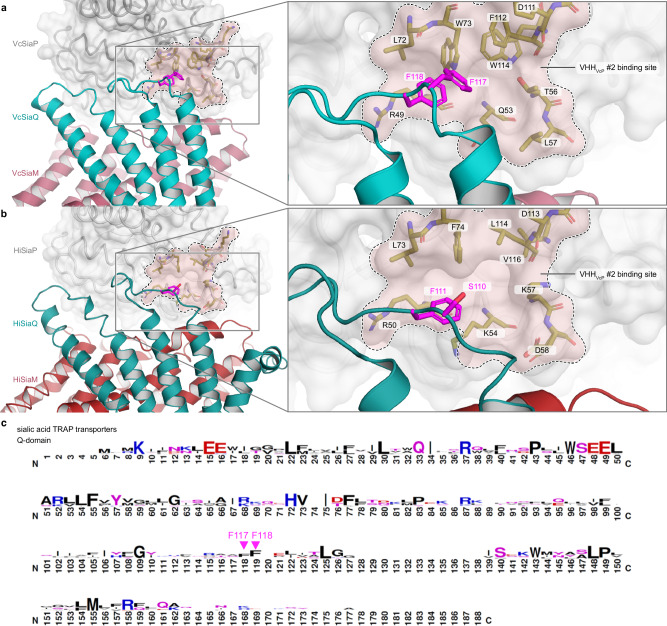
Fig. 8. Structural conservation of SiaPQM around the allosteric pocket that forms the VHHVcP #2 epitope.
a Model of the tripartite SiaPQM complex from Vibrio cholerae. The model was built up from AlphaFold2 predictions and experimental structures. The binding area of VHHVcP #2 is indicated and labelled. The Q (teal) and M(red) transmembrane domains are depicted as cartoon model and the P-domain is shown as surface representation. On the right-hand-side, a magnification of the region of interest is shown and the highlighted amino acid residues are labeled. b Same as (a) but for the tripartite SiaPQM complex from Haemophilus influenzae. c Sequence logo to visualize the hydrophobic loop of the Q-domain that is conserved among sialic acid TRAP transporters and includes the conserved Phe residue 118 (111 in HiSiaQ). This image was created with WebLogo47 and was adapted from Peter et al.21 (http://creativecommons.org/licenses/by/4.0/).