Abstract
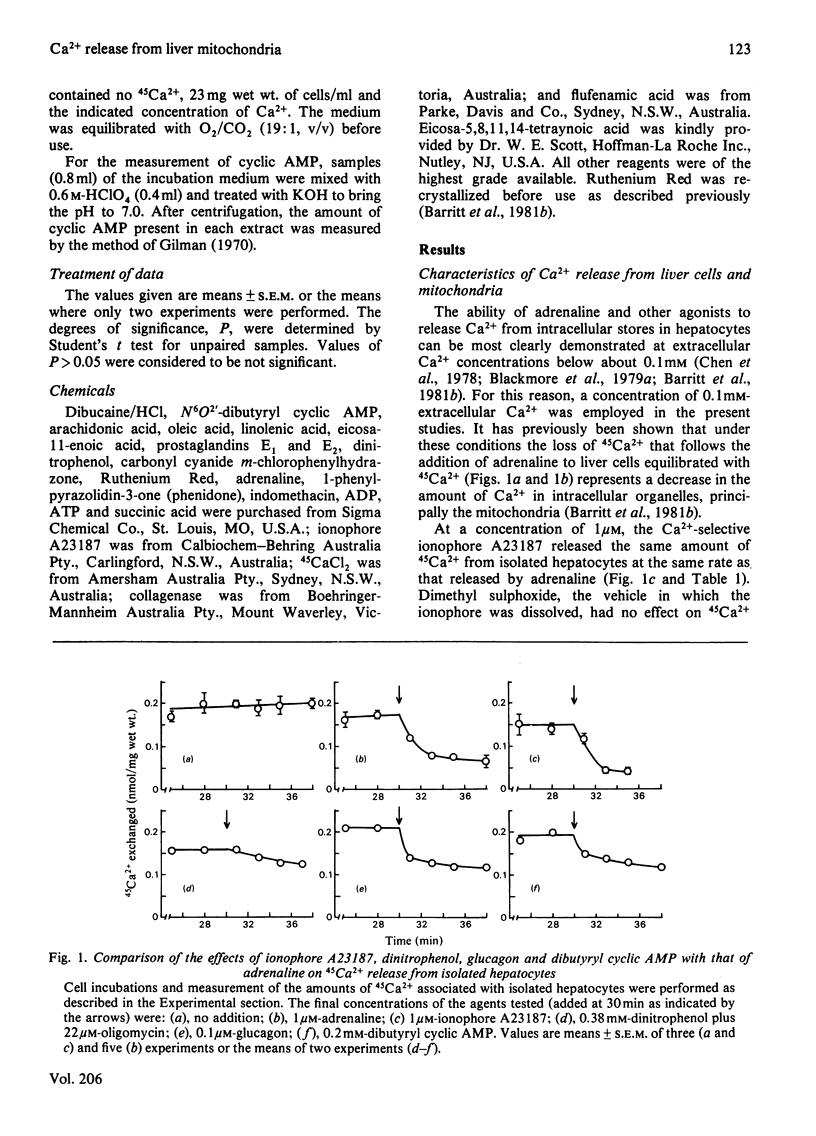
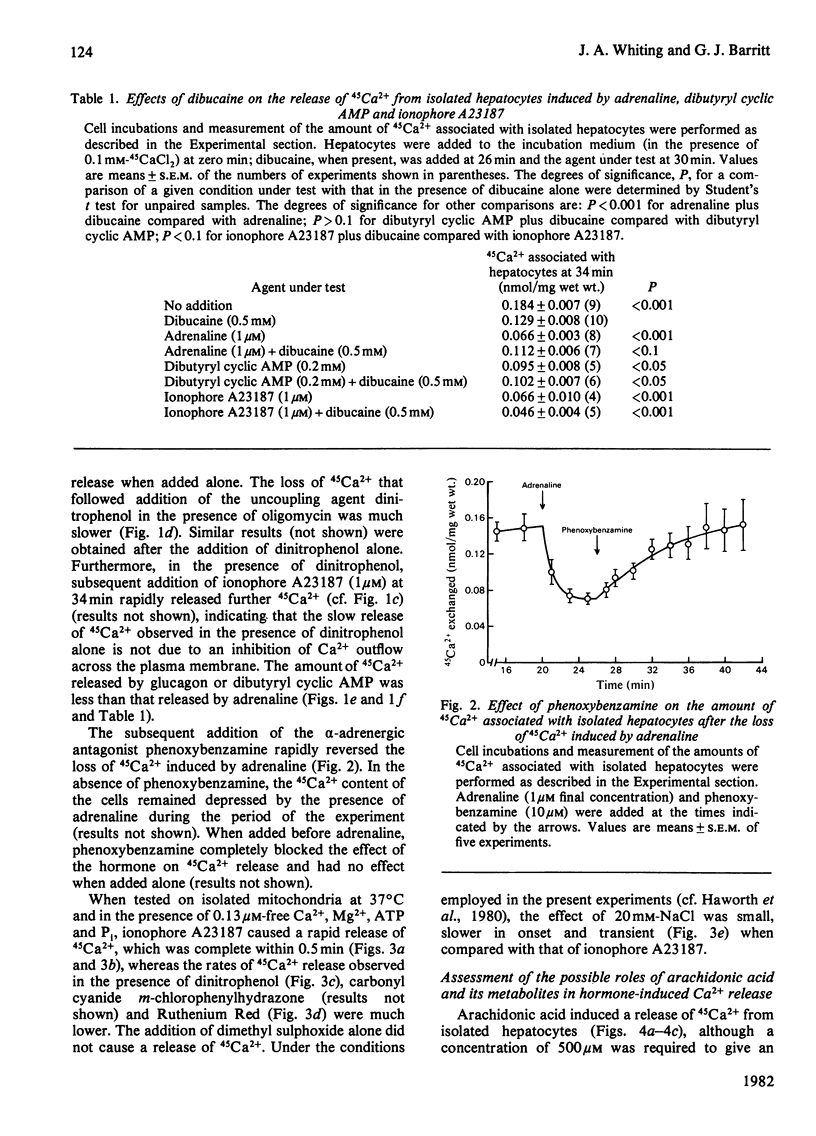
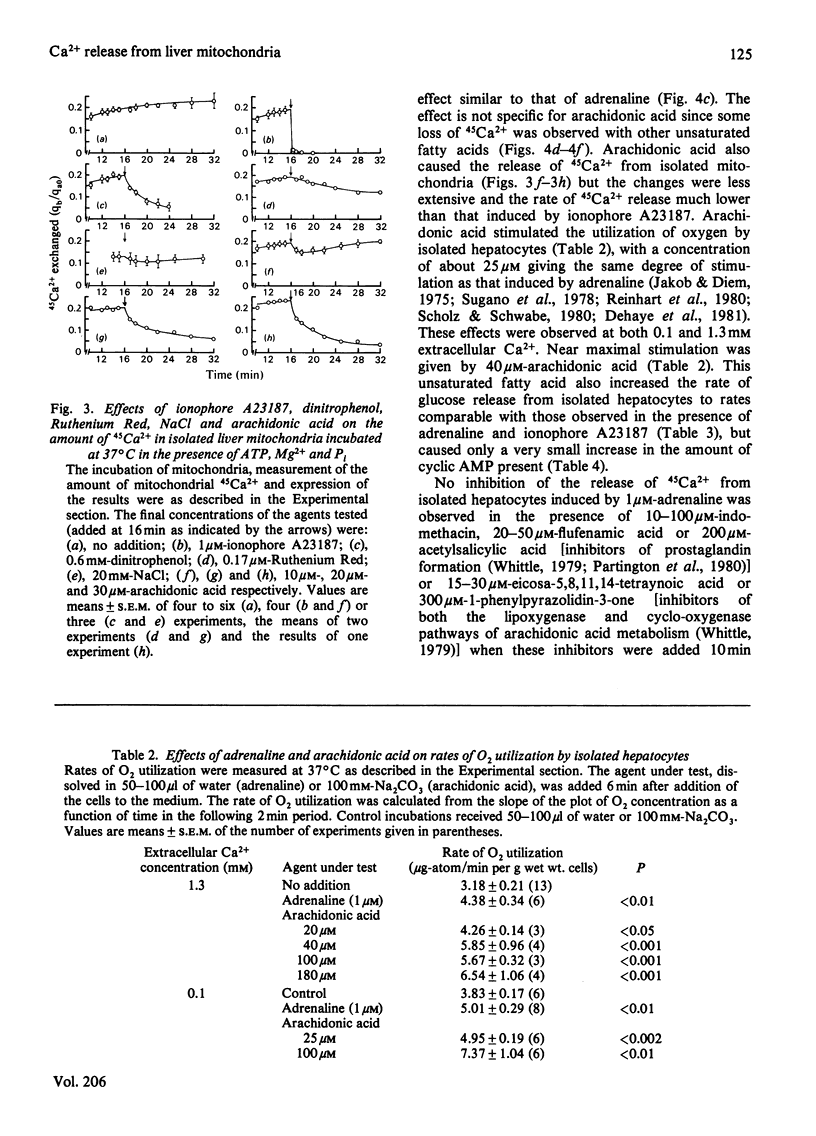
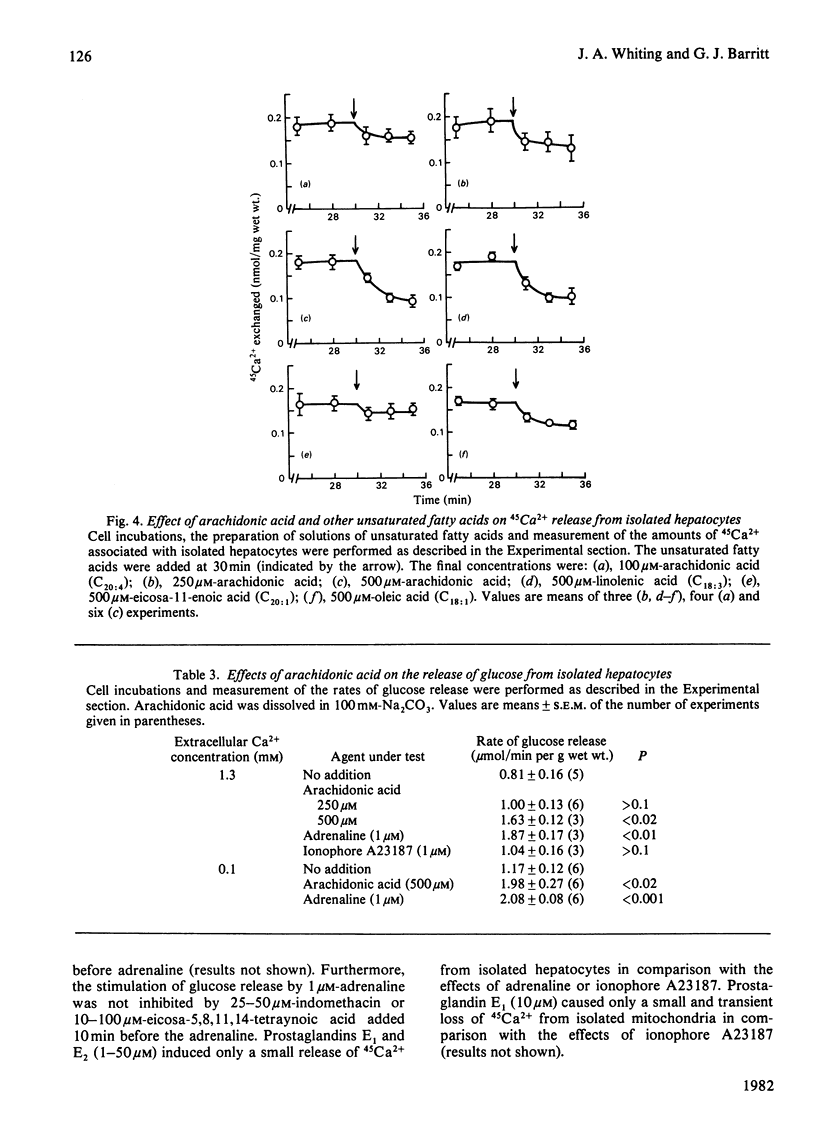
1. The abilities of dinitrophenol, NaCl, Ruthenium Red and the Ca2+-selective ionophore A23187 to release 45 Ca2+ from isolated hepatocytes and liver mitochondria (incubated at 37 degrees C in the presence of 0.1 microM-free Ca2+, Mg2+, ATP and phosphate ions) were compared with the action of adrenaline on 45Ca2+ release from isolated hepatocytes. The effects of adrenaline were most closely described by those of the ionophore A23187. 2. In isolated hepatocytes, a release of 45Ca2+ and stimulation of O2 utilization similar to that induced by adrenaline was observed in the presence of 500 and 20 microM-arachidonic acid respectively. The effect of arachidonic acid on 45Ca2+ release was not specific for this unsaturated fatty acid. 3. Inhibitors of arachidonic acid metabolism, including indomethacin and eicosa-5,811,14-tetraynoic acid, did not block the effects of adrenaline on 45Ca2+ or glucose release from isolated hepatocytes. 4. The ability of adrenaline to stimulate 45Ca2+ release from isolated hepatocytes was rapidly reversed after the subsequent addition of phenoxybenzamine to the cell suspension, and was completely blocked by 0.5 mM-dibucaine. 5. The results are consistent with the action of a Ca2+-selective ionophore in the mechanism by which adrenaline induces the release of Ca2+ from mitochondria in the liver cell and indicate that it is unlikely that arachidonic acid or a metabolite of arachidonic acid is involved in this process.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Althaus-Salzmann M., Carafoli E., Jakob A. Ca2+, K+ redistributions and alpha-adrenergic activation of glycogenolysis in perfused rat livers. Eur J Biochem. 1980 May;106(1):241–248. doi: 10.1111/j.1432-1033.1980.tb06015.x. [DOI] [PubMed] [Google Scholar]
- Babcock D. F., Chen J. L., Yip B. P., Lardy H. A. Evidence for mitochondrial localization of the hormone-responsive pool of Ca2+ in isolated hepatocytes. J Biol Chem. 1979 Sep 10;254(17):8117–8120. [PubMed] [Google Scholar]
- Barritt G. J., Dalton K. A., Whiting J. A. Evidence that phosphatidic acid stimulates the uptake of calcium by liver cells but not calcium release from mitochondria. FEBS Lett. 1981 Mar 23;125(2):137–140. doi: 10.1016/0014-5793(81)80703-x. [DOI] [PubMed] [Google Scholar]
- Barritt G. J. Evidence for two compartments of exchangeable calcium in isolated rat liver mitochondria obtained using a 45Ca exchange technique in the presence of magnesium, phosphate, and ATPase at 37 degrees C. J Membr Biol. 1981;62(1-2):53–63. doi: 10.1007/BF01870199. [DOI] [PubMed] [Google Scholar]
- Berry M. N., Friend D. S. High-yield preparation of isolated rat liver parenchymal cells: a biochemical and fine structural study. J Cell Biol. 1969 Dec;43(3):506–520. doi: 10.1083/jcb.43.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billah M. M., Michell R. H. Phosphatidylinositol metabolism in rat hepatocytes stimulated by glycogenolytic hormones. Effects of angiotensin, vasopressin, adrenaline, ionophore A23187 and calcium-ion deprivation. Biochem J. 1979 Sep 15;182(3):661–668. doi: 10.1042/bj1820661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackmore P. F., Dehaye J. P., Exton J. H. Studies on alpha-adrenergic activation of hepatic glucose output. The role of mitochondrial calcium release in alpha-adrenergic activation of phosphorylase in perfused rat liver. J Biol Chem. 1979 Aug 10;254(15):6945–6950. [PubMed] [Google Scholar]
- Blackmore P. F., El-Refai M. F., Dehaye J. P., Strickland W. G., Hughes B. P., Exton J. H. Blockade of hepatic alpha-adrenergic receptors and responses by chlorpromazine and trifluoperazine. FEBS Lett. 1981 Jan 26;123(2):245–248. doi: 10.1016/0014-5793(81)80298-0. [DOI] [PubMed] [Google Scholar]
- Blackmore P. F., El-Refai M. F., Exton J. H. alpha-Adrenergic blockade and inhibition of A23187 mediated Ca2+ uptake by the calcium antagonist verapamil in rat liver cells. Mol Pharmacol. 1979 May;15(3):598–606. [PubMed] [Google Scholar]
- Blackmore P. F., Exton J. H. Alpha 1-Adrenergic stimulation of Ca2+ mobilization without phosphorylase activation in hepatocytes from phosphorylase b kinase-deficient gsd/gsd rats. Biochem J. 1981 Aug 15;198(2):379–383. doi: 10.1042/bj1980379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. L., Babcock D. F., Lardy H. A. Norepinephrine, vasopressin, glucagon, and A23187 induce efflux of calcium from an exchangeable pool in isolated rat hepatocytes. Proc Natl Acad Sci U S A. 1978 May;75(5):2234–2238. doi: 10.1073/pnas.75.5.2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherrington A. D., Assimacopoulos F. D., Harper S. C., Corbin J. D., Park C. R., Exton J. H. Studies on the alpha-andrenergic activation of hepatic glucose output. II. Investigation of the roles of adenosine 3':5'-monophosphate and adenosine 3':5'-monophosphate-dependent protein kinase in the actions of phenylephrine in isolated hepatocytes. J Biol Chem. 1976 Sep 10;251(17):5209–5218. [PubMed] [Google Scholar]
- Exton J. H. Mechanisms involved in alpha-adrenergic phenomena: role of calcium ions in actions of catecholamines in liver and other tissues. Am J Physiol. 1980 Jan;238(1):E3–12. doi: 10.1152/ajpendo.1980.238.1.E3. [DOI] [PubMed] [Google Scholar]
- Gilman A. G. A protein binding assay for adenosine 3':5'-cyclic monophosphate. Proc Natl Acad Sci U S A. 1970 Sep;67(1):305–312. doi: 10.1073/pnas.67.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon L. M., Dipple I., Sauerheber R. D., Esgate J. A., Houslay M. D. The selective effects of charged local anaesthetics on the glucagon- and fluoride-stimulated adenylate cyclase activity of rat-liver plasma membranes. J Supramol Struct. 1980;14(1):21–32. doi: 10.1002/jss.400140104. [DOI] [PubMed] [Google Scholar]
- Hamberg M., Svensson J., Samuelsson B. Thromboxanes: a new group of biologically active compounds derived from prostaglandin endoperoxides. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2994–2998. doi: 10.1073/pnas.72.8.2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haworth R. A., Hunter D. R., Berkoff H. A. Na+ releases Ca2+ from liver, kidney and lung mitochondria. FEBS Lett. 1980 Feb 11;110(2):216–218. doi: 10.1016/0014-5793(80)80076-7. [DOI] [PubMed] [Google Scholar]
- Hughes B. P., Barritt G. J. Effects of glucagon and N6O2'-dibutyryladenosine 3':5'-cyclic monophosphate on calcium transport in isolated rat liver mitochondria. Biochem J. 1978 Oct 15;176(1):295–304. doi: 10.1042/bj1760295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes B. P., Blackmore P. F., Exton J. H. Exploration of the role of sodium in the alpha-adrenergic regulation of hepatic glycogenolysis. FEBS Lett. 1980 Dec 1;121(2):260–264. doi: 10.1016/0014-5793(80)80357-7. [DOI] [PubMed] [Google Scholar]
- Jakob A., Diem S. Metabolic responses of perfused rat livers to alpha- and beta-adrenergic agonists, glucagon and cyclic AMP. Biochim Biophys Acta. 1975 Sep 8;404(1):57–66. doi: 10.1016/0304-4165(75)90147-6. [DOI] [PubMed] [Google Scholar]
- Lehninger A. L., Vercesi A., Bababunmi E. A. Regulation of Ca2+ release from mitochondria by the oxidation-reduction state of pyridine nucleotides. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1690–1694. doi: 10.1073/pnas.75.4.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall P. J., Boatman D. E., Hokin L. E. Direct demonstration of the formation of prostaglandin E2 due to phosphatidylinositol breakdown associated with stimulation of enzyme secretion in the pancreas. J Biol Chem. 1981 Jan 25;256(2):844–847. [PubMed] [Google Scholar]
- Marshall P. J., Dixon J. F., Hokin L. E. Evidence for a role in stimulus--secretion coupling of prostaglandins derived from release of arachidonoyl residues as a result of phosphatidylinositol breakdown. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3292–3296. doi: 10.1073/pnas.77.6.3292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore C. L. Specific inhibition of mitochondrial Ca++ transport by ruthenium red. Biochem Biophys Res Commun. 1971 Jan 22;42(2):298–305. doi: 10.1016/0006-291x(71)90102-1. [DOI] [PubMed] [Google Scholar]
- Murphy E., Coll K., Rich T. L., Williamson J. R. Hormonal effects on calcium homeostasis in isolated hepatocytes. J Biol Chem. 1980 Jul 25;255(14):6600–6608. [PubMed] [Google Scholar]
- Nemeth E. F., Douglas W. W. Differential inhibitory effects of the arachidonic acid analog ETYA on rat mast cell exocytosis evoked by secretagogues utilizing cellular or extracellular calcium. Eur J Pharmacol. 1980 Oct 31;67(4):439–450. doi: 10.1016/0014-2999(80)90185-5. [DOI] [PubMed] [Google Scholar]
- Orly J., Schramm M. Fatty acids as modulators of membrane functions: catecholamine-activated adenylate cyclase of the turkey erythrocyte. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3433–3437. doi: 10.1073/pnas.72.9.3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partington C. R., Edwards M. W., Daly J. W. Regulation of cyclic AMP formation in brain tissue by alpha-adrenergic receptors: requisite intermediacy of prostaglandins of the E series. Proc Natl Acad Sci U S A. 1980 May;77(5):3024–3028. doi: 10.1073/pnas.77.5.3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poggioli J., Berthon B., Claret M. Calcium movements in in situ mitochondria following activation of alpha-adrenergic receptors in rat liver cells. FEBS Lett. 1980 Jun 30;115(2):243–246. doi: 10.1016/0014-5793(80)81178-1. [DOI] [PubMed] [Google Scholar]
- Prpić V., Spencer T. L., Bygrave F. L. Stable enhancement of calcium retention in mitochondria isolated from rat liver after the administration of glucagon to the intact animal. Biochem J. 1978 Dec 15;176(3):705–714. doi: 10.1042/bj1760705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhart P. H., Taylor W. M., Bygrave F. L. Trifluoperazine, an inhibitor of calmodulin action, antagonises phenylephrine-induced metabolic responses and mitochondrial calcium fluxes in liver. FEBS Lett. 1980 Oct 20;120(1):71–74. doi: 10.1016/0014-5793(80)81049-0. [DOI] [PubMed] [Google Scholar]
- Roman I., Gmaj P., Nowicka C., Angielski S. Regulation of Ca2+ efflux from kidney and liver mitochondria by unsaturated fatty acids and Na+ ions. Eur J Biochem. 1979 Dec 17;102(2):615–623. doi: 10.1111/j.1432-1033.1979.tb04279.x. [DOI] [PubMed] [Google Scholar]
- Sies H., Graf P., Estrela J. M. Hepatic calcium efflux during cytochrome P-450-dependent drug oxidations at the endoplasmic reticulum in intact liver. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3358–3362. doi: 10.1073/pnas.78.6.3358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugano T., Suda K., Shimada M., Oshino N. Biochemical and ultrastructural evaluation of isolated rat liver systems perfused with a hemoglobin-free medium. J Biochem. 1978 Apr;83(4):995–1007. doi: 10.1093/oxfordjournals.jbchem.a132028. [DOI] [PubMed] [Google Scholar]
- Vanderhoek J. Y., Feinstein M. B. Local anesthetics, chlorpromazine and propranolol inhibit stimulus-activation of phospholipase A2 in human platelets. Mol Pharmacol. 1979 Jul;16(1):171–180. [PubMed] [Google Scholar]
- Whittle B. J. Prostaglandin synthetase inhibitors. Drugs which affect arachidonic acid metabolism. Acta Obstet Gynecol Scand Suppl. 1979;87:21–26. doi: 10.3109/00016347909157785. [DOI] [PubMed] [Google Scholar]


