation (Dingledine et al. 1999). NMDAR can evoke cellular depolarization upon binding to glutamate or NMDA, altering the membrane voltage and relieving Mg2+ blockade of voltage-dependent channels.
This leads to a significant influx of Ca2+ and subsequent modulation of cellular functions (Golovynska et al. 2019; Paoletti et al. 2007). Golovynska et al. (2019, 2021) demonstrated that 808 nm laser irradiation of neuronal and HeLa cells in vitro induced a substantial Ca2+ influx. Application of dizocilpine, a specific NMDAR blocker, reduced this influx, indicating that the laser’s effect on Ca2+ influx primarily occurs via NMDAR activation. NMDAR’s rapid response and low depolarization potential highlight its importance in the initial stages of Ca2+ influx. Subsequently, the influx of Ca2+ activates AMPAR, enhancing its synthesis and promoting Na+ and K+ influx, thereby maintaining membrane depolarization (Nanou et al. 2007). Zhang et al. (Zhang et al. 2021) demonstrated that PBM can alleviate glutamatergic dysfunction in mice with depression induced by chronic unpredictable mild stress. This effect is achieved by stimulating ATP synthesis through activation of CCO, leading to elevated cAMP levels. This subsequently enhances AMPA receptor phosphorylation and surface expression through the cAMP/PKA signaling pathway. Separately, Shen et al. (2021) investigated the impact of PBM using a 635 nm semiconductor laser on an Alzheimer’s disease mouse model. Their findings revealed that PBM effectively inhibited JNK3 phosphorylation, which reduced AMPA receptor endocytosis and restored surface levels. This treatment also significantly decreased amyloid accumulation, neuroinflammation, and synaptic loss in APP/PS1 transgenic mice.
Neuroplasticity refers to the brain's remarkable capacity to reorganize and adapt its neural connections in response to environmental changes, including injuries and various experiences that affect the nervous system (Chou et al. 2024). This inherent plasticity plays a crucial role in how organisms react to both internal and external stimuli. Recent studies have provided valuable insights into the mechanisms underlying neuroplasticity. For instance, research by Pascal Jorratt et al. (2023) highlights that prolonged stimulation of NMDAR can intensify activity in glutamatergic neurons, leading to dendritic field expansion that may worsen psychiatric conditions such as anxiety. Conversely, findings from Hongli Chen et al. (2024a, b) demonstrate that PBM can alleviate behavioral deficits, reduce neuroinflammation, and restore synaptic function in the hippocampus of depressed mice, likely through the enhancement of brain-derived neurotrophic factor signaling. Moreover, Katayoon Montazeri et al. (2024) showed that the expression of doublecortin, a neuroplasticity marker, was significantly reduced in the dentate gyrus of rats experiencing tinnitus, suggesting that PBM may promote neuroplasticity that is compromised by sodium salicylate-induced auditory disorders. In summary, these studies emphasize the complex interplay between neural plasticity and PBM in the treatment of neurological and psychiatric disorders, with glutamate receptors potentially playing a significant role. Further research is necessary to explore how PBM influences neural plasticity through its effects on glutamate receptors.
Excitotoxicity, a series of pathologic changes in the CNS triggered by neuroinflammation or ischemic trauma, can result from the over-synthesis of glutamate by microglia or abnormally active glutamate function in neuronal cells (Neves et al. 2023). This abnormal activation of glutamate receptors can lead to elevated intracellular Ca2+ levels, ultimately causing cell death and contributing to the pathogenesis of conditions such as epilepsy (Sarlo et al. 2021). Furthermore, chronic noise damage affecting the cochlear nucleus can elevate glutamate concentrations and increase glutamate receptor expression, potentially leading to conditions like tinnitus (Muly et al. 2004). Research on PBM as a treatment for CNS disorders has shown promising results by improving mitochondrial function, boosting ATP synthesis, and countering neuroinflammation (Lim 2024). However, the impact of this therapy on ion channels and the specific mechanisms involved in the therapeutic process require further investigation. Studies have indicated that PBM can both promote and inhibit glutamate receptor activity, with its biological effects likely dependent on the light regimen employed (Amaroli et al. 2019).
AChR
AChR plays a crucial role in various bodily functions, particularly in synapses, where they are pivotal in parasympathetic action and motor function (Sine 2012). These receptors are categorized into M and N receptors (Ved et al. 2020). M receptors, upon binding with acetylcholine, elicit parasympathetic excitatory effects such as inhibiting cardiac contraction and promoting smooth muscle contraction in the digestive tract (Eglen et al. 2001). On the other hand, N receptors trigger skeletal muscle contraction or excitation of postganglionic neurons following acetylcholine binding (Changeux et al. 1992). Pissulin et al. (2017) have shown promising outcomes in using GaAs laser therapy to restore the morphology of the neuromuscular junction and AChR post-injury caused by bupivacaine. Their studies on PBM for nerve injury due to local anesthetics demonstrated that irradiation with 904 nm laser light reduced myonecrosis and significantly increased the expression of the ε-subunit of the AChR. The ε-subunit of the nAChR ion channel plays a crucial role in regulating calcium ion influx, thereby ensuring stable initial interactions between nerves and muscles, leading to synaptic maturation (Castro et al. 2022). These findings shed light on potential therapeutic interventions for nerve injuries and underscore the intricate mechanisms underlying nerve–muscle interactions.
The role of AChR in cognitive processes has been widely acknowledged in the scientific community. By either increasing acetylcholine synthesis or directly activating AChR, researchers have found that these approaches can be effective in treating neurodegenerative diseases such as Alzheimer’s disease (Castro et al. 2022), schizophrenia (Dean 2023) and Parkinson’s disease (Castro et al. 2022; Vallés et al. 2023). In the case of Parkinson’s disease, the lack of dopamine synthesis in the brain leads to an imbalance with acetylcholine neurotransmitters, resulting in symptoms like tremors (Morris et al. 2024). Chiao-Hsin Lan et al. (2022) reported a myasthenia gravis patient treated with PBM, who regained muscle strength. SPECT brain imaging revealed a significant increase in perfusion in the prefrontal lobe and anterior cingulate gyrus. We speculate that this may be linked to PBM’s activation of AChR, but further investigation is needed to clarify the underlying mechanism. While the exact mechanism of this treatment method is still being explored, it offers a potential alternative for diseases related to AChR dysfunction. Further research into the use of PBM for neurodegenerative diseases holds great promise and may lead to new therapeutic approaches for patients in the future.
Potassium Channels
Potassium channels play a crucial role in the regulation of cellular excitability within all cells (Abbott 2021). These channels can be categorized into four main types based on their structure and function: calcium-activated potassium channels, inwardly rectifying potassium channels, tandem pore domain potassium channels, and voltage-gated potassium channels (Griffith 2001). Among these, calcium-activated and inwardly rectifying potassium channels are particularly influenced by photobiological regulation. (1) Calcium-activated potassium channels are activated through an indirect pathway involving the absorption of specific wavelengths by CCO within the mitochondrial oxidative respiratory chain (Liebert et al. 2023; Salehpour et al. 2018). This activation leads to the release of NO, a key intracellular signaling molecule that triggers a cascade resulting in the opening of potassium channels and the regulation of vasodilation (Hennessy et al. 2017). NO plays a crucial role as a significant intracellular signaling molecule by triggering soluble guanylate cyclase to produce cyclic guanosine monophosphate (cGMP). This cGMP then initiates the activation of protein kinase G (PKG), which subsequently facilitates the influx of calcium ions, opens calcium-activated potassium channels, and governs the process of vasorelaxation (Bardou et al. 2001). (2) ATP-sensitive potassium channels, a subtype of inwardly rectifying potassium channels, are also activated indirectly. Laís et al. (2018) have demonstrated that CB1 receptors play a role in modulating calcium and potassium channels in response to light exposure, with the ATP-sensitive potassium channel inhibitor glibenclamide reversing certain effects.
Potassium channels are pivotal in the modulation of neuronal electroactivity, with aberrant potassium channels being intricately linked to the pathogenesis of numerous CNS disorders. Notably, acquired mutations in the α1 subunit of calcium-activated potassium channels have been associated with 50% of individuals afflicted by petit mal seizure (Du et al. 2005), while mutations in the β4 subunit have been shown to induce epileptiform behavior in mice (Wang et al. 2009). Various potassium channels are implicated in conditions such as epilepsy (Gribkoff et al. 2023), tinnitus (Lai et al. 2024), and Alzheimer’s disease (Taylor et al. 2022), although a comprehensive exploration of the interplay between these ion channels and diseases is beyond the scope of this discourse. For further insights, the commentary paper authored by Yian Huang et al. (2018) provides a valuable resource. While a plethora of potassium channel activators have been developed for treating CNS disorders, their utility is hampered by non-specific drug targets, severe side effects, and the emergence of drug resistance. Consequently, pharmacotherapy may not always represent the optimal treatment modality (Sills et al. 2020). PBM emerges as a promising alternative, enabling precise modulation of neuronal activity without affecting non-target sites, thereby reducing adverse reactions. Moreover, PBM circumvents the issue of drug resistance, offering novel avenues for managing CNS ailments. Future investigations in neuromodulation are poised to unravel the intricate mechanisms governing the regulation of distinct potassium channel subtypes, paving the way for tailored interventions in various diseases.
Sodium Channels
Sodium channels play a crucial role in cellular function by facilitating the entry of sodium ions into the cell through an electrochemical gradient. These membrane proteins are essential for various physiological processes and can be classified into different types based on their mode of activation, such as ionic and ligand-type sodium channels (McDougall et al. 2024). Cervetto et al. (2023) have demonstrated how the irradiation of nerve endings with an 810 nm laser in adult mice cultured in vitro can activate ligand-type sodium channels and Ca2+ channels in the postsynaptic membrane. This activation process may involve photon-induced stimulation of the CCO in the presynaptic membrane, leading to ATP synthesis and the release of glutamate into the cytosol. Additionally, Li et al. (2013, 2014) have revealed the association between the opening of sodium channels and the photothermal effect. Neuronal cells depolarized by electrical pulses from − 70 to − 30 mV exhibit peak Na+ currents, with the 980 nm near-infrared laser appearing to decrease ion channel resistance. Furthermore, there is a positive correlation between the accelerated inward flow of Na+ in the extracellular fluid induced by irradiation and the transient temperature rise.
Maintaining the normal function of sodium channels is crucial for the proper functioning of the CNS. Numerous research studies have highlighted the significance of mutations or irregular activities in sodium channels in the onset and progression of various CNS disorders. Conditions such as epilepsy, Parkinson’s disease, and Alzheimer’s disease have been linked to abnormalities in sodium channels, as evidenced by several studies (Barbieri et al. 2023; Thompson et al. 2023; Vaidya et al. 2024). In response to these findings, pharmaceutical interventions in the form of sodium channel blockers like lidocaine, quinidine, and phenytoin sodium have been developed for the management of epilepsy, tinnitus, and other associated disorders. Sodium channels, akin to potassium channels, have emerged as pivotal therapeutic targets in the treatment of CNS disorders (Zierath et al. 2023).
Sodium channels are crucial for neuronal excitability, and studying their structure and function has revealed important insights into this process. These complex protein molecules, which consist of about 3000 amino acids, typically comprise four subunits, each featuring intra- and extra-membrane transmembrane regions (Schott et al. 2024). This arrangement forms a channel that allows sodium ions to flow across the cell membrane during potential changes, triggering electrical activity in neurons and modulating their excitability. Overactivation of sodium channels can lower the threshold for action potentials, enhancing neuronal firing and leading to abnormal excitation (Miralles et al. 2024). Conversely, reduced sodium channel function can impede normal action potential firing, resulting in diminished excitability. Thus, studying sodium channel’s role in regulating neuronal excitability is vital for understanding related diseases and developing new therapies.
Despite the acknowledged importance of sodium channels in CNS disorder pathogenesis, the exploration of the effects of PBM on their modulation remains relatively uncharted territory, as indicated by our comprehensive review of the existing literature. We posit that further fundamental research in this area will pave the way for a more seamless transition of PBM technology from theoretical understanding to practical clinical applications.
TRPs
TRPs, initially discovered in Drosophila mutants and predominantly associated with vision development in insects, have now been recognized as pivotal light- and heat-gated ion channels crucial for modulating cellular functions (Feng 2014). The PBM process works with chromophore CCOs and heat/light-gated ion channels to raise secondary messengers like calcium ions, cAMP, and ROS. These secondary messengers play a vital role in initiating the activation of transcription factors and signaling molecules, which subsequently trigger downstream signaling cascades leading to profound photobiological effects within the cell (Ma et al. 2024). Notably, TRPs exhibit responsiveness to both green and near-infrared light, although the limited penetration capability of green light through the cranium necessitates the predominant utilization of near-infrared light in PBM practices to activate TRPs effectively (Gholami et al. 2022). Wang et al. (2017a) have demonstrated that when subjecting adipose-derived stem cells to laser irradiation in vitro using wavelengths exceeding 900 nm, the primary chromophore absorbing the light is the TRPs located on the cell membrane. Della Pietra A et al. (2024) have highlighted a significant correlation between TRPs and migraine. Their findings suggest that individuals suffering from migraines exhibit elevated levels of TRPs compared to those without the condition. Moreover, these TRPs appear to be more responsive to external stimuli such as light, heat, and mechanical pressure in migraine patients. Our research also highlights the critical role of TRPs in mediating the analgesic properties associated with PBM.
However, it is puzzling that the activation of TRPs appears to be triggered not only by light stimulation but also by capsaicin, other vanilloids, acid, and heat (Caterina et al. 1997; Gunthorpe et al. 2007; Julius et al. 2001). This contradicts the concept of PBM, which focuses on the non-thermal effects of light. The debate continues on whether the regulation of TRPs through photobiological means truly falls under PBM. Despite this, our study includes relevant TRPs studies for the reference of other researchers. Delving into the regulation of TRPs through photobiological methods presents significant challenges, particularly in completely eliminating non-light stimuli. Even with the most rigorous experimental setups, controlling cell temperature may have limitations, and subtle temperature changes undetectable by instruments could activate TRPs, leading to the erroneous attribution of biological effects to light exposure. Therefore, further precise and meticulous experimental designs are essential for studying the regulation of TRPs through photobiological means.
Protocols for PBM of Effects on Ion Channels
PBM is a sophisticated therapeutic technique that requires careful consideration of multiple parameters to effectively harness its potential in non-invasive neuromodulation. In Table 3, we outline the key parameters influencing PBM. It is concerning that, despite previous research on PBM parameters, a consensus has yet to be reached on a reasonable and standardized set of parameters. This lack of agreement not only introduces systematic bias, diminishing result reliability, but also hampers experimental reproducibility and impedes in-depth investigation into the mechanism of action. Among the reviewed studies, only five papers included all parameters, with spot area and distance being the most commonly omitted (de Oliveira et al. 2021; Pires de Sousa et al. 2016; Pissulin et al. 2017; Shen et al. 2021; Zhang et al. 2021). This paper aims to establish a detailed and standardized experimental design framework for PBM to assist researchers in conducting more effective experiments.
Table 3.
Description of the parameters in PBM
| Parameters | Descriptions |
|---|---|
| Laser sources | Light sources can be classified into two categories: diode lasers and semiconductor lasers. Each type has distinct biological effects. Diode lasers emit coherent light with an uneven energy distribution from the center to the edge of the spot, while semiconductor lasers emit light energy that is evenly distributed throughout the spot. Diode lasers have greater penetration capabilities compared to semiconductor lasers; however, semiconductor lasers are more cost-effective and are advantageous for creating array laser sources. Depending on the specific research requirements, both diode and semiconductor lasers can be utilized effectively in experiments |
| Wavelength (nm) | Defined as the distance between two consecutive wave peaks, wavelength plays a significant role in determining the depth of penetration and biological effects of these waves. In the field of PBM, a wavelength range of 620–1270 nm is commonly chosen for its therapeutic benefits |
| Mode | Lighting modes are commonly classified into two categories: continuous and pulsed wave. In the continuous wave mode, the light source emits light continuously during the illumination process, whereas in the pulse wave mode, light emission occurs periodically according to a specific time wave. In this context, the frequency at which these pulses occur is referred to as the pulse frequency, measured in Hertz (Hz), while the duration of each pulse is known as the pulse width, measured in seconds (s). The duty cycle, which is the ratio of the total time of illumination to the total time, reflects the actual degree of illumination achieved. Typically, the pulse frequency ranges from 1 to 3000 Hz (Sommer et al. 2012). Research suggests that pulsed light may influence the electronic oscillations within nerve cells, potentially enhancing the opening or closing of ion channels(Kampa et al. 2004) |
| Power density (mW/cm2) | Power density is a crucial parameter that must be carefully considered in any application involving electromagnetic fields. It plays a significant role in determining the potential effects on living organisms, including modulation effects and the risk of tissue damage. Typically, power density levels are maintained within the range of 0.1–100 mW/cm2 to minimize the risk of thermal damage |
| Radiant exposure (J/cm2) | Radiant exposure serves as a crucial parameter in quantifying the accumulation of light energy on the surface of action. It is defined as the product of power density, which represents the amount of power per unit area, and the actual illumination time |
| Duration | The determination of illumination time stands as a pivotal parameter in experimental design, directly shaping the course of research endeavors. This factor, which encompasses both the number and duration of illuminations, exhibits remarkable flexibility, adapting to the unique demands of each study subject |
| Spot area (cm2) | The spot area is a crucial parameter that is often underestimated in its importance. Even with identical power levels, variations in spot area can lead to substantial differences in power density. Moreover, the size of the spot area directly influences the precision of PBM manipulation. In research involving rodents as experimental subjects, maintaining the spot diameter at the millimeter level is imperative. If the spot size is too large, multiple areas may inadvertently receive irradiation simultaneously, introducing a notable system bias due to the anatomical curvature of the irradiation point. This factor cannot be disregarded, as it may contribute to the diminished credibility of numerous experiments |
| Location | The location of illumination in a study can vary depending on the specific research objectives. Common areas for illumination include the head, limbs, chest, and abdomen. Researchers should carefully consider the purpose of their study when selecting the appropriate location for illumination to ensure accurate and reliable results |
| Distance (cm) | Distance refers to the space separating the light source from the subject, impacting the spot size and the thermal influence of the rising ambient temperature from the light source |
Promising Applications of PBM
Recently, PBM applications have expanded beyond traditional CNS irradiation, demonstrating potential in various areas, particularly brain–gut axis intervention and enhancing blood–brain barrier (BBB) permeability. (1) PBM modulates interactions between the nervous system and gut microbiota, improving mood and cognitive function (Huang et al. 2024). Qianqian Chen et al. (2021) found that PBM on the abdomen of amyloid β protein (Aβ)-induced Alzheimer’s disease mice led to significant changes in intestinal microflora after 8 weeks, affecting pathways related to hormone synthesis, phagocytosis, and metabolism. This suggests that PBM can diversify the intestinal flora and mitigate Alzheimer’s-related damage. (2) Additionally, PBM enhances blood–brain barrier permeability, enabling more efficient drug delivery to the CNS. Ting Zhou et al. (2021) showed that an 808 nm laser significantly increased permeability in a cellular model of the BBB, likely due to inhibited metalloproteinase activity and altered tight junction protein expression in endothelial cells. These findings position PBM as a promising method for improving drug delivery through the blood–brain barrier, providing innovative treatment avenues for neurological diseases, and paving the way for further exploration of PBM mechanisms in clinical settings. Despite these preliminary results, the specific mechanisms behind these applications require further investigation, particularly concerning the potential role of ion channels.
Limitations and Potential for Future Research
Despite the promising therapeutic effects that PBM has demonstrated in various central nervous system disorders, there remains a notable lack of convincing evidence from randomized controlled trials to support its efficacy (Figueiro Longo et al. 2020). Consequently, the effectiveness of PBM requires more rigorous scientific investigation and well-structured experimental designs, aspects that are currently insufficiently addressed in the existing research. The variability in parameters across different experimental protocols, coupled with the frequent omission of critical factors such as spot size and the distance between the light source and the treatment area, significantly hampers the comparability of PBM treatments and does not accurately reflect their therapeutic potential. Moving forward, it is imperative to standardize the selection of treatment parameters for PBM and conduct more scientifically robust randomized controlled trials to compare PBM with widely accepted treatment modalities, ultimately elucidating its therapeutic efficacy.
Research on the relationship between PBM and ion channels remains limited, primarily addressing voltage-gated, ligand-gated, and TRP channels. Further investigation into the roles of different channels in PBM is necessary. The P2X7 receptor has garnered significant attention due to its link with inflammation, particularly in the context of chronic obstructive pulmonary disease (COPD) and temporomandibular arthritis (da Cunha Moraes et al. 2018; Mazuqueli Pereira et al. 2021). However, GABAR, acid-sensitive ion channels, chloride channels, and mechanosensitive ion channels have yet to be explored, presenting opportunities for future research.
The opening of many ion channels relies on ATP, so PBM not only modifies ion channel protein structure through photon interactions but also enhances ATP synthesis, influencing ion channel function. Different cell types exhibit significant variations in mitochondrial number and activity, and it remains unclear how PBM affects mitochondria in these diverse cell types—a topic that requires further investigation in future research.
Conclusions
PBM demonstrates the ability to effectively regulate the excitatory and inhibitory states of neurons by directly influencing the activity of ion channels, thereby fine-tuning the transmission and processing of neural signals. A plethora of studies have underscored the capacity of PBM to influence an array of ion channels, encompassing glutamate receptors, AChR, potassium and sodium channels, and TRPs, among others. This modulation intricately adjusts the dynamics of neuronal membrane potentials, impacting the initiation and propagation of action potentials. Such a regulatory mechanism assumes a pivotal role in the pathological progression of neurological disorders. This article endeavors to present the available data in a comprehensible manner, serving as a springboard for future investigations and offering insights into potential therapeutic avenues for combating neurological conditions arising from aberrant excitability. Of the 19 studies we reviewed, all involved rodent and cellular models, indicating a lack of clinical studies on the mechanisms of ion channels following PBM treatment for various CNS disorders. This is why we have not focused extensively on clinical research when discussing the mechanisms of action of different PBMs on ion channels. The limited exploration may stem from PBM’s experimental status in the CNS, focusing more on treatment efficacy than on mechanistic understanding. Therefore, this paper summarizes all PBM studies on ion channels conducted to date, aiming to inspire researchers and broaden applications and clinical inquiries into other ion channels yet to be studied. While numerous aspects remain to be elucidated or refined, the evolving comprehension of PBM mechanisms and the exploration of diverse ion channels represent a quest to unveil novel and promising therapeutic domains. Therefore, a thorough exploration of the interplay between PBM and ion channels is of paramount significance in unveiling the principles governing nervous system activity and in devising innovative strategies for the treatment and prevention of neurological ailments.
Acknowledgements
All authors were involved in the design of this review. All authors read and approved the final manuscript. Specific division of labor is as follows: Zhixin Zhang: Conceptualization, Visualization, Review, Writing—original draft. Zhiyu Zhang: Writing—original draft, Visualization. Peng Liu: Review, Writing—original draft, Writing—review & editing. Xinmiao Xue: Visualization, Writing—original draft. Chi Zhang: Conceptualization, Writing—original draft. Lili Peng: Conceptualization, Writing—original draft. Weidong Shen: Writing—review & editing. Shiming Yang: Conceptualization, Funding acquisition, Writing—review & editing. Fangyuan Wang: Conceptualization, Funding acquisition, Review, Writing—review & editing. We extend our heartfelt gratitude to Prof. Shiming Yang and Prof. Fangyuan Wang for their insightful comments and dedication in preparing the manuscript. Additionally, we would like to thank Jintao Li, Minyue Qiu, Xiaotang Fan, and Chuanqi Liu from the Army Medical University for their ongoing encouragement and inspiration.
Abbreviations
- PBM
Photobiomodulation
- CCO
Cytochrome c oxidase
- TRPs
Transient receptor potential channels
- ROS
Reactive oxygen species
- AChR
Acetylcholine receptor
- ChR
Channelrhodopsins
- KCR
Kalium channelrhodopsin
- UVB
Ultraviolet radiation b
- FDA
U.S. food and drug administration
- ASICs
Acid-sensitive ion channels
- CFTRs
Cystic fibrosis transmembrane conductance regulators
- CW
Continuous wave
- PW
Pulsed wave
- DM
Diabetic mellitus
- SCI
Spinal cord injury
- CCI
Chronic constriction injury
- DC
Duty cycle
- DRG
Dorsal root ganglia
- hASCs
Human adipose-derived stem cells
- BMV
Bothrops moojeni venom
- MOR
μ-Opioid receptor
- GluR1
Glutamate receptor 1
- ACC
Anterior cingulate cortex
- CNS
Central nervous system
- MMP
Mitochondrial membrane potential
- NIR
Near-infrared
- ATP
Adenosine triphosphate
- NMJ
Neuromuscular junction
- nAChR
Nicotinic acetylcholine receptor
- iGluRs
Ionotropic glutamate receptors
- mGluRs
Metabotropic glutamate receptors
- NMDARs
N-methyl-D-aspartame receptors
- AMPARs
α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors
- cGMP
Cyclic guanosine monophosphate
- PKG
Protein kinase G
- cAMP
Cyclic adenosine monophosphate
- BBB
Blood–brain barrier
- Aβ
Amyloid β protein
- COPD
Chronic obstructive pulmonary disease
Author Contributions
CRediT authorship contribution statement Zhixin Zhang: Conceptualization, Visualization, Review, Writing—original draft. Zhiyu Zhang: Writing—original draft, Visualization. Peng Liu: Review, Writing—original draft, Writing—review & editing. Xinmiao Xue: Visualization, Writing—original draft. Chi Zhang: Conceptualization, Writing—original draft. Lili Peng: Conceptualization, Writing—original draft. Weidong Shen: Writing—review & editing. Shiming Yang: Conceptualization, Funding acquisition, Writing—review & editing. Fangyuan Wang: Conceptualization, Funding acquisition, Review, Writing—review & editing. All authors reviewed the manuscript.
Funding
This research was financially supported by the National Key Research and Development Program (Project: 2020YFC2005203, 2022YFC2402704, and 2022YFC2402701) and National Natural Science Foundation of China (Project: NO.81820108009).
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request. This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-Non-Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, if appropriate credit is given and the new creations are licensed under the identical terms.
Declarations
Conflict of interest
The authors do not have any financial support or relationships that may pose conflict of interest to disclose.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Zhixin Zhang and Zhiyu Zhang have contributed equally to this work.
Contributor Information
Zhixin Zhang, Email: zhangzhixin0246@126.com.
Shiming Yang, Email: shm_yang@163.com.
Fangyuan Wang, Email: fangyuanwang05@163.com.
References
- Abbott GW (2021) Control of biophysical and pharmacological properties of potassium channels by ancillary subunits. Handb Exp Pharmacol 267:445–480. 10.1007/164_2021_512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aggarwal I, Lio PA (2023) Photobiomodulation therapy and low-level light therapy in wound healing. Lasers Med Sci 38(1):239. 10.1007/s10103-023-03909-9 [DOI] [PubMed] [Google Scholar]
- Amaroli A, Ferrando S, Benedicenti S (2019) Photobiomodulation affects key cellular pathways of all life-forms: considerations on old and new laser light targets and the calcium issue. Photochem Photobiol 95(1):455–459. 10.1111/php.13032 [DOI] [PubMed] [Google Scholar]
- Barbieri R, Nizzari M, Zanardi I, Pusch M, Gavazzo P (2023) Voltage-gated sodium channel dysfunctions in neurological disorders. Life (Basel). 10.3390/life13051191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbora A, Bohar O, Sivan AA, Magory E, Nause A, Minnes R (2021) Higher pulse frequency of near-infrared laser irradiation increases penetration depth for novel biomedical applications. PLoS ONE 16(1):e0245350. 10.1371/journal.pone.0245350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardou M, Goirand F, Marchand S, Rouget C, Devillier P, Dumas JP, Morcillo EJ, Rochette L, Dumas M (2001) Hypoxic vasoconstriction of rat main pulmonary artery: role of endogenous nitric oxide, potassium channels, and phosphodiesterase inhibition. J Cardiovasc Pharmacol 38(2):325–334. 10.1097/00005344-200108000-00018 [DOI] [PubMed] [Google Scholar]
- Barolet D (2021) Near-infrared light and skin: why intensity matters. Curr Probl Dermatol 55:374–384. 10.1159/000517645 [DOI] [PubMed] [Google Scholar]
- Bathini M, Raghushaker CR, Mahato KK (2022) The molecular mechanisms of action of photobiomodulation against neurodegenerative diseases: a systematic review. Cell Mol Neurobiol 42(4):955–971. 10.1007/s10571-020-01016-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behroozi Z, Rahimi B, Motamednezhad A, Ghadaksaz A, Hormozi-Moghaddam Z, Moshiri A, Jafarpour M, Hajimirzaei P, Ataie A, Janzadeh A (2024) Combined effect of cerium oxide nanoparticles loaded scaffold and photobiomodulation therapy on pain and neuronal regeneration following spinal cord injury: an experimental study. Photochem Photobiol Sci 23(2):225–243. 10.1007/s43630-023-00501-6 [DOI] [PubMed] [Google Scholar]
- Berman MH, Nichols TW (2019) Treatment of neurodegeneration: integrating photobiomodulation and neurofeedback in Alzheimer’s dementia and Parkinson’s: a review. Photobiomodul Photomed Laser Surg 37(10):623–634. 10.1089/photob.2019.4685 [DOI] [PubMed] [Google Scholar]
- Blivet G, Roman FJ, Lelouvier B, Ribière C, Touchon J (2024) Photobiomodulation therapy: a novel therapeutic approach to alzheimer’s disease made possible by the evidence of a brain-gut interconnection. J Integr Neurosci 23(5):92. 10.31083/j.jin2305092 [DOI] [PubMed] [Google Scholar]
- Bullock-Saxton J, Lehn A, Laakso EL (2021) Exploring the effect of combined transcranial and intra-oral photobiomodulation therapy over a four-week period on physical and cognitive outcome measures for people with Parkinson’s disease: a randomized double-blind placebo-controlled pilot study. J Alzheimers Dis 83(4):1499–1512. 10.3233/jad-210170 [DOI] [PubMed] [Google Scholar]
- Cardoso FDS, Salehpour F, Coimbra NC, Gonzalez-Lima F, Gomes da Silva S (2022) Photobiomodulation for the treatment of neuroinflammation: a systematic review of controlled laboratory animal studies. Front Neurosci 16:1006031. 10.3389/fnins.2022.1006031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castaño-Castaño S, Zorzo C, Martínez-Esteban J, Arias JL (2024) Dosimetry in cranial photobiomodulation therapy: effect of cranial thickness and bone density. Lasers Med Sci 39(1):76. 10.1007/s10103-024-04024-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro P, Machanocker DH, Luna GF, Barbosa GM, Cunha JE, Cunha TM, Cunha FQ, Russo TL, Salvini TF (2022) Clinical-like cryotherapy in acute knee arthritis protects neuromuscular junctions of quadriceps and reduces joint inflammation in mice. Biomed Res Int 2022:7442289. 10.1155/2022/7442289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D (1997) The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389(6653):816–824. 10.1038/39807 [DOI] [PubMed] [Google Scholar]
- Ceranoglu TA, Hutt Vater C (2024) Dr. Joseph Biederman’s enduring legacy: illuminating the path to addressing autistic traits in attention deficit hyperactivity disorder with transcranial photobiomodulation. J Atten Disord. 10.1177/10870547231222599 [DOI] [PubMed] [Google Scholar]
- Cervetto C, Amaroli A, Amato S, Gatta E, Diaspro A, Maura G, Signore A, Benedicenti S, Marcoli M (2023) Photons induce vesicular exocytotic release of glutamate in a power-dependent way. Int J Mol Sci. 10.3390/ijms241310977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty P, Dey A, Gopalakrishnan AV, Swati K, Ojha S, Prakash A, Kumar D, Ambasta RK, Jha NK, Jha SK, Dewanjee S (2023) Glutamatergic neurotransmission: a potential pharmacotherapeutic target for the treatment of cognitive disorders. Ageing Res Rev 85:101838. 10.1016/j.arr.2022.101838 [DOI] [PubMed] [Google Scholar]
- Chamkouri H, Liu Q, Zhang Y, Chen C, Chen L (2024) Brain photobiomodulation therapy on neurological and psychological diseases. J Biophotonics 17(1):e202300145. 10.1002/jbio.202300145 [DOI] [PubMed] [Google Scholar]
- Chan ST, Mercaldo N, Figueiro Longo MG, Welt J, Avesta A, Lee J, Lev MH, Ratai EM, Wenke MR, Parry BA, Drake L, Anderson RR, Rauch T, Diaz-Arrastia R, Kwong KK, Hamblin M, Vakoc BJ, Gupta R (2024) Effects of low-level light therapy on resting-state connectivity following moderate traumatic brain injury: secondary analyses of a double-blinded placebo-controlled study. Radiology 311(2):e230999. 10.1148/radiol.230999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Changeux JP, Devillers-Thiéry A, Galzi JL, Revah F (1992) The acetylcholine receptor: a model of an allosteric membrane protein mediating intercellular communication. Ciba Found Symp 164:66–89. 10.1002/9780470514207.ch6 [DOI] [PubMed] [Google Scholar]
- Chen Q, Wu J, Dong X, Yin H, Shi X, Su S, Che B, Li Y, Yang J (2021) Gut flora-targeted photobiomodulation therapy improves senile dementia in an Aß-induced Alzheimer’s disease animal model. J Photochem Photobiol B 216:112152. 10.1016/j.jphotobiol.2021.112152 [DOI] [PubMed] [Google Scholar]
- Chen H, Shi X, Liu N, Jiang Z, Ma C, Luo G, Liu S, Wei X, Liu Y, Ming D (2024a) Photobiomodulation therapy mitigates depressive-like behaviors by remodeling synaptic links and mitochondrial function. J Photochem Photobiol B 258:112998. 10.1016/j.jphotobiol.2024.112998 [DOI] [PubMed] [Google Scholar]
- Chen Z, Li M, Wu C, Su Y, Feng S, Deng Q, Zou P, Liu TC, Duan R, Yang L (2024b) Photobiomodulation therapy alleviates repeated closed head injury-induced anxiety-like behaviors. J Biophotonics 17(2):e202300343. 10.1002/jbio.202300343 [DOI] [PubMed] [Google Scholar]
- Choi JE, Chang SY, Lee MY, Park I, Jung JY (2024) Photobiomodulation with near-infrared laser for tinnitus management: preliminary animal experiments and randomized clinical trials. Lasers Med Sci 39(1):224. 10.1007/s10103-024-04175-z [DOI] [PubMed] [Google Scholar]
- Chou TH, Epstein M, Fritzemeier RG, Akins NS, Paladugu S, Ullman EZ, Liotta DC, Traynelis SF, Furukawa H (2024) Molecular mechanism of ligand gating and opening of NMDA receptor. Nature 632(8023):209–217. 10.1038/s41586-024-07742-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correia Rocha IR, Chacur M (2021) Modulatory effects of photobiomodulation in the anterior cingulate cortex of diabetic rats. Photochem Photobiol Sci 20(6):781–790. 10.1007/s43630-021-00059-1 [DOI] [PubMed] [Google Scholar]
- da Cunha Moraes G, Vitoretti LB, de Brito AA, Alves CE, de Oliveira NCR, Dos Santos Dias A, Matos YST, Oliveira-Junior MC, Oliveira LVF, da Palma RK, Candeo LC, Lino-Dos-Santos-Franco A, Horliana A, Gimenes Júnior JA, Aimbire F, Vieira RP, Ligeiro-de-Oliveira AP (2018) Low-level laser therapy reduces lung inflammation in an experimental model of chronic obstructive pulmonary disease involving P2X7 receptor. Oxid Med Cell Longev 2018:6798238. 10.1155/2018/6798238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva TG, Rodrigues JA, Siqueira PB, Dos Santos Soares M, Mencalha AL, de Souza Fonseca A (2023) Effects of photobiomodulation by low-power lasers and LEDs on the viability, migration, and invasion of breast cancer cells. Lasers Med Sci 38(1):191. 10.1007/s10103-023-03858-3 [DOI] [PubMed] [Google Scholar]
- de Oliveira ME, Da Silva JT, Brioschi ML, Chacur M (2021) Effects of photobiomodulation therapy on neuropathic pain in rats: evaluation of nociceptive mediators and infrared thermography. Lasers Med Sci 36(7):1461–1467. 10.1007/s10103-020-03187-9 [DOI] [PubMed] [Google Scholar]
- de Sousa MVP, Kawakubo M, Ferraresi C, Kaippert B, Yoshimura EM, Hamblin MR (2018) Pain management using photobiomodulation: mechanisms, location, and repeatability quantified by pain threshold and neural biomarkers in mice. J Biophotonics 11(7):e201700370. 10.1002/jbio.201700370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean B (2023) Muscarinic M1 and M4 receptor agonists for schizophrenia: promising candidates for the therapeutic arsenal. Expert Opin Investig Drugs 32(12):1113–1121. 10.1080/13543784.2023.2288074 [DOI] [PubMed] [Google Scholar]
- Dehghanpour HR, Parvin P, Ganjali P, Golchini A, Eshghifard H, Heidari O (2023) Evaluation of photobiomodulation effect on cesarean-sectioned wound healing: a clinical study. Lasers Med Sci 38(1):171. 10.1007/s10103-023-03774-6 [DOI] [PubMed] [Google Scholar]
- Della Pietra A, Gómez Dabó L, Mikulenka P, Espinoza-Vinces C, Vuralli D, Baytekin I, Martelletti P, Giniatullin R (2024) Mechanosensitive receptors in migraine: a systematic review. J Headache Pain 25(1):6. 10.1186/s10194-023-01710-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingledine R, Borges K, Bowie D, Traynelis SF (1999) The glutamate receptor ion channels. Pharmacol Rev 51(1):7–61 [PubMed] [Google Scholar]
- do Nascimento THO, Pereira-Figueiredo D, Veroneze L, Nascimento AA, De Logu F, Nassini R, Campello-Costa P, Faria-Melibeu ADC, Monteiro S, de Araújo D, Calaza KC (2024) Functions of TRPs in retinal tissue in physiological and pathological conditions. Front Mol Neurosci 17:1459083. 10.3389/fnmol.2024.1459083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du W, Bautista JF, Yang H, Diez-Sampedro A, You SA, Wang L, Kotagal P, Lüders HO, Shi J, Cui J, Richerson GB, Wang QK (2005) Calcium-sensitive potassium channelopathy in human epilepsy and paroxysmal movement disorder. Nat Genet 37(7):733–738. 10.1038/ng1585 [DOI] [PubMed] [Google Scholar]
- Du G, Liu M, Qi Y, Lin M, Wu J, Xie W, Ren D, Du S, Jia T, Zhang F, Song W, Liu H (2024) BMP4 up-regulated by 630 nm LED irradiation is associated with the amelioration of rheumatoid arthritis. J Photochem Photobiol B 250:112828. 10.1016/j.jphotobiol.2023.112828 [DOI] [PubMed] [Google Scholar]
- Eglen RM, Choppin A, Watson N (2001) Therapeutic opportunities from muscarinic receptor research. Trends Pharmacol Sci 22(8):409–414. 10.1016/s0165-6147(00)01737-5 [DOI] [PubMed] [Google Scholar]
- Escalé MR, Sergeyev A, Geiss R, Grange R (2017) Nonlinear mode switching in lithium niobate nanowaveguides to control light directionality. Opt Express 25(4):3013–3023. 10.1364/oe.25.003013 [DOI] [PubMed] [Google Scholar]
- Feng Q (2014) Temperature sensing by thermal TRP channels: thermodynamic basis and molecular insights. Curr Top Membr 74:19–50. 10.1016/b978-0-12-800181-3.00002-6 [DOI] [PubMed] [Google Scholar]
- Figueiro Longo MG, Tan CO, Chan ST, Welt J, Avesta A, Ratai E, Mercaldo ND, Yendiki A, Namati J, Chico-Calero I, Parry BA, Drake L, Anderson R, Rauch T, Diaz-Arrastia R, Lev M, Lee J, Hamblin M, Vakoc B, Gupta R (2020) Effect of transcranial low-level light therapy vs sham therapy among patients with moderate traumatic brain injury: a randomized clinical trial. JAMA Netw Open 3(9):e2017337. 10.1001/jamanetworkopen.2020.17337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaggi NL, Collins KA, Gonzalez-Castillo J, Hurtado AM, Castellanos FX, Osorio R, Cassano P, Iosifescu DV (2024) Transcranial photobiomodulation increases intrinsic brain activity within irradiated areas in early Alzheimer’s disease: potential link with cerebral metabolism. Brain Stimul 17(2):208–210. 10.1016/j.brs.2024.02.012 [DOI] [PubMed] [Google Scholar]
- Ghaderi AH, Jahan A, Akrami F, Moghadam Salimi M (2021) Transcranial photobiomodulation changes topology, synchronizability, and complexity of resting state brain networks. J Neural Eng. 10.1088/1741-2552/abf97c [DOI] [PubMed] [Google Scholar]
- Gholami L, Afshar S, Arkian A, Saeidijam M, Hendi SS, Mahmoudi R, Khorsandi K, Hashemzehi H, Fekrazad R (2022) NIR irradiation of human buccal fat pad adipose stem cells and its effect on TRP ion channels. Lasers Med Sci 37(9):3681–3692. 10.1007/s10103-022-03652-7 [DOI] [PubMed] [Google Scholar]
- Goldsmith PJ (2019) NMDAR PAMs: multiple chemotypes for multiple binding sites. Curr Top Med Chem 19(24):2239–2253. 10.2174/1568026619666191011095341 [DOI] [PubMed] [Google Scholar]
- Golovynska I, Golovynskyi S, Stepanov YV, Garmanchuk LV, Stepanova LI, Qu J, Ohulchanskyy TY (2019) Red and near-infrared light induces intracellular Ca(2+) flux via the activation of glutamate N-methyl-d-aspartate receptors. J Cell Physiol 234(9):15989–16002. 10.1002/jcp.28257 [DOI] [PubMed] [Google Scholar]
- Golovynska I, Golovynskyi S, Stepanov YV, Stepanova LI, Qu J, Ohulchanskyy TY (2021) Red and near-infrared light evokes Ca(2+) influx, endoplasmic reticulum release and membrane depolarization in neurons and cancer cells. J Photochem Photobiol B 214:112088. 10.1016/j.jphotobiol.2020.112088 [DOI] [PubMed] [Google Scholar]
- Golovynska I, Stepanov YV, Golovynskyi S, Zhou T, Stepanova LI, Garmanchuk LV, Ohulchanskyy TY, Qu J (2022) Macrophages modulated by red/NIR light: phagocytosis, cytokines, mitochondrial activity, Ca(2+) influx. Membrane Depolarizat Viability Photochem Photobiol 98(2):484–497. 10.1111/php.13526 [DOI] [PubMed] [Google Scholar]
- González-Cota AL, Martínez-Flores D, Rosendo-Pineda MJ, Vaca L (2024) NMDA receptor-mediated Ca(2+) signaling: impact on cell cycle regulation and the development of neurodegenerative diseases and cancer. Cell Calcium 119:102856. 10.1016/j.ceca.2024.102856 [DOI] [PubMed] [Google Scholar]
- Govorunova EG, Gou Y, Sineshchekov OA, Li H, Lu X, Wang Y, Brown LS, St-Pierre F, Xue M, Spudich JL (2022) Kalium channelrhodopsins are natural light-gated potassium channels that mediate optogenetic inhibition. Nat Neurosci 25(7):967–974. 10.1038/s41593-022-01094-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gribkoff VK, Winquist RJ (2023) Potassium channelopathies associated with epilepsy-related syndromes and directions for therapeutic intervention. Biochem Pharmacol 208:115413. 10.1016/j.bcp.2023.115413 [DOI] [PubMed] [Google Scholar]
- Griffith LC (2001) Potassium channels: the importance of transport signals. Curr Biol 11(6):R226-228. 10.1016/s0960-9822(01)00111-7 [DOI] [PubMed] [Google Scholar]
- Gunthorpe MJ, Hannan SL, Smart D, Jerman JC, Arpino S, Smith GD, Brough S, Wright J, Egerton J, Lappin SC, Holland VA, Winborn K, Thompson M, Rami HK, Randall A, Davis JB (2007) Characterization of SB-705498, a potent and selective vanilloid receptor-1 (VR1/TRPV1) antagonist that inhibits the capsaicin-, acid-, and heat-mediated activation of the receptor. J Pharmacol Exp Ther 321(3):1183–1192. 10.1124/jpet.106.116657 [DOI] [PubMed] [Google Scholar]
- Gurung S, Agbaga MP, Myers DA (2016) Cognitive differences between Sprague-Dawley rats selectively bred for sensitivity or resistance to diet induced obesity. Behav Brain Res 311:122–130. 10.1016/j.bbr.2016.05.018 [DOI] [PubMed] [Google Scholar]
- Gutiérrez-Menéndez A, Martínez JA, Méndez M, Arias JL (2022) No effects of photobiomodulation on prefrontal cortex and hippocampal cytochrome C oxidase activity and expression of c-fos protein of young male and female rats. Front Neurosci 16:897225. 10.3389/fnins.2022.897225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamblin MR (2016) Shining light on the head: photobiomodulation for brain disorders. BBA Clin 6:113–124. 10.1016/j.bbacli.2016.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamblin MR (2018) Mechanisms and mitochondrial redox signaling in photobiomodulation. Photochem Photobiol 94(2):199–212. 10.1111/php.12864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamblin MR (2024) Transcranial photobiomodulation for the brain: a wide range of clinical applications. Neural Regen Res 19(3):483–484. 10.4103/1673-5374.380891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han DS, Lee CH, Shieh YD, Chen CC (2019) Involvement of substance P in the analgesic effect of low-level laser therapy in a mouse model of chronic widespread muscle pain. Pain Med 20(10):1963–1970. 10.1093/pm/pnz056 [DOI] [PubMed] [Google Scholar]
- Henderson TA, Morries LD (2015) Near-infrared photonic energy penetration: can infrared phototherapy effectively reach the human brain? Neuropsychiatr Dis Treat 11:2191–2208. 10.2147/ndt.S78182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennessy M, Hamblin MR (2017) Photobiomodulation and the brain: a new paradigm. J Opt 19(1):013003. 10.1088/2040-8986/19/1/013003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YY, Chen AC, Carroll JD, Hamblin MR (2009) Biphasic dose response in low level light therapy. Dose Response 7(4):358–383. 10.2203/dose-response.09-027.Hamblin [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YY, Gupta A, Vecchio D, de Arce VJ, Huang SF, Xuan W, Hamblin MR (2012) Transcranial low level laser (light) therapy for traumatic brain injury. J Biophotonics 5(11–12):827–837. 10.1002/jbio.201200077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YY, Nagata K, Tedford CE, Hamblin MR (2014) Low-level laser therapy (810 nm) protects primary cortical neurons against excitotoxicity in vitro. J Biophotonics 7(8):656–664. 10.1002/jbio.201300125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Tao J, Zhao R (2018) Potassium channels and CNS diseases. CNS Neurol Disord Drug Targets 17(4):245–247. 10.2174/187152731704180706105155 [DOI] [PubMed] [Google Scholar]
- Huang Z, Hamblin MR, Zhang Q (2024) Photobiomodulation in experimental models of Alzheimer’s disease: state-of-the-art and translational perspectives. Alzheimers Res Ther 16(1):114. 10.1186/s13195-024-01484-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignatov YD, Vislobokov AI, Vlasov TD, Kolpakova ME, Mel’nikov KN, Petrishchev IN (2005) Effects of helium-neon laser irradiation and local anesthetics on potassium channels in pond snail neurons. Neurosci Behav Physiol 35(8):871–875. 10.1007/s11055-005-0137-7 [DOI] [PubMed] [Google Scholar]
- Ishibashi N, Uta D, Sawahata M, Kume T (2024) Photobiomodulation transiently increases the spontaneous firing in the superficial layer of the rat spinal dorsal horn. Biochem Biophys Res Commun 729:150362. 10.1016/j.bbrc.2024.150362 [DOI] [PubMed] [Google Scholar]
- Jorratt P, Ricny J, Leibold C, Ovsepian SV (2023) Endogenous modulators of NMDA receptor control dendritic field expansion of cortical neurons. Mol Neurobiol 60(3):1440–1452. 10.1007/s12035-022-03147-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julius D, Basbaum AI (2001) Molecular mechanisms of nociception. Nature 413(6852):203–210. 10.1038/35093019 [DOI] [PubMed] [Google Scholar]
- Kampa BM, Clements J, Jonas P, Stuart GJ (2004) Kinetics of Mg2+ unblock of NMDA receptors: implications for spike-timing dependent synaptic plasticity. J Physiol 556(Pt 2):337–345. 10.1113/jphysiol.2003.058842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kariev AM, Green ME (2024) Water, protons, and the gating of voltage-gated potassium channels. Membranes (Basel). 10.3390/membranes14020037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khuman J, Zhang J, Park J, Carroll JD, Donahue C, Whalen MJ (2012) Low-level laser light therapy improves cognitive deficits and inhibits microglial activation after controlled cortical impact in mice. J Neurotrauma 29(2):408–417. 10.1089/neu.2010.1745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Choi S, Lee E, Koh W, Lee CJ (2024) Tonic NMDAR currents in the brain: regulation and cognitive functions. Biol Psychiatry. 10.1016/j.biopsych.2024.03.009 [DOI] [PubMed] [Google Scholar]
- Ladagu AD, Olopade FE, Adejare A, Olopade JO (2023) GluN2A and GluN2B N-methyl-d-aspartate receptor (NMDARs) subunits: their roles and therapeutic antagonists in neurological diseases. Pharmaceuticals (Basel). 10.3390/ph16111535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai H, Gao M, Yang H (2024) The potassium channels: neurobiology and pharmacology of tinnitus. J Neurosci Res 102(1):e25281. 10.1002/jnr.25281 [DOI] [PubMed] [Google Scholar]
- Lan CH, Wu YC, Chiang CC, Chang ST (2022) Effects of intravascular photobiomodulation on motor deficits and brain perfusion images in intractable myasthenia gravis: a case report. World J Clin Cases 10(24):8718–8727. 10.12998/wjcc.v10.i24.8718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanzafame RJ, Stadler I, Kurtz AF, Connelly R, Peter TA Sr, Brondon P, Olson D (2007) Reciprocity of exposure time and irradiance on energy density during photoradiation on wound healing in a murine pressure ulcer model. Lasers Surg Med 39(6):534–542. 10.1002/lsm.20519 [DOI] [PubMed] [Google Scholar]
- Li X, Liu J, Liang S, Guan K, An L, Wu X, Li S, Sun C (2013) Temporal modulation of sodium current kinetics in neuron cells by near-infrared laser. Cell Biochem Biophys 67(3):1409–1419. 10.1007/s12013-013-9674-9 [DOI] [PubMed] [Google Scholar]
- Li X, Liu J, Liang S, Sun C (2014) 980-nm infrared laser modulation of sodium channel kinetics in a neuron cell linearly mediated by photothermal effect. J Biomed Opt 19(10):105002. 10.1117/1.Jbo.19.10.105002 [DOI] [PubMed] [Google Scholar]
- Liebert A, Capon W, Pang V, Vila D, Bicknell B, McLachlan C, Kiat H (2023) Photophysical mechanisms of photobiomodulation therapy as precision medicine. Biomedicines. 10.3390/biomedicines11020237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim L (2024) Traumatic brain injury recovery with photobiomodulation: cellular mechanisms, clinical evidence, and future potential. Cells. 10.3390/cells13050385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P, Kong XY, Jiang L, Wen L (2024) Ion transport in nanofluidics under external fields. Chem Soc Rev. 10.1039/d3cs00367a [DOI] [PubMed] [Google Scholar]
- Ma H, Du Y, Xie D, Wei ZZ, Pan Y, Zhang Y (2024) Recent advances in light energy biotherapeutic strategies with photobiomodulation on central nervous system disorders. Brain Res 1822:148615. 10.1016/j.brainres.2023.148615 [DOI] [PubMed] [Google Scholar]
- Maiorov SA, Kairat BK, Berezhnov AV, Zinchenko VP, Gaidin SG, Kosenkov AM (2024) Peculiarities of ion homeostasis in neurons containing calcium-permeable AMPA receptors. Arch Biochem Biophys. 10.1016/j.abb.2024.109951 [DOI] [PubMed] [Google Scholar]
- Marques DP, Chacur M, Martins DO (2023) Photobiomodulation and vitamin B treatment alleviate both thermal and mechanical orofacial pain in rats. Photochem Photobiol Sci 22(10):2315–2327. 10.1007/s43630-023-00452-y [DOI] [PubMed] [Google Scholar]
- Martin SP, Leeman-Markowski BA (2023) Proposed mechanisms of tau: relationships to traumatic brain injury, Alzheimer’s disease, and epilepsy. Front Neurol 14:1287545. 10.3389/fneur.2023.1287545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazuqueli Pereira ESB, Basting RT, Abdalla HB, Garcez AS, Napimoga MH, Clemente-Napimoga JT (2021) Photobiomodulation inhibits inflammation in the temporomandibular joint of rats. J Photochem Photobiol B 222:112281. 10.1016/j.jphotobiol.2021.112281 [DOI] [PubMed] [Google Scholar]
- McDougall JJ, O’Brien MS (2024) Analgesic potential of voltage gated sodium channel modulators for the management of pain. Curr Opin Pharmacol 75:102433. 10.1016/j.coph.2024.102433 [DOI] [PubMed] [Google Scholar]
- McElroy DL, Sabir H, Glass AE, Greba Q, Howland JG (2024) The anterior retrosplenial cortex is required for short-term object in place recognition memory retrieval: role of ionotropic glutamate receptors in male and female long-evans rats. Eur J Neurosci. 10.1111/ejn.16284 [DOI] [PubMed] [Google Scholar]
- Meng C, Xia Q, Wu H, Huang H, Liu H, Li Y, Zhang F, Song W (2020) Photobiomodulation with 630-nm LED radiation inhibits the proliferation of human synoviocyte MH7A cells possibly via TRPV4/PI3K/AKT/mTOR signaling pathway. Lasers Med Sci 35(9):1927–1936. 10.1007/s10103-020-02977-5 [DOI] [PubMed] [Google Scholar]
- Mineroff J, Maghfour J, Ozog DM, Lim HW, Kohli I, Jagdeo J (2024) Photobiomodulation CME part II: clinical applications in dermatology. J Am Acad Dermatol. 10.1016/j.jaad.2023.10.074 [DOI] [PubMed] [Google Scholar]
- Miralles RM, Boscia AR, Kittur S, Hanflink JC, Panchal PS, Yorek MS, Deutsch TCJ, Reever CM, Vundela SR, Wengert ER, Patel MK (2024) Parvalbumin interneuron impairment causes synaptic transmission deficits and seizures in SCN8A developmental and epileptic encephalopathy. JCI Insight. 10.1172/jci.insight.181005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montazeri K, Farhadi M, Majdabadi A, Akbarnejad Z, Fekrazad R, Shahbazi A, Mahmoudian S (2024) Photobiomodulation therapy in improvement of harmful neural plasticity in sodium salicylate-induced tinnitus. PLoS ONE 19(4):e0296607. 10.1371/journal.pone.0296607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moos WH, Faller DV, Glavas IP, Kanara I, Kodukula K, Pernokas J, Pernokas M, Pinkert CA, Powers WR, Sampani K, Steliou K, Vavvas DG (2023) Epilepsy: mitochondrial connections to the ‘Sacred’ disease. Mitochondrion 72:84–101. 10.1016/j.mito.2023.08.002 [DOI] [PubMed] [Google Scholar]
- Morris HR, Spillantini MG, Sue CM, Williams-Gray CH (2024) The pathogenesis of Parkinson’s disease. Lancet 403(10423):293–304. 10.1016/s0140-6736(23)01478-2 [DOI] [PubMed] [Google Scholar]
- Muly SM, Gross JS, Potashner SJ (2004) Noise trauma alters D-[3H]aspartate release and AMPA binding in chinchilla cochlear nucleus. J Neurosci Res 75(4):585–596. 10.1002/jnr.20011 [DOI] [PubMed] [Google Scholar]
- Nanou E, El Manira A (2007) A postsynaptic negative feedback mediated by coupling between AMPA receptors and Na+-activated K+ channels in spinal cord neurones. Eur J Neurosci 25(2):445–450. 10.1111/j.1460-9568.2006.05287.x [DOI] [PubMed] [Google Scholar]
- Navarro G, Gómez-Autet M, Morales P, Rebassa JB, Llinas Del Torrent C, Jagerovic N, Pardo L, Franco R (2024) Homodimerization of CB(2) cannabinoid receptor triggered by a bivalent ligand enhances cellular signaling. Pharmacol Res 208:107363. 10.1016/j.phrs.2024.107363 [DOI] [PubMed] [Google Scholar]
- Negandhi J, Harrison AL, Allemang C, Harrison RV (2014) Time course of cochlear injury discharge (excitotoxicity) determined by ABR monitoring of contralateral cochlear events. Hear Res 315:34–39. 10.1016/j.heares.2014.06.002 [DOI] [PubMed] [Google Scholar]
- Neves LMS, Gonçalves ECD, Cavalli J, Vieira G, Laurindo LR, Simões RR, Coelho IS, Santos ARS, Marcolino AM, Cola M, Dutra RC (2018) Photobiomodulation therapy improves acute inflammatory response in mice: the role of cannabinoid receptors/ATP-sensitive K(+) channel/p38-MAPK signalling pathway. Mol Neurobiol 55(7):5580–5593. 10.1007/s12035-017-0792-z [DOI] [PubMed] [Google Scholar]
- Neves D, Salazar IL, Almeida RD, Silva RM (2023) Molecular mechanisms of ischemia and glutamate excitotoxicity. Life Sci 328:121814. 10.1016/j.lfs.2023.121814 [DOI] [PubMed] [Google Scholar]
- Oppermann J, Rozenberg A, Fabrin T, González-Cabrera C, Parker R, Béjà O, Prigge M, Hegemann P (2024) Robust optogenetic inhibition with red-light-sensitive anion-conducting channelrhodopsins. Elife. 10.7554/eLife.90100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oron U, Yaakobi T, Oron A, Hayam G, Gepstein L, Rubin O, Wolf T, Ben Haim S (2001) Attenuation of infarct size in rats and dogs after myocardial infarction by low-energy laser irradiation. Lasers Surg Med 28(3):204–211. 10.1002/lsm.1039 [DOI] [PubMed] [Google Scholar]
- Paoletti P, Neyton J (2007) NMDA receptor subunits: function and pharmacology. Curr Opin Pharmacol 7(1):39–47. 10.1016/j.coph.2006.08.011 [DOI] [PubMed] [Google Scholar]
- Paoletti P, Bellone C, Zhou Q (2013) NMDA receptor subunit diversity: impact on receptor properties, synaptic plasticity and disease. Nat Rev Neurosci 14(6):383–400. 10.1038/nrn3504 [DOI] [PubMed] [Google Scholar]
- Parent HH, Niswender CM (2024) Therapeutic potential for metabotropic glutamate receptor 7 (mGlu(7)) modulators in cognitive disorders. Mol Pharmacol. 10.1124/molpharm.124.000874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pires de Sousa MV, Ferraresi C, Kawakubo M, Kaippert B, Yoshimura EM, Hamblin MR (2016) Transcranial low-level laser therapy (810 nm) temporarily inhibits peripheral nociception: photoneuromodulation of glutamate receptors, prostatic acid phophatase, and adenosine triphosphate. Neurophotonics 3(1):015003. 10.1117/1.NPh.3.1.015003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pissulin CN, de Souza Castro PA, Codina F, Pinto CG, Vechetti-Junior IJ, Matheus SM (2017) GaAs laser therapy reestablishes the morphology of the NMJ and nAChRs after injury due to bupivacaine. J Photochem Photobiol B 167:256–263. 10.1016/j.jphotobiol.2016.12.024 [DOI] [PubMed] [Google Scholar]
- Powner MB, Jeffery G (2024) Light stimulation of mitochondria reduces blood glucose levels. J Biophotonics. 10.1002/jbio.202300521 [DOI] [PubMed] [Google Scholar]
- Razzaghi M, Sheibani F, Kimia N, Razzaghi Z, Chenari Z, Ashrafi F, Barati M, Advani S (2024) Photobiomodulation’s potential as a non-invasive therapy for Alzheimer’s disease and minimal cognitive impairment: a 12-week investigation. Photodiagnosis Photodyn Ther 46:103991. 10.1016/j.pdpdt.2024.103991 [DOI] [PubMed] [Google Scholar]
- Rhee YH, Moon JH, Jung JY, Oh C, Ahn JC, Chung PS (2019) Effect of photobiomodulation therapy on neuronal injuries by ouabain: the regulation of Na, K-ATPase; Src; and mitogen-activated protein kinase signaling pathway. BMC Neurosci 20(1):19. 10.1186/s12868-019-0499-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochkind S, Shainberg A (2013) Protective effect of laser phototherapy on acetylcholine receptors and creatine kinase activity in denervated muscle. Photomed Laser Surg 31(10):499–504. 10.1089/pho.2013.3537 [DOI] [PubMed] [Google Scholar]
- Rodríguez-Fernández L, Zorzo C, Arias JL (2024) Photobiomodulation in the aging brain: a systematic review from animal models to humans. Geroscience 46(6):6583–6623. 10.1007/s11357-024-01231-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Clavijo L, Martín-Suárez S (2023) The differential response to neuronal hyperexcitation and neuroinflammation of the hippocampal neurogenic niche. Front Neurosci 17:1186256. 10.3389/fnins.2023.1186256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salehpour F, Mahmoudi J, Kamari F, Sadigh-Eteghad S, Rasta SH, Hamblin MR (2018) Brain photobiomodulation therapy: a narrative review. Mol Neurobiol 55(8):6601–6636. 10.1007/s12035-017-0852-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarlo GL, Holton KF (2021) Brain concentrations of glutamate and GABA in human epilepsy: a review. Seizure 91:213–227. 10.1016/j.seizure.2021.06.028 [DOI] [PubMed] [Google Scholar]
- Savić AM, Novičić M, Ðorđević O, Konstantinović L, Miler-Jerković V (2023) Novel electrotactile brain-computer interface with somatosensory event-related potential based control. Front Hum Neurosci 17:1096814. 10.3389/fnhum.2023.1096814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schott K, Usher SG, Serra O, Carnevale V, Pless SA, Chua HC (2024) Unplugging lateral fenestrations of NALCN reveals a hidden drug binding site within the pore region. Proc Natl Acad Sci USA 121(22):e2401591121. 10.1073/pnas.2401591121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Q, Liu L, Gu X, Xing D (2021) Photobiomodulation suppresses JNK3 by activation of ERK/MKP7 to attenuate AMPA receptor endocytosis in Alzheimer’s disease. Aging Cell 20(1):e13289. 10.1111/acel.13289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sills GJ, Rogawski MA (2020) Mechanisms of action of currently used antiseizure drugs. Neuropharmacology 168:107966. 10.1016/j.neuropharm.2020.107966 [DOI] [PubMed] [Google Scholar]
- Sine SM (2012) End-plate acetylcholine receptor: structure, mechanism, pharmacology, and disease. Physiol Rev 92(3):1189–1234. 10.1152/physrev.00015.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sineshchekov OA, Jung KH, Spudich JL (2002) Two rhodopsins mediate phototaxis to low- and high-intensity light in Chlamydomonas reinhardtii. Proc Natl Acad Sci USA 99(13):8689–8694. 10.1073/pnas.122243399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer AP, Bieschke J, Friedrich RP, Zhu D, Wanker EE, Fecht HJ, Mereles D, Hunstein W (2012) 670 nm laser light and EGCG complementarily reduce amyloid-β aggregates in human neuroblastoma cells: basis for treatment of Alzheimer’s disease? Photomed Laser Surg 30(1):54–60. 10.1089/pho.2011.3073 [DOI] [PubMed] [Google Scholar]
- Souto-Neto JA, David DD, Zanetti G, Sua-Cespedes C, Freret-Meurer NV, Moraes MN, de Assis LVM, Castrucci AML (2024) Light-specific wavelengths differentially affect the exploration rate, opercular beat, skin color change, opsin transcripts, and the oxi-redox system of the longsnout seahorse Hippocampus reidi. Comp Biochem Physiol A Mol Integr Physiol 288:111551. 10.1016/j.cbpa.2023.111551 [DOI] [PubMed] [Google Scholar]
- Tang L, Jiang H, Sun M, Liu M (2023) Pulsed transcranial photobiomodulation generates distinct beneficial neurocognitive effects compared with continuous wave transcranial light. Lasers Med Sci 38(1):203. 10.1007/s10103-023-03865-4 [DOI] [PubMed] [Google Scholar]
- Taylor JL, Pritchard HAT, Walsh KR, Strangward P, White C, Hill-Eubanks D, Alakrawi M, Hennig GW, Allan SM, Nelson MT, Greenstein AS (2022) Functionally linked potassium channel activity in cerebral endothelial and smooth muscle cells is compromised in Alzheimer’s disease. Proc Natl Acad Sci USA 119(26):e2204581119. 10.1073/pnas.2204581119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson CH, Potet F, Abramova TV, DeKeyser JM, Ghabra NF, Vanoye CG, Millichap JJ, George AL (2023) Epilepsy-associated SCN2A (NaV1.2) variants exhibit diverse and complex functional properties. J Gen Physiol. 10.1085/jgp.202313375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uozumi Y, Nawashiro H, Sato S, Kawauchi S, Shima K, Kikuchi M (2010) Targeted increase in cerebral blood flow by transcranial near-infrared laser irradiation. Lasers Surg Med 42(6):566–576. 10.1002/lsm.20938 [DOI] [PubMed] [Google Scholar]
- Vaidya B, Padhy DS, Joshi HC, Sharma SS, Singh JN (2024) Ion channels and metal ions in Parkinson’s disease: historical perspective to the current scenario. Methods Mol Biol 2761:529–557. 10.1007/978-1-0716-3662-6_36 [DOI] [PubMed] [Google Scholar]
- Vallés AS, Barrantes FJ (2023) Nicotinic acetylcholine receptor dysfunction in addiction and in some neurodegenerative and neuropsychiatric diseases. Cells. 10.3390/cells12162051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasilenko T, Slezák M, Kovác I, Bottková Z, Jakubco J, Kostelníková M, Tomori Z, Gál P (2010) The effect of equal daily dose achieved by different power densities of low-level laser therapy at 635 and 670 nm on wound tensile strength in rats: a short report. Photomed Laser Surg 28(2):281–283. 10.1089/pho.2009.2489 [DOI] [PubMed] [Google Scholar]
- Ved HS, Doshi GM (2020) A review on emerging drug targets in treatment of schizophrenia. Curr Drug Targets 21(15):1593–1605. 10.2174/1389450121666200615150429 [DOI] [PubMed] [Google Scholar]
- Wang B, Rothberg BS, Brenner R (2009) Mechanism of increased BK channel activation from a channel mutation that causes epilepsy. J Gen Physiol 133(3):283–294. 10.1085/jgp.200810141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Huang YY, Wang Y, Lyu P, Hamblin MR (2017a) Photobiomodulation of human adipose-derived stem cells using 810 and 980 nm lasers operates via different mechanisms of action. Biochim Biophys Acta Gen Subj 1861(2):441–449. 10.1016/j.bbagen.2016.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Huang YY, Wang Y, Lyu P, Hamblin MR (2017b) Red (660 nm) or near-infrared (810 nm) photobiomodulation stimulates, while blue (415 nm), green (540 nm) light inhibits proliferation in human adipose-derived stem cells. Sci Rep 7(1):7781. 10.1038/s41598-017-07525-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W, Wang Y, Liu Y, Guo H, Li Z, Zou W, Liu J, Song Z (2023) TRPA1 promotes UVB-induced skin pigmentation by regulating melanosome luminal pH. Exp Dermatol 32(2):165–176. 10.1111/exd.14693 [DOI] [PubMed] [Google Scholar]
- Yan X, Liu J, Zhang Z, Li W, Sun S, Zhao J, Dong X, Qian J, Sun H (2017) Low-level laser irradiation modulates brain-derived neurotrophic factor mRNA transcription through calcium-dependent activation of the ERK/CREB pathway. Lasers Med Sci 32(1):169–180. 10.1007/s10103-016-2099-0 [DOI] [PubMed] [Google Scholar]
- Yoo M, Koo H, Kim M, Kim HI, Kim S (2013) Near-infrared stimulation on globus pallidus and subthalamus. J Biomed Opt 18(12):128005. 10.1117/1.Jbo.18.12.128005 [DOI] [PubMed] [Google Scholar]
- Zhang D, Shen Q, Wu X, Xing D (2021) Photobiomodulation therapy ameliorates glutamatergic dysfunction in mice with chronic unpredictable mild stress-induced depression. Oxid Med Cell Longev 2021:6678276. 10.1155/2021/6678276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou T, Ohulchanskyy TY, Qu J (2021) Effect of NIR light on the permeability of the blood-brain barriers in in vitro models. Biomed Opt Express 12(12):7544–7555. 10.1364/boe.438445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zierath D, Mizuno S, Barker-Haliski M (2023) Frontline sodium channel-blocking antiseizure medicine use promotes future onset of drug-resistant chronic seizures. Int J Mol Sci. 10.3390/ijms24054848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorzo C, Rodríguez-Fernández L, Martínez JA, Arias JL (2024) Photobiomodulation increases brain metabolic activity through a combination of 810 and 660 wavelengths: a comparative study in male and female rats. Lasers Med Sci 39(1):26. 10.1007/s10103-023-03966-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zupin L, Ottaviani G, Rupel K, Biasotto M, Zacchigna S, Crovella S, Celsi F (2019) Analgesic effect of photobiomodulation therapy: an in vitro and in vivo study. J Biophotonics 12(10):e201900043. 10.1002/jbio.201900043 [DOI] [PubMed] [Google Scholar]
- Zupin L, Barbi E, Sagredini R, Ottaviani G, Crovella S, Celsi F (2021) In vitro effects of photobiomodulation therapy on 50B11 sensory neurons: evaluation of cell metabolism, oxidative stress, mitochondrial membrane potential (MMP), and capsaicin-induced calcium flow. J Biophotonics 14(2):e202000347. 10.1002/jbio.202000347 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request. This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-Non-Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, if appropriate credit is given and the new creations are licensed under the identical terms.