Abstract
Photothermal catalysis is a promising strategy to combine the advantages of both thermal-catalysis and photocatalysis. Herein we achieve the protolignin conversion to aromatics via the photothermal catalytic transfer hydrogenolysis process intensified by the in-situ protection strategy. The Pd/TiO2 at 140 °C with UV irradiation can catalyze the reforming of primary alcohols to aldehydes and active H* species, which further participate in the acetalation protection of the 1,3-diol group of β-O-4 linkage and mediate the hydrogenolysis of Cβ–OAr bonds, respectively. The conversion of birch sawdust with ethanol as the hydrogen donor provides a 40 wt% yield of phenolic monomers, compared with an 11 wt% monomer yield obtained from the conversion of extracted 1,3-diol-protected lignin under the same conditions. The synergistic effect of photocatalysis and thermal-catalysis contributes to the prior cleavage of the Cβ–OAr bond before other C–O bonds. The feasibility of solar-light-driven photothermal catalytic system is demonstrated.
Subject terms: Photocatalysis, Heterogeneous catalysis, Catalytic mechanisms
Photothermal catalysis has emerged as a promising strategy to combine the advantages of single thermal catalysis with that of photocatalysis. Herein, the authors report the protolignin direct conversion to aromatics via the photothermal catalytic transfer hydrogenolysis process intensified by the in-situ protection strategy.
Introduction
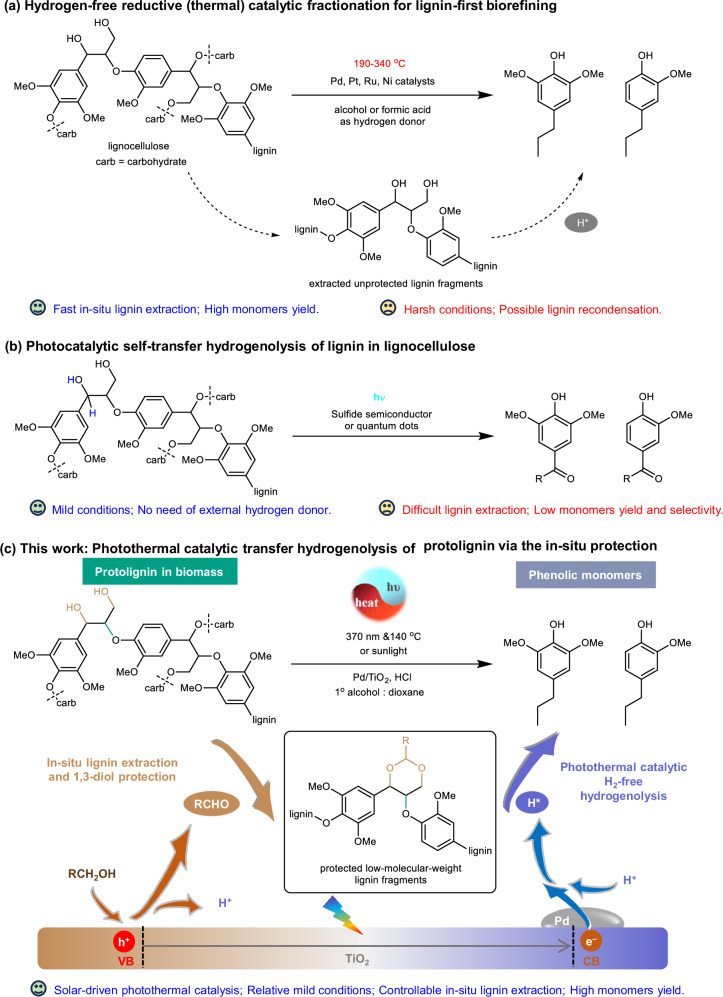
Lignin, the third largest biomass resource containing abundant functionalized aromatic structures, has received much attention as it can be potentially used to prepare the functionalized aromatic chemicals1–4, which could reduce reliance on fossil-based resources. Focusing on the catalytic lignin conversion to chemicals, the key issue lies in the development of strategies and catalysts to selectively cleave the stable and ubiquitous C–O/C–C bonds, and meanwhile to keep the aromaticity unconverted5–9. In nature, the lignin, cellulose, and hemicellulose are intricately cross-linked to constitute the lignocellulose in wood and grass. Although the transformation of isolated lignin can avoid the interference of polysaccharides, the lignin extraction under harsh conditions can simultaneously cause the breakup of the intrinsic β-O-4 linkage and the nonnegligible condensation reactions via the formation of recalcitrant C–C bonds10–12, which will prevent the further sufficient depolymerization of lignin. Therefore, compared with the isolated lignin conversion, the direct transformation of protolignin from raw lignocellulosic biomass to chemicals is appealing but more challenging, especially for the preferential conversion of lignin and retention of cellulose based on the concept of lignin-first biorefinery13–17.
Compared with oxidation18,19, reduction20,21, hydrolysis22,23, and other depolymerization methods24 or combined strategies25–29, catalytic hydrogenolysis30–38 including transfer hydrogenolysis39–43 featured a higher yield of phenolic monomers. For example, Li44 reported that the Ni-W2C-mediated hydrogenolysis of birch powders at 235 °C and 6 MPa H2 could efficiently deliver aliphatic diols (75.6 wt%) and monophenols (45.6 wt%). The high-temperature hydrogenolysis process has obvious advantages in converting all main components. Still, the goal of the lignin-first biorefinery with lignin preferential conversion requires further development of systems to regulate the hydrogenolysis capacity and selectivity. Compared with the high-temperature hydrogenolysis with excess H2 to directly generate active H* on the catalyst surface, the H* generation through the hydrogen transfer mechanism involving a higher Ea could be a choice39–41. We once reported that Ni/AC could catalyze fragmentation-hydrogenolysis of birch sawdust to provide a 50 wt% lignin conversion and a 97% selectivity toward phenolic monomers at 200 °C with methanol solvent under argon atmosphere14, during which the typical lignin oligomers were firstly generated from the wood solvolysis and underwent further transfer hydrogenolysis with C–O bond cleavage to form phenolic monomers. The quick lignin release from solid biomass at 200 °C led to a high concentration of the lignin fragments14, which was not only critical for further lignin depolymerization but also conducive to the corresponding condensation reactions among lignin fragments45 (Fig. 1a). In addition, the high temperature could also cause high energy consumption and over-hydrogenation with aromatic ring breakdown. Although decreasing the temperature to 120~140 °C can ensure the lignin extraction from solid biomass, it will affect the generation and performance of the active H* on the catalyst surface, making the relatively low temperature unfavorable to the overall lignin transformation. One potential method to restrain the side reactions at a high temperature is employing the flowthrough system with the tubular reactor46,47, which involves pre-extraction-fragmentation of lignin from solid biomass to lignin oligomers with hot alcohol and allows the reaction time of the following hydrogenolysis to be precisely controlled. However, to obtain a high reaction selectivity by controlling the reaction time before the total conversion, it is usually necessary to sacrifice a certain amount of conversion. As another method to regulate the reduction capacity, the semiconductor-mediated lignin photocatalytic hydrogen transfer depolymerization could be an alternative48–54, during which the photo-generated holes (h+) and electrons (e−) can oxidize the hydrogen donor and reduce proton to the active H*, respectively. Nevertheless, the current photocatalytic transformation methods at the near-room temperature delivered a modest yield of monomers due to the difficult solvolysis of lignin or the bad collision contact between catalyst and protolignin (Fig. 1b), especially for the internal protolignin below the solid biomass surface, which requires a certain temperature to achieve the dissolution and initial fragmentation of lignin from the solid lignocellulose.
Fig. 1. A comparison of catalytic methods for the transfer hydrogenolysis of protolignin in lignocellulose.
a Thermal catalytic methods. b Photocatalytic methods. c Our developed photothermal catalytic method. The advantage and disadvantage are denoted as smile mark and sad marks, respectively.
Given the above contradiction, one potential and simple improvement strategy is combining the advantages of photocatalytic systems and thermal catalytic systems, achieving the efficient extraction-fragmentation-conversion of protolignin by a photothermal catalytic transfer hydrogenolysis system at a relatively mild temperature with light irradiation. In the proposed photo-thermal system, a mild but high enough temperature can ensure the release of lignin, and light irradiation over the semiconductor catalyst can induce the oxidation of sacrifice reagent by the hole (h+) and provide active H* species from proton and electron (e−) for the consequent hydrogenolysis of lignin linkages, which can overcome the challenge in efficient generation of active H* under a mild condition for the thermal catalysis and the challenge in efficient extraction of lignin from solid wood powder during the traditional photocatalysis. At the same time, the release of lignin from lignocellulosic solids can be controlled to a certain extent by lowering the temperature appropriately, which may decrease the condensation of lignin fragments.
Here we show the photothermal catalytic transfer hydrogenolysis system for the protolignin conversion (Fig. 1c). After the reaction condition optimization, it is found that the Pd/TiO2 could efficiently catalyze the transfer hydrogenolysis of protolignin in birch sawdust under a relatively mild condition with 370 nm light irradiation at 140 °C, which provides a 40 wt% monophenols (including 30 wt% yield of propyl-substituted phenols) by using primary alcohols as hydrogen donor. Compared to reported works on lignin and protolignin valorization via transfer hydrogenolysis, this photothermal system delivers a close to theoretical yield of monomers under milder conditions (Supplementary Table S1, for more information). At the same time, the roles of the primary alcohol are proposed, which include the solvent in combination with dioxane for the lignin extraction, the hydrogen donor for the photothermal catalytic transfer hydrogenolysis of released lignin, and the precursor of the aldehyde as the protecting agent of diol structures in lignin. Besides, the released lignin from the mild in-situ solvolysis has a lower molecular weight than externally extracted lignin, which makes the conversion of the in-situ extracted protolignin more efficient than the extracted lignin under the same conditions. Furthermore, the synergistic effect of photocatalysis and thermal catalysis in this process is supported by controlled experiments. The heating not only mediates the extraction-fragmentation of lignin but also affects the photocatalytic reaction mechanism of β-O-4 linkage cleavage. In brief, we realize the efficient photothermal catalytic transfer hydrogenolysis of protolignin to aromatics intensified by the in-situ extraction-protection strategy, which could overcome the shortcomings of single thermal catalysis or photocatalysis.
Results
Photothermal catalytic transfer hydrogenolysis of lignin β-O-4 model
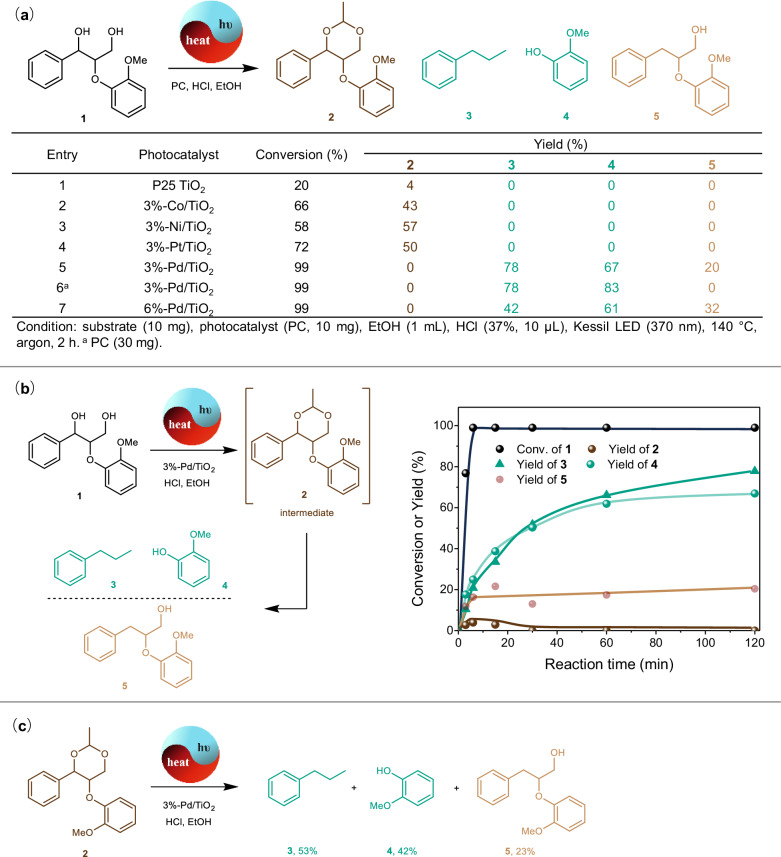
According to previous reports10–12, the aldehyde additive can protect the 1,3-diol structure from condensation and greatly increase the monomers yield in the lignin hydrogenolysis. In our proposed photothermal system, the aldehyde can be generated from the photocatalytic oxidation of primary alcohol when the alcohol is utilized as the hydrogen donor, so the aldehyde-mediated 1,3-diol protection and selective lignin hydrogenolysis could be realized in one pot. Based on this conjecture, the photothermal catalytic transfer hydrogenolysis of the lignin β-O-4 diol model 1 was tested under 370 nm light irradiation at 140 °C, during which HCl was added to accelerate the potential diol acetalation mediated by the acetaldehyde from the hydrogen donor-ethanol (Fig. 2a). The acetal product 2 was then observed when TiO2 was used as the photocatalyst (Fig. 2a, entry 1), which verified the feasibility of 1,3-diol protection under photothermal catalytic conditions. To further accelerate the generation of active H* species for the β-O-4 ether linkage cleavage, the metal cocatalysts including Co, Ni, Pt, and Pd particles were loaded on the TiO2 surface, respectively. It was found that the Co, Ni, and Pt cocatalysts just promoted the formation of 2 (Fig. 2a, entries 2–4) and did not initiate the critical Cβ–OAr bond cleavage, which could be attributed to their ability to promote the ethanol dehydrogenation to acetaldehyde and then the acetalation of diol 1 to acetal 2 was accelerated. Besides ethanol, the utilization of other primary alcohols (1-propanol and 1-butanol) as the hydrogen donor could also provide corresponding acetal products using Pt/TiO2 as the photocatalyst (Supplementary Figs. S1 and S2). However, when the Pd particle (3 wt%) was used as the co-catalyst (Fig. 2a, entry 5), the transfer hydrogenolysis of 1 with Cβ–OAr bond cleavage was aroused, which provided a 78% yield of n-propylbenzene 3 and a 67% yield of guaiacol 4 as the main products. At the same time, a 20% yield of byproduct 5 without Cβ–OAr bond cleavage but with benzylic Cα–O bond hydrogenolysis cleavage was obtained. No other byproducts can be identified in the gas chromatography data (Supplementary Figs. S3 and S4). Following reaction optimization results showed that the yield of guaiacol 4 could be increased to 83% and the generation of byproduct 5 could be avoided by adding twice as much photocatalyst (Fig. 2a, entry 6). The structure and morphology characterizations (Supplementary Figs. S5–S7 and Supplementary Table S2) of the optimal catalyst indicated that the metallic Pd nanoparticles with a mean size of 5.1 nm were evenly dispersed on the surface of TiO2 particles. Although the loading of Pd particles could induce visible light absorption and promote the charge-carriers separation (Supplementary Fig. S8), no visible light-responsive catalytic activity was observed for Pd/TiO2 (Supplementary Table S3). Meanwhile, the photocatalytic transfer hydrogenolysis occurred only when Pd particles were loaded on semiconductors (ZnO, Nb2O5, CeO2, and C3N4) rather than inert carriers (SiO2 and ZrO2) under UV or visible light irradiation (Supplementary Table S4). Meanwhile, just increasing the Pd loading amount on the TiO2 failed to increase the products of Cβ–OAr bond cleavage (Fig. 2a, entry 7), which may show the critical synergistic effect between the semiconductor TiO2 and loading Pd NPs. The higher Pd loading amount may not induce more exposed active sites due to the increase of Pd particle mean size to 8.1 nm (Supplementary Fig. S6). Meanwhile, the larger size of Pd particles could affect the light absorption of semiconductor support and decrease the density of photo-generated electrons (Supplementary Fig. S8). Besides ethanol, other simple aliphatic alcohols also can be utilized as the hydrogen donor and solvent, delivering slightly lower yields of hydrogenolysis products (Supplementary Fig. S9).
Fig. 2. The formation and transformation of 1,3-diol protected intermediate during the photothermal catalytic transfer hydrogenolysis of β-O-4 diol model compound.
a The photothermal catalytic transfer hydrogenolysis of β-O-4 1,3-diol model over different photocatalysts; b The time course of Pd/TiO2 catalyzed transfer hydrogenolysis of model 1. Reaction conditions: substrate 1 (10 mg), 3%-Pd/TiO2 (10 mg), EtOH (1 mL), HCl (37%, 10 μL), Kessil LED (370 nm), 140 °C, Ar; c The conversion of 1,3-diol-protected intermediate 2 on Pd/TiO2. Reaction conditions: substrate 2 (10 mg), 3%-Pd/TiO2 (10 mg), EtOH (1 mL), HCl (37%, 10 μL), Kessil LED (370 nm), 140 °C, argon, 2 h.
In addition, according to the time course of Pd/TiO2 catalyzed transfer hydrogenolysis of the β-O-4 diol model (Fig. 2b), it can be concluded that the acetal intermediate 2 was initially formed and rapidly transformed into hydrogenated products (3, 4, and 5). The apparent quantum efficiency was 16.7% based on the proposed elementary reaction steps (Supplementary Fig. S10) and kinetic data obtained at the initial stage (Supplementary Fig. S11). Besides, when acetal 2 was utilized as the substrate (Fig. 2c), the product distribution was similar to that of 1, which further verified that this photothermal catalytic hydrogenolysis involved the in-situ protection of β-O-4 diol structure before its further reductive cleavage.
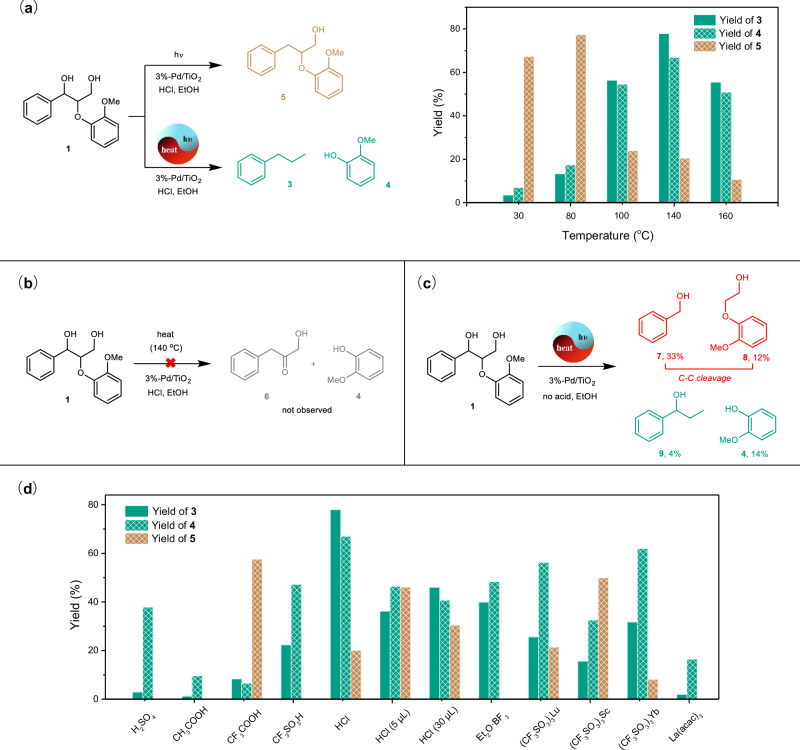
Meanwhile, the synergistic effect between photocatalysis and thermal catalysis played a critical role in this reaction (Fig. 3). Initially, this transformation diminished with decreasing of 370 nm light intensity and failed after switching the UV light to visible light (Supplementary Table S3), which suggests the UV-triggered photocatalysis was essential for the whole transformation. The Pd/TiO2 system tended to catalyze the hydrogenolysis of Cα–OH into CαH2 (5, 67% yield) rather than the Cβ–OAr ether bond cleavage at room temperature under UV light irradiation (Fig. 3a), which was consistent with our previous results that the benzylic Cα–O bond was reactive on Pd/TiO2 at room temperature55,56. However, the hydrogenolysis selectivity of the Cβ–OAr bond rather than the conversion of the substrate without linkage cleavage was dramatically improved by increasing the reaction temperature under light irradiation (Fig. 3a). With the assistance of heat by raising the temperature to 140 °C, the Pd/TiO2 system tended to catalyze the Cβ–OAr ether bond cleavage and provided n-propylbenzene and guaiacol as the monomer products. Furthermore, considering that heat might not only promote the transfer hydrogenolysis but also trigger the acidolysis because of the HCl additive, the controlled experiment without light irradiation was performed at 140 °C, and the unobvious conversion result suggested that the pure thermal catalytic acidolysis and transfer hydrogenolysis of the lignin model did not occur in the photothermal conditions (Fig. 3b), which excluded the reaction pathway of tandem thermal catalytic acidolysis followed by photocatalytic hydrogenation. For the promotion effect of the temperature on the photothermal system, further photocurrent characterization of the Pd/TiO2 catalyst at 30, 94, and 140 °C indicated the photocurrent slightly increased with the temperature, which suggested that heating favored the separation of photo-generated charge carriers (Supplementary Fig. S12).
Fig. 3. The photothermal effects on the transfer hydrogenolysis of the lignin model.
a The effect of heating on the photocatalytic hydrogenolysis pathways. Reaction conditions: substrate 1 (10 mg), 3%-Pd/TiO2 (10 mg), EtOH (1 mL), HCl (37%, 10 μL), Kessil LED (370 nm), 30–160 °C, argon, 2 h; b The controlled experiment under dark conditions; c The controlled photothermal catalytic reaction without acid; d The performance of different acids (liquid, 10 μL; solid, 10 mg) under the standard photothermal conditions.
Further acid optimization with various Brönsted acids and Lewis acids showed the importance of the acid additive (Fig. 3d), especially for the HCl, which could efficiently promote the hydrogenolysis cleavage of the Cβ–OAr linkage. In addition, when the acid additive was removed from the photothermal system (Fig. 3c), monomer products were generated in a much lower yield from the Cβ–OAr cleavage, and Cα–Cβ bond cleavage occurred simultaneously. Given the redox properties of the photothermal system, the benzyl alcohol may be generated from the hydrogenation of benzaldehyde, which could be derived from the β-scission of the CαO• fragment after the hydrogen atom transfer (HAT) reaction between the 1,3-diol 1 and photogenerated holes (h+)4.
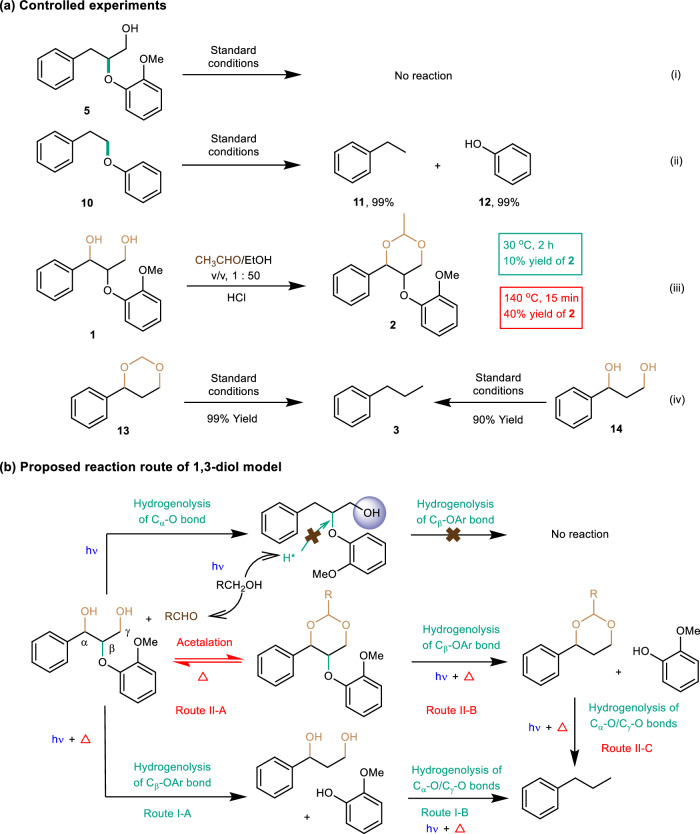
To further study the photothermal process focusing on the relevant bonds cleavage and hydrogenolysis pathway of β-O-4 linkage, controlled experiments with different substrates were performed. Compared with the normal β-O-4 diol model 1, the β-O-4 dimer model 5 without the benzylic hydroxyl group showed no conversion (Fig. 4a, i), while the simultaneous absence of benzylic hydroxyl and Cγ–OH in β-O-4 dimer model 10 could facilitate the hydrogenolysis cleavage of the Cβ–OAr bond (Fig. 4a, ii), delivering close to equivalent cleaved products. Based on these results, it can be deduced that the presence of benzylic hydroxyl is not essential to the cleavage of the Cβ–OAr bond, but the single Cγ–OH existence may inhibit the hydrogenolysis of the Cβ–OAr bond in the β-O-4 dimer. In addition, acetal 2 could be generated from the reaction between 1,3-diol 1 with acetaldehyde at room temperature and 140 °C, and the heating conditions were beneficial to the fast formation of acetal 2 (Fig. 4a, iii). In addition, when the potential Cβ–OAr cleavage products including 4-phenyl-1,3-dioxane 13 and 1-phenylpropane-1,3-diol 14 were added into the photothermal catalytic system, both of them were efficiently converted into propylbenzene via hydrogenolysis of Cα–O/Cγ–O bonds (Fig. 4a, iv). Therefore, the hydrogenolysis of β-O-4 models including the 1,3-diol model and protected model may undergo the direct cleavage of Cβ–O bond followed by hydrogenolysis of Cα–O/Cγ–O bonds (Fig. 4b). Based on the obtained results, there could exist two reasonable routes for the photothermal catalytic transfer hydrogenolysis of the β-O-4 diol dimer. The first route involves the direct reductive cleavage of the Cβ–OAr bond and following hydrogenolysis of other C–O bonds under the photothermal synergistic catalysis with ethanol as the hydrogen donor (Fig. 4b, route I). The second route was the tandem process consisting of photocatalytic oxidation of primary alcohols, acetalation of the 1,3-diol structure of β-O-4 motif with aldehyde, photothermal hydrogenolysis cleavage of Cβ–OAr bond, and further hydrogenolysis of monomer products (Fig. 4b, route II).
Fig. 4. Controlled experiments and proposed reaction routes of β-O-4 diol model.
a Controlled experiments. Standard conditions: substrate (10 mg), 3%-Pd/TiO2 (10 mg), EtOH (1 mL), HCl (37%, 10 μL), Kessil LED (370 nm), 140 °C, argon, 2 h. b Proposed reaction routes.
Photothermal catalytic transfer hydrogenolysis of birch protolignin
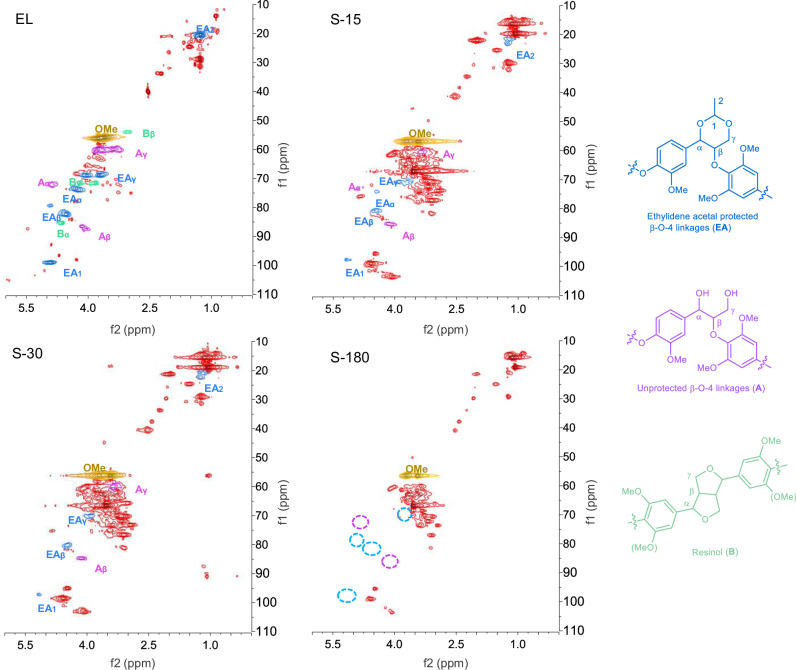
Then the photothermal catalytic transfer hydrogenolysis method was applied for the conversion of the solid birch sawdust, which was composed of 19.2 wt% lignin, 38.6 wt% cellulose, and 23.8 wt% hemicellulose as determined by the NREL method. Different from the β-O-4 model conversion, the addition of 1,4-dioxane besides the ethanol as solvent was proposed to extract lignin in situ during transfer hydrogenolysis. Firstly, the 2D HSQC NMR spectra of in-situ extracted lignin samples during hydrogenolysis were recorded and compared with the externally extracted 1,3-diol-protected lignin sample which was prepared according to previous works (Fig. 5)10,11. The ethylidene acetal-protected β-O-4 linkage (blue regions) was identified in the NMR spectra of externally extracted lignin (Fig. 5, EL), in combination with a small amount of unprotected β-O-4 linkage (purple regions) and resinol linkage (green regions). The ethylidene acetal protected β-O-4 linkage was also found in the in-situ extracted lignin samples at 15 and 30 min (Fig. 5, S-15 and S-30), proving the 1,3-diol protection of lignin fragments under photothermal catalytic conditions. Furthermore, both protected and unprotected β-O-4 linkages diminished after 3 hours of reaction (Fig. 5, S-180), suggesting the hydrogenolysis of these linkages.
Fig. 5. The 2D HSQC NMR spectra of external extracted birch lignin with 1,3-diol protection (EL) and soluble lignin samples at 15, 30, and 180 minutes during photothermal catalytic transfer hydrogenolysis of birch sawdust (S-15, S-30 and S-180).
Reaction conditions of lignin transformation: birch sawdust (60 mg), 3%-Pd/TiO2 (10 mg), EtOH (1 mL), dioxane (0.6 mL), HCl (37%, 10 μL), Kessil LED (370 nm), 140 °C, argon.
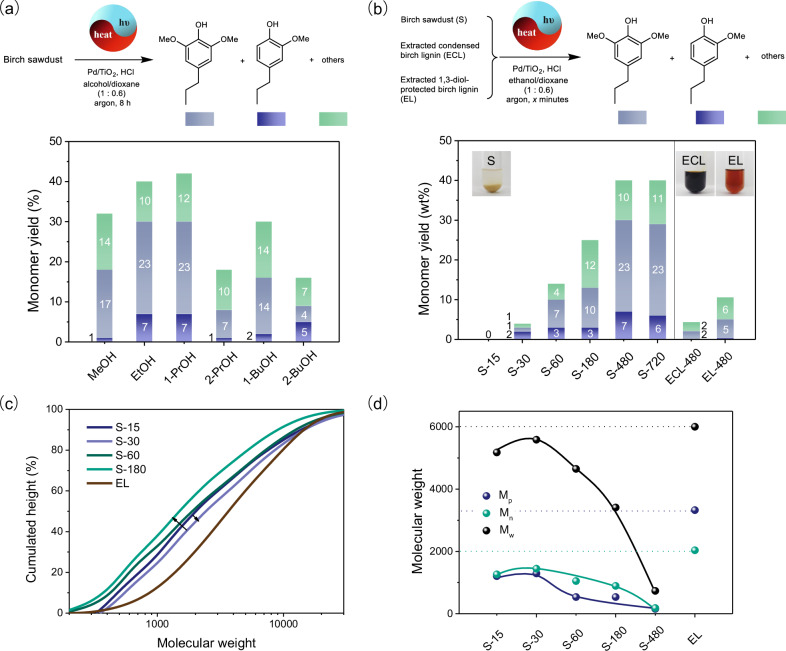
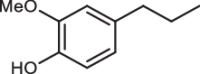
Then, the effect of in-situ extraction and protection on lignin transfer hydrogenolysis was embodied in the yield of monomers using different alcohols as the hydrogen donor (Fig. 6a, Supplementary Figs. S13 and S14). The monomer yields obtained in primary alcohols (30-42%) were much higher than those obtained in secondary alcohols (16–18 wt%). For example, the yields of 2,6-dimethoxy-4-propylphenol and 2-methoxy-4-propylphenol in 1-propanol/1,4-dioxane were 23 wt% and 7 wt%, respectively. However, when 1-propanol was replaced by 2-propanol, the conversion just provided a 7 wt% yield of 2,6-dimethoxy-4-propylphenol and a 1 wt% yield of 2-methoxy-4-propylphenol. This phenomenon was contrary to the hydrogenolysis result of the lignin model 1 in different alcohols (Supplementary Fig. S9). Even the photocatalytic dehydrogenation product ketone from secondary alcohol was a modest protection reagent, the secondary alcohol was a better hydrogen donor than primary alcohol. Besides, the hydrogenolysis performance of the ethylidene acetal-protected model was slightly worse than that of the unprotected model (Fig. 2b, c). Thus, the ability of hydrogen donating rather than protecting effect dominated the model conversion. In contrast, the higher yields of monomers from protolignin conversion in primary alcohol with modest hydrogen donating ability demonstrated the importance of protecting effect in this tandem extraction/hydrogenation process of real biomass11. These results also indicated the difference between lignin model transformation with protolignin transformation.
Fig. 6. The photothermal catalytic transfer hydrogenolysis of lignin samples.
a The transformation of birch sawdust into phenolic monomers using different alcohols as the hydrogen donor. Birch sawdust (60 mg), 3%-Pd/TiO2 (10 mg), alcohol (1 mL), dioxane (0.6 mL), HCl (37%, 10 μL), Kessil LED (370 nm), 140 °C, argon, 8 h; b The transformation of birch sawdust at different reaction times (15, 30, 60, 180, 480, and 720 min, the samples are denoted as S-15, S-30, S-60, S-180, S-480, and S-720) and the transformation of extracted condensed lignin (ECL) and 1,3-diol-protected lignin (EL) samples for 480 min in ethanol/dioxane. The illustrations are initial wood/lignin samples dispersed in ethanol/dioxane (v/v, 1:0.6); c, d The molecular weight analysis of extracted 1,3-diol-protected lignin and soluble lignin samples during the transformation of birch sawdust by GPC. Mp, molecular weight of the peak maxima; Mn, number average molecular weight; Mw, weight average molecular weight. Conditions for b, c, and d: birch sawdust (60 mg) or lignin samples (10 mg), 3%-Pd/TiO2 (10 mg), ethanol (1 mL), dioxane (0.6 mL), HCl (37%, 10 μL), Kessil LED (370 nm), 140 °C, argon.
Furthermore, although the in-situ extraction and 1,3-diol protection can efficiently promote the depolymerization of protolignin in birch sawdust, the direct transfer hydrogenolysis of externally extracted birch lignin with or without 1,3-diol protection just delivered monomers in yields of 11 wt% and 4 wt% in the same photothermal catalytic system which was much lower than that from birch wood conversion with a 40 wt% yield of phenolic monomers at the same reaction time of 480 min (Fig. 6b). Then, the GPC characterization focusing on the molecule weight change was carried out to reveal the reason. The results indicated the molecular weight of in-situ extracted lignin increased before 30 minutes and then decreased gradually (Fig. 6c, d), which suggested that the timely conversion of in-situ extracted lignin could restrain the recondensation of fragments. At the same time, all the in-situ extracted lignin samples showed a lower molecular weight distribution than the externally pre-extracted lignin with 1,3-diol protection (EL) (Fig. 6c, d). Given the fact that the releasing of protolignin from wood was a slow and gradual process and the released lignin macromolecules could be depolymerized to the smaller ones efficiently, the lignin concentration during the in-situ extraction-protection-hydrogenolysis should be lower than the solution of the pre-isolated lignin. Therefore, the better performance of in-situ extracted lignin than the pre-isolated lignin is not only because of the decrease of lignin fragment size favoring its transformation over the heterogeneous catalyst but also related to the controllable lignin release and low lignin concentration that can reduce the occurrence of undesired fragments condensation.
In addition, HCl may play multiple roles during the photothermal catalytic conversion of protolignin (Supplementary Fig. S15). The above tests on the lignin model have proved the promoting effect of HCl on the selective ether bond cleavage (Fig. 3C). As for the lignocellulosic substrate, the lignin was barely extracted from birch sawdust without HCl under the heating conditions. Therefore, HCl was vital for the in-situ lignin extraction. Besides, the condensation of lignin 1,3-diol motif with acetaldehyde can be improved by HCl. However, HCl has a negative effect on the hemicellulose and even cellulose retaining (vide infra). The photothermal experiment of birch sawdust without the addition of photocatalysts but with HCl indicated that hemicellulose was totally decomposed and cellulose was partly decomposed (Supplementary Table S6).
The recycling of solid photocatalysts
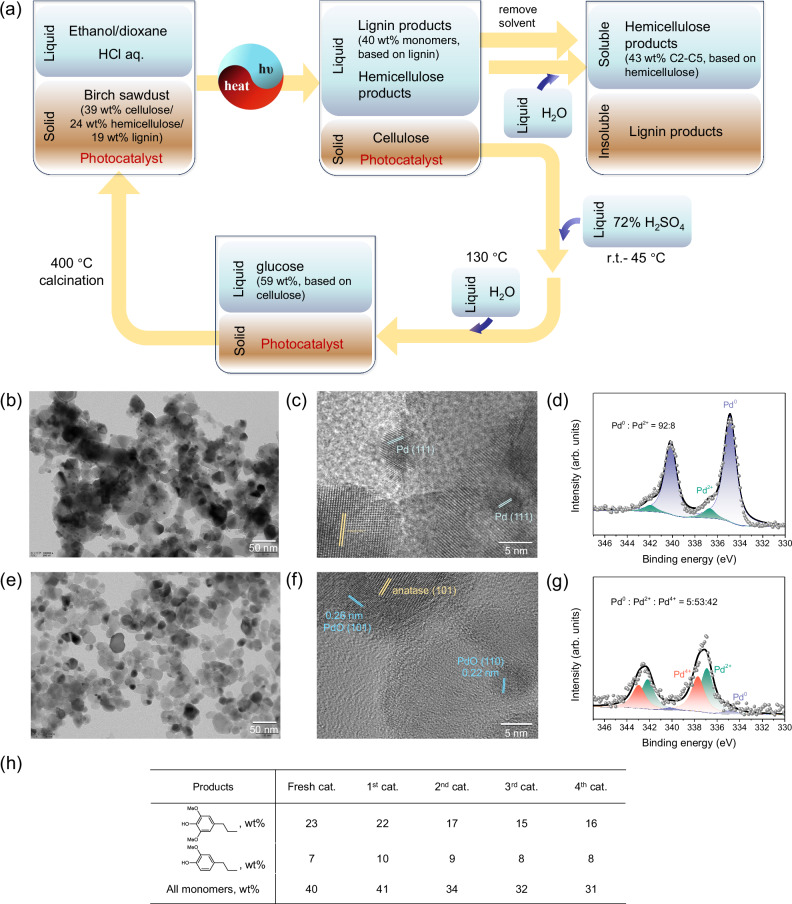
Given the fact that the usage of a solid heterogeneous catalyst in solid biomass transformation usually meets the challenges in the separation of catalyst from the post-reaction mixture and the deactivation of the active catalyst, the recycling of solid photocatalyst in this photothermal system was further checked. After the photothermal reaction, both lignin and hemicellulose were depolymerized into small molecules that were soluble in the liquid phase (the analysis of products from hemicellulose, see Supplementary Table S6), leaving the cellulose mixed with the photocatalyst in the solid phase (Fig. 7a). The first solvent removal in the liquid phase and the following mixture redissolution in water could separate hemicellulose products from lignin products. The isolation of the photocatalyst was performed via acid-catalyzed hydrolysis of cellulose, which delivered a 59 wt% yield of glucose in the aqueous solution. The solid residue underwent further calcination in air to achieve photocatalyst recycling. The comparison of TEM images (Fig. 7b, c, e, f) and XPS spectra (Fig. 7d, g) indicated that the loaded Pd particles aggregated and transformed into PdOx species after the calcination regeneration. Nevertheless, the photocatalyst could be used for another four cycles (Fig. 7h), during which the PdOx could be in-situ reduced in the transfer hydrogenolysis system. Therefore, the cycle stability of Pd/TiO2 can decrease the cost of precious metal in further large-scale biomass utilization.
Fig. 7. The procedure of photocatalyst recycling and characterizations of Pd species in fresh and recycled photocatalysts.
The photocatalyst recycling procedure and transformations of lignin, hemicellulose, and cellulose (a); TEM images of fresh Pd/TiO2 (b, c) and recycled Pd/TiO2 (e, f); Pd 3d XPS results of fresh Pd/TiO2 (d) and recycled Pd/TiO2 (g); and the catalytic performance of the recycled photocatalyst (h). The catalysts which undergo one, two, three and four cycles are denoted as 1st cat., 2nd cat., 3rd cat., and 4th cat.
Catalytic conversion of protolignin in other lignocellulosic biomass
To explore the application scope of this Pd/TiO2-catalyzed photothermal system in the protolignin depolymerization, other inedible lignocellulosic biomass including pine sawdust, bark of Platanus orientalis Linn, walnut shell, reeds straw, wheat straw, and corn straw powders were also tested in the photothermal catalytic system (Table 1). Before the depolymerization test, the lignin content of the selected biomass samples was determined according to the NREL method (Supplementary Table S7), which showed that the lignin contents in the selected wood biomass and reed straw were around 20 wt% and the corn and wheat straws have a lower lignin content of 13 wt% and 7 wt%, respectively. During the following photothermal catalytic transformation, the yields of phenolic monomers from the selected biomass samples were 17–42 wt% based on the weight of initial lignin in the biomass substrate (Table 1), and the main products from various biomass substrates were propyl-substituted phenols, which further verified the developed photothermal catalytic system being a promising method to upgrade the protolignin in diverse biomass to valuable aromatic chemicals.
Table 1.
The transformation of various biomass under photothermal catalytic conditions
| Biomass substrate | Monomers yield (wt%) |
 (wt%) (wt%) |
 (wt%) (wt%) |
|---|---|---|---|
| Pine sawdust | 29 | 8 | 13 |
| Platanus orientalis Linn. | 17 | 6 | 6 |
| Walnut shell | 23 | 5 | 7 |
| Reed straw | 19 | 7 | 9 |
| Wheat straw | 42 | 19 | 20 |
| Corn straw | 30 | 24 | 0 |
Reaction conditions: Biomass powders (60 mg), Pd/TiO2 (10 mg), HCl (37 wt%, 10 μL), EtOH/dioxane (5:3, 1.6 mL), Kessil LED 370 nm, 140 °C, argon, 8 h. Note: The GC yields of aromatic monomers were calculated based on the weight of initial lignin in the biomass substrate.
Photothermal transfer hydrogenolysis of proto-lignin driven by pure solar light
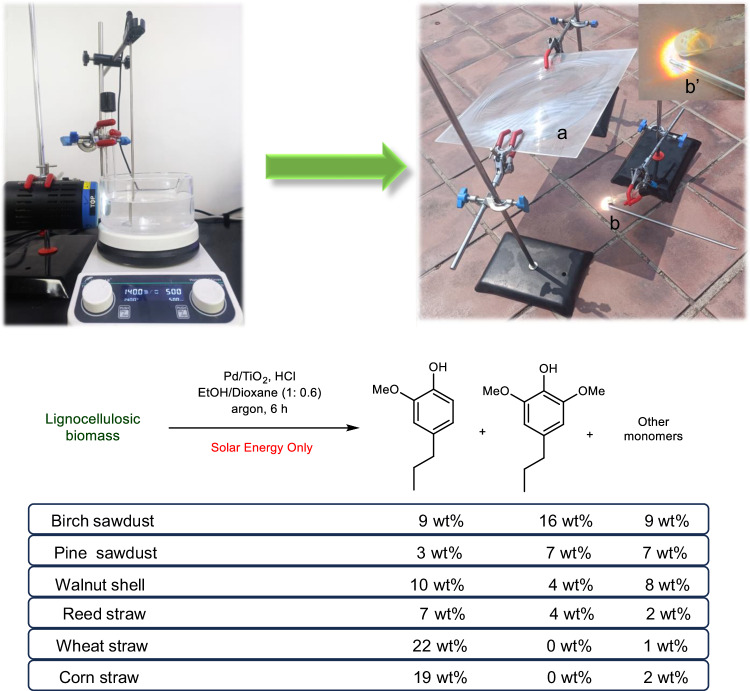
Although the current photothermal system can efficiently promote protolignin depolymerization with a close to ideal yield of phenolic monomers, the above transformation system still needs extra electrical energy input to drive the heating device, LED light, and stirrer device (Fig. 8). Given the fact that solar energy is one typical renewable primary energy that can synchronously provide the necessary heat and irradiation conditions, we then used a Fresnel lens (300 × 300 mm2) to concentrate solar light and provide sufficient temperature and irradiation for the transfer hydrogenolysis of birch protolignin (Fig. 8). By simply adjusting the distance and angle between the lens and the reaction tube to maintain the reaction at 140 °C for 6 h, the outdoor test of birch based on the pure solar energy provided a 34 wt% yield of phenolic monomers from birch sawdusts, including a 16 wt% yield of 2,6-dimethoxy-4-propylphenol and a 9 wt% yield of 2-methoxy-4-propylphenol. Furthermore, the performance of the concise pure solar-light-driven system in the transformation of other lignocellulosic biomass, including walnut shell, reed straw, wheat straw, and corn straw, was also slightly inferior to those of electrical-driven devices, but the obtained results demonstrated the feasibility of utilizing greener solar energy to achieve the photothermal catalytic transformation of protolignin. The utilization of solar light makes our method environmentally friendly and economically viable, which could provide guidance for further large-scale and low-cost lignin transformation.
Fig. 8. The changing of driving energy for the photothermal transfer hydrogenolysis of biomass substrates from electric energy (left) to pure solar light (right), and the performance of pure solar energy system.
Reaction conditions: biomass (60 mg), Pd/TiO2 (10 mg), HCl (37 wt%, 10 μL), EtOH/dioxane (5:3, 1.6 mL), argon, sunlight irradiation collected with a Fresnel lens. GC yields. Picture notes: (a) Fresnel lens 300 × 300 mm2; (b) Reaction tube with a thermometer; (b’) Boiling reaction solution at 140 °C maintained by controlling the light focusing.
Techno-economic analysis and life cycle assessment (LCA)
An industrial-scale process flow has been designed and simulated to evaluate the practical potential of 4-propylguaiacol production from the solar-driven photothermal conversion of wheat straw, which featured the extensive source feedstock and high selectivity of a single product. The model employed wheat straw as feedstock with 100,000 metric tons per annual (t/a), which approximates 2800 t/a of 4-propylguaiacol yield. With feedstock pretreatment, photothermal reaction, consumable recovery, 4-propylguaiacol purification, cellulose hydrolysis, and photocatalyst recovery units (Supplementary Fig. S16), the model was performed through steady-state simulation using Aspen plus to obtain material and energy inventories according to current reaction conditions and parameters (Supplementary Tables S8 and 9). Techno-economic analysis and life cycle carbon emissions were subsequently carried out to identify the opportunity for industrialization. The results reveal that the total production cost (TPC) of 4-propylguaiacol can be lower at 44354 CNY/t, and consumables and depreciation costs are two constraints due to the small scale designed based on the biomass supply and product demand (Supplementary Table S10, Supplementary Fig. S17). Life cycle greenhouse gases (GHG) emission is 8.70 t CO2eq/t 4-propylguaiacol, of which wheat straw accounts for the largest contribution of 85.28% (Supplementary Figs. S18 and 19). The life cycle GHG emissions of wheat straw approximates 170 kg CO2eq/t due to plant, harvest, and transport. High wheat straw input causes more GHG emissions than the carbon credit of bio-derived chemicals, thus a higher conversion ratio is attractive to facilitate this work.
Discussion
In conclusion, focusing on the efficient protolignin conversion in the lignocellulosic biomass to aromatic monomers, we herein developed a photothermal catalytic method to achieve protolignin in-situ extraction, 1,3-diol acetalation protection, and transfer hydrogenolysis to phenolic monomers in one pot. After the catalyst screening and reaction condition optimization, it was found that the Pd/TiO2 at 140 °C with 370 nm light irradiation can efficiently catalyze the reforming of primary alcohols to the corresponding aldehydes and active H* species, which can further participate in the acetalation protection of the 1,3-diol group in the in-situ extracted β-O-4 linkage and mediate the following selective hydrogenolysis of the Cβ–OAr bonds to aromatic monomers, respectively. According to the controlled experiments, the synergistic effect of photocatalysis and thermal catalysis is crucial to the prior cleavage of the Cβ–OAr bond, avoiding the formation of an inert benzylic dehydroxylated byproduct to prevent the Cβ–OAr bond cleavage. Furthermore, the slow or controllable in-situ release of low-molecular-weight lignin fragments during biomass conversion benefited the lignin hydrogenolysis, which makes the whole biomass a better starting material than the externally isolated lignin. This method allowed the hydrogenolysis of lignin in various biomass into propyl-substituted monomers at 140 °C under H2-free conditions. In addition, this work also applied the developed system in the conversion of lignin from more kinds of biomass resources and studied the recycling and reuse of the Pd/TiO2 catalyst. The pure solar-light-driven photothermal catalytic transfer hydrogenolysis of protolignin was feasible without the extra electric energy input, showing its potentiality in large-scale applications. Although more efforts are still needed to update the simple photothermal system, the relevant photothermal research can further expand methods for converting and utilizing lignocellulosic biomass in the future.
Methods
The preparation of photocatalyst
All photocatalysts were prepared via the impregnation-reduction method. As for the 3%-Pd/TiO2, TiO2 (0.1 g, Degussa P25) was added into a 5 mL methanol solution of palladium (II) acetate containing 3 mg of Pd. After ultrasonic treatment for 30 min, the mixture was stirred for another two hours. The solvent was removed under vacuum, and the collected powder was calcined in H2 flow (20 mL·min−1) at 400 °C (heating rate 10 °C·min−1) for 4 h. The preparation procedures of other metal/TiO2 samples were similar to that of Pd/TiO2 except for using Co(NO3)2·6H2O, NiCl2·6H2O, and H2PtCl6·6H2O as metal precursors.
Characterizations of lignin samples and catalysts
Transmission electron microscopy (TEM) was carried out using a Jem-2100F electron microscope with an accelerating voltage of 200 kV. X-ray photoelectron spectroscopy (XPS) was collected on Thermofisher escalab 250xi. ICP-OES was performed on Thermo Fisher iCAP 7400. The 1H-, 13C-, and 2D HSQC NMR spectra of compounds and lignin samples were measured on a Bruker AVIII 400 spectrometer (1H: 400 MHz, 13C: 101 MHz). The Gel Permeation Chromatography (GPC) was measured on Agilent GPC 50.
The procedure of the photothermal catalytic test
In a typical procedure, a 20 mL quartz reactor equipped with a stir bar was loaded with lignin model (10 mg) or wood sawdust (60 mg), photocatalyst (10 mg), acid (10 μL) and solvent (1.0 or 1.6 mL). Then the atmosphere was switched to argon before sealing the reactor. This mixture was irradiated using Kessil PR160L LED lamps (λmax = 370 nm, 40 W) for a certain time. After the reaction, the standard solution (naphthalene in ethanol) was added. After filtration using a Nylon syringe filter, the solution was analyzed by gas chromatography (Shimadzu GC-2014C) and GC/MS (GC: Shimadzu 2030AM, MS: Shimadzu, QP2020NX).
The experimental procedure of protolignin transformation using solar energy was similar to the above method except for using the combination of sunlight with a Fresnel lens to replace the LED lamp and the heating device.
All aromatic products were quantified using a GC (Shimadzu GC-2014) equipped with an HP-5 capillary column (30 m × 0.32 mm × 0.25 μm) and a flame ionization detector. The injector and detector temperatures were set at 280 °C. The column temperature was initially maintained at 80 °C for 2 min, then heated to 260 °C at a rate of 10 °C min–1, and then maintained for another 8 min. Molar yields of products from the lignin model reaction were calculated as
| 1 |
where n(i) and n(s) are the mole of product i and the initial lignin model substrate, respectively. Mass yields of aromatic monomer products from biomass reaction were calculated as
| 2 |
where m(i), m(b), and c(l) are the mass of product i, the mass of initial biomass, and the content of lignin determined by the NREL method, respectively. The water-soluble depolymerized products from hemicellulose were quantified using a high performance liquid chromatography (Shimadzu LC-20AT) equipped with a C18 column. Mass yields of aliphatic monomer products from biomass reaction were calculated as
| 3 |
where m(i), m(b), and c(h) are the mass of product i, mass of initial biomass, and the content of hemicellulose determined by the NREL method, respectively.
The photocatalyst recycling procedure
After the photothermal reaction, the organic solution and solid residue were separately collected through filtration of the reaction mixture. The organic solution was directly analyzed by GC to determine the yield of lignin monomers. Besides, the compounds from hemicellulose conversion were collected via the removal of organic solvent and redissolution in water, and this aqueous solution was analyzed by HPLC to determine the yield of hemicellulose products. The solid residue after the reaction was subjected to acid-catalyzed hydrolysis which was similar to the NREL method to separate the photocatalyst from unreacted cellulose and other components. The filtrated solid from the hydrolysis mixture underwent calcination to obtain the used photocatalyst without organic impurities. The filtrated hydrolysis aqueous solution was analyzed by HPLC to determine the content of cellulose in the solid after the photothermal reaction of biomass. The used photocatalyst was subjected to the next run without further treatment.
Supplementary information
Acknowledgements
This work was supported by the National Natural Science Foundation of China (22109139) (H.L.), the National Natural Science Foundation of China (22025206) (F.W.), the National Natural Science Foundation of China (32471809) (C.Z.), the Research Fund for High-level Talents Introduction of Nanjing Forestry University (163105107, 163105164) (C.Z.), the Dalian Innovation Support Plan for High-level Talents (2022RG13) (F.W.), and the Liaoning Revitalization Talents Program (XLYC2002012) (F.W.).
Author contributions
H.L., C.Z., and F.W. conceived and designed the project. H.L., X.S., H.W., T.L., and X.Y. conducted the experiments. Z.Z. conducted the life cycle assessment (LCA) and techno-economic analysis (TEA). H.L. and C.Z. wrote the manuscript. All authors contributed to analyzing the data and editing the manuscript.
Peer review
Peer review information
Nature Communications thanks Shanjun Mao, Gang Xiao, and the other, anonymous, reviewer for their contribution to the peer review of this work. A peer review file is available.
Data availability
The data reported in this paper including experimental details, characterization data for the catalysts and organic compounds are available in the main text and Supplementary Information files, or from the corresponding author upon request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Hongji Li, Email: hongjili@zzu.edu.cn.
Chaofeng Zhang, Email: zhangchaofeng@njfu.edu.cn.
Feng Wang, Email: wangfeng@dicp.ac.cn.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-024-54664-6.
References
- 1.Li, C., Zhao, X., Wang, A., Huber, G. W. & Zhang, T. Catalytic Transformation of Lignin for the Production of Chemicals and Fuels. Chem. Rev.115, 11559–11624 (2015). [DOI] [PubMed] [Google Scholar]
- 2.Sun, Z., Fridrich, B., de Santi, A., Elangovan, S. & Barta, K. Bright side of lignin depolymerization: Toward new platform chemicals. Chem. Rev.118, 614–678 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang, C. F. & Wang, F. Lignin Conversion Catalysis: Transformation to Aromatic Chemicals (Wiley, 2022).
- 4.Zhang, C. F. et al. Catalytic Strategies and Mechanism Analysis Orbiting the Center of Critical Intermediates in Lignin Depolymerization. Chem. Rev.123, 4510–4601 (2023). [DOI] [PubMed] [Google Scholar]
- 5.Zhang, C. F. & Wang, F. Catalytic Lignin Depolymerization to Aromatic Chemicals. Acc. Chem. Res.53, 470–484 (2020). [DOI] [PubMed] [Google Scholar]
- 6.Zhang, B., Meng, Q. L., Liu, H. Z. & Han, B. X. Catalytic Conversion of Lignin into Valuable Chemicals: Full Utilization of Aromatic Nuclei and Side Chains. Acc. Chem. Res.56, 3558–3571 (2023). [DOI] [PubMed] [Google Scholar]
- 7.Dong, L. et al. Breaking the Limit of Lignin Monomer Production via Cleavage of Interunit Carbon–Carbon Linkages. Chem5, 1521–1536 (2019). [Google Scholar]
- 8.Gu, N. X. et al. Autoxidation Catalysis for Carbon−Carbon Bond Cleavage in Lignin. ACS Cent. Sci.9, 2277–2285 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang, C. F. & Wang, F. Sell a dummy: Adjacent functional group modification strategy for the catalytic cleavage of lignin β-O-4 linkage. Chin. J. Catal.38, 1102–1107 (2017). [Google Scholar]
- 10.Shuai, L. et al. Formaldehyde stabilization facilitates lignin monomer production during biomass depolymerization. Science354, 329–333 (2016). [DOI] [PubMed] [Google Scholar]
- 11.Lan, W., Amiri, M. T., Hunston, C. M. & Luterbacher, J. S. Protection Group Effects During α,γ-Diol Lignin Stabilization Promote High-Selectivity Monomer Production. Angew. Chem. Int. Ed.57, 1356–1360 (2018). [DOI] [PubMed] [Google Scholar]
- 12.Questell-Santiago, Y. M., Galkin, M. V., Barta, K. & Luterbacher, J. S. Stabilization strategies in biomass depolymerization using chemical functionalization. Nat. Rev. Chem.4, 311–330 (2020). [DOI] [PubMed] [Google Scholar]
- 13.Renders, T., Van den Bosch, S., Koelewijn, S. F., Schutyser, W. & Sels, B. F. Lignin-first biomass fractionation: the advent of active stabilisation strategies. Energy Environ. Sci.10, 1551–1557 (2017). [Google Scholar]
- 14.Song, Q. et al. Lignin depolymerization (LDP) in alcohol over nickel-based catalysts via a fragmentation-hydrogenolysis process. Energy Environ. Sci.6, 994–1007 (2013). [Google Scholar]
- 15.Huang, X. M. et al. Reductive fractionation of woody biomass into lignin monomers and cellulose by tandem metal triflate and Pd/C catalysis. Green. Chem.19, 175–187 (2017). [Google Scholar]
- 16.Abu-Omar, M. M. et al. Guidelines for performing lignin-first biorefining. Energy Environ. Sci.14, 262–292 (2021). [Google Scholar]
- 17.Chen, L. et al. Lignin First: Confirming the Role of the Metal Catalyst in Reductive Fractionation. JACS Au1, 729–733 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luo, H. et al. Oxidative Catalytic Fractionation of Lignocellulosic Biomass under Non-alkaline Conditions. J. Am. Chem. Soc.143, 15462–15470 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhu, Y. et al. Oxidative Catalytic Fractionation of Lignocellulose to High-Yield Aromatic Aldehyde Monomers and Pure Cellulose. ACS Catal.13, 7929–7941 (2023). [Google Scholar]
- 20.Ren, Y. L., Yan, M. J., Wang, J. J., Zhang, Z. C. & Yao, K. S. Selective Reductive Cleavage of Inert Aryl C−O Bonds by an Iron Catalyst. Angew. Chem. Int. Ed.52, 12674–12678 (2013). [DOI] [PubMed] [Google Scholar]
- 21.Lancefield, C. S., Ojo, O. S., Tran, F. & Westwood, N. J. Isolation of Functionalized Phenolic Monomers through Selective Oxidation and C-O Bond Cleavage of the beta-O-4 Linkages in Lignin. Angew. Chem. Int. Ed.54, 258–262 (2015). [DOI] [PubMed] [Google Scholar]
- 22.Deuss, P. J. et al. Aromatic Monomers by in Situ Conversion of Reactive Intermediates in the Acid-Catalyzed Depolymerization of Lignin. J. Am. Chem. Soc.137, 7456–7467 (2015). [DOI] [PubMed] [Google Scholar]
- 23.Lahive, C. W. et al. Advanced Model Compounds for Understanding Acid-Catalyzed Lignin Depolymerization: Identification of Renewable Aromatics and a Lignin-Derived Solvent. J. Am. Chem. Soc.138, 8900–8911 (2016). [DOI] [PubMed] [Google Scholar]
- 24.Liao, Y. H. et al. A sustainable wood biorefinery for low-carbon footprint chemicals production. Science367, 1385–1390 (2020). [DOI] [PubMed] [Google Scholar]
- 25.Rahimi, A., Ulbrich, A., Coon, J. J. & Stahl, S. S. Formic-acid-induced depolymerization of oxidized lignin to aromatics. Nature515, 249–252 (2014). [DOI] [PubMed] [Google Scholar]
- 26.Lancefield, C. S., Ojo, O. S., Tran, F. & Westwood, N. J. Isolation of Functionalized Phenolic Monomers through Selective Oxidation and C−O Bond Cleavage of the β-O-4 Linkages in Lignin. Angew. Chem. Int. Ed.54, 258–262 (2015). [DOI] [PubMed] [Google Scholar]
- 27.Song, S., Zhang, J. G., Gozaydin, G. & Yan, N. Production of Terephthalic Acid from Corn Stover Lignin. Angew. Chem. Int. Ed.58, 4934–4937 (2019). [DOI] [PubMed] [Google Scholar]
- 28.Zhang, B. et al. Transition-metal-free synthesis of pyrimidines from lignin beta-O-4 segments via a one-pot multi-component reaction. Nat. Commun.13, 3365 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dong, L. et al. Sustainable production of dopamine hydrochloride from softwood lignin. Nat. Commun.14, 4996 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Song, Q., Wang, F. & Xu, J. Hydrogenolysis of lignosulfonate into phenols over heterogeneous nickel catalysts. Chem. Commun.48, 7019–7021 (2012). [DOI] [PubMed] [Google Scholar]
- 31.Li, N. et al. Selective lignin arylation for biomass fractionation and benign bisphenols. Nature630, 381–386 (2024). [DOI] [PubMed] [Google Scholar]
- 32.Zhang, J. et al. A Series of NiM (M = Ru, Rh, and Pd) Bimetallic Catalysts for Effective Lignin Hydrogenolysis in Water. ACS Catal.4, 1574–1583 (2014). [Google Scholar]
- 33.Zhang, C. et al. Cleavage of the lignin β-O-4 ether bond via a dehydroxylation-hydrogenation strategy over a NiMo sulfide catalyst. Green. Chem.18, 6545–6555 (2016). [Google Scholar]
- 34.Xia, Q. N. et al. Direct hydrodeoxygenation of raw woody biomass into liquid alkanes. Nat. Commun.7, 11162 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shao, Y. et al. Selective production of arenes via direct lignin upgrading over a niobium-based catalyst. Nat. Commun.8, 16104 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shivhare, A. et al. Hydrogenolysis of Lignin-Derived Aromatic Ethers over Heterogeneous Catalysts. ACS Sustain. Chem. Eng.9, 3379–3407 (2021). [Google Scholar]
- 37.Wang, S., Zhang, K., Li, H., Xiao, L.-P. & Song, G. Selective hydrogenolysis of catechyl lignin into propenylcatechol over an atomically dispersed ruthenium catalyst. Nat. Commun.12, 416 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu, L. et al. Aqueous amine enables sustainable monosaccharide, monophenol, and pyridine base coproduction in lignocellulosic biorefineries. Nat. Commun.15, 734 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shen, X., Zhang, C., Han, B. & Wang, F. Catalytic self-transfer hydrogenolysis of lignin with endogenous hydrogen: road to the carbon-neutral future. Chem. Soc. Rev.51, 1608–1628 (2022). [DOI] [PubMed] [Google Scholar]
- 40.Li, L. et al. Hydrogen-Free Production of 4-Alkylphenols from Lignin via Self-Reforming-Driven Depolymerization and Hydrogenolysis. ACS Catal.10, 15197–15206 (2020). [Google Scholar]
- 41.Meng, Q. et al. Sustainable production of benzene from lignin. Nat. Commun.12, 4534 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peng, Z., Wu, Z., Sun, X. & Li, H. Photocatalytic transfer hydrogenolysis of aryl ethers. Green. Chem.25, 6869–6880 (2023). [Google Scholar]
- 43.Zhou, H., Liu, X., Guo, Y. & Wang, Y. Self-Hydrogen Supplied Catalytic Fractionation of Raw Biomass into Lignin-Derived Phenolic Monomers and Cellulose-Rich Pulps. JACS Au3, 1911–1917 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li, C., Zheng, M., Wang, A. & Zhang, T. One-pot catalytic hydrocracking of raw woody biomass into chemicals over supported carbide catalysts: simultaneous conversion of cellulose, hemicellulose and lignin. Energy Environ. Sci.5, 6383–6390 (2012). [Google Scholar]
- 45.Zhang, C. F. et al. Promoting Lignin Depolymerization and Restraining the Condensation via an Oxidation-Hydrogenation Strategy. ACS Catal.7, 3419–3429 (2017). [Google Scholar]
- 46.Kumaniaev, I. et al. Lignin depolymerization to monophenolic compounds in a flow-through system. Green. Chem.19, 5767–5771 (2017). [Google Scholar]
- 47.Anderson, E. M. et al. Flowthrough Reductive Catalytic Fractionation of Biomass. Joule1, 613–622 (2017). [Google Scholar]
- 48.Luo, N. C. et al. Photocatalytic Oxidation–Hydrogenolysis of Lignin β-O-4 Models via a Dual Light Wavelength Switching Strategy. ACS Catal.6, 7716–7721 (2016). [Google Scholar]
- 49.Luo, N. et al. Visible-Light-Driven Self-Hydrogen Transfer Hydrogenolysis of Lignin Models and Extracts into Phenolic Products. ACS Catal.7, 4571–4580 (2017). [Google Scholar]
- 50.Huang, Z., Luo, N., Zhang, C. & Wang, F. Radical generation and fate control for photocatalytic biomass conversion. Nat. Rev. Chem.6, 197–214 (2022). [DOI] [PubMed] [Google Scholar]
- 51.Wu, X. J. et al. Solar energy-driven lignin-first approach to full utilization of lignocellulosic biomass under mild conditions. Nat. Catal.1, 772–780 (2018). [Google Scholar]
- 52.Wu, X. et al. Photocatalytic transformations of lignocellulosic biomass into chemicals. Chem. Soc. Rev.49, 6198–6223 (2020). [DOI] [PubMed] [Google Scholar]
- 53.Li, T. et al. Matching the metal oxides with a conjugated and confined N-oxyl radical for the photocatalytic C(sp3)–H bond activation. Appl. Catal. B: Environ.342, 123432 (2024). [Google Scholar]
- 54.Jiang, H., Liu, M., Lian, X., Zhu, M. & Zhang, F. CsPbBr3 Quantum Dots Promoted Depolymerization of Oxidized Lignin via Photocatalytic Semi-Hydrogenation/Reduction Strategy. Angew. Chem. Int. Ed.63, e202318850 (2024). [DOI] [PubMed] [Google Scholar]
- 55.Fu, H. et al. Selectivity Control in Photocatalytic Transfer Hydrogenation of Bio-based Aldehydes. ChemCatchem14, e202200120 (2022). [Google Scholar]
- 56.Li, H. et al. Photocatalytic transfer hydrogenolysis of aromatic ketones using alcohols. Green. Chem.22, 3802–3808 (2020). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data reported in this paper including experimental details, characterization data for the catalysts and organic compounds are available in the main text and Supplementary Information files, or from the corresponding author upon request.