Abstract
Background
The carrying rate of thalassemia is high in Quanzhou city. However, there are few large-scale studies on the correlation analysis between genotype and phenotype of thalassemia in Quanzhou. In this study, the genotype and phenotype data of 1076 individuals with thalassemia in Quanzhou city were analyzed to provide reference data for screening and diagnosis of thalassemia in this region.
Material and methods
Reverse dot blot hybridization (RDB-PCR), Gap-PCR and nested PCR were used to detect the thalassemia genotype. Clinical and hematological parameters of 1076 individuals of thalassemia were collected to analyze the correlation between genotype and phenotype.
Results
Among 2997 subjects, 1076 cases diagnosed as thalassemia gene carrier or patients, with detection rate 35.9 %, among which Southeast Asian deletion (--SEA)/αα was the most common α-thalassemia genotype (48.4 %) and one rare genotype was detected: HKαα/--SEA (0.1 %). Subjects with thalassemia alone showed the least severe symptoms of anemia with higher red blood cell count (RBC) and hemoglobin (Hb), lower red blood cell distribution width (RDW) than those with iron deficiency (ID) or iron overload (IO) (p < 0.05). The Hb, mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH) levels in gene carriers of α-thalassemia were higher than those of β-thalassemia, while RBC, RDW and serum ferritin (SF) levels were lower than the later(p < 0.05). Among individuals with --SEA/αα, the Hb, MCV, MCH, mean corpuscular hemoglobin concentration (MCHC) and SF levels of subjects≥19 years old were higher than those of ≤18 years old. For cases ≥19 years old, the RBC, Hb and SF levels in male were higher than that in female, while MCHC level was lower than female(p < 0.05).
Conclusion
The difference of hematological phenotypes in patients with thalassemia is not only affected by their genotype, but also the influence of gender and age.
Keywords: Thalassemia, Genotype, Phenotype
Name of Departhalassemiaent and Institution where work was done: Departhalassemiaent of Clinical Laboratory, Quanzhou First Hospital Affiliated to Fujian Medical University.
1. Background
Thalassemia is a group of autosomal recessive hereditary diseases that seriously threaten the health of human. Due to deletion or mutation of α and β genes encoding globin peptide chains, the function of hemoglobin is affected, leading to ineffective hematopoiesis and red blood cell destruction [1]. The α-gene cluster is located on chromosome 16 (16p13.3), while the β-gene cluster is situated on chromosome 11 (11p15.3). The primary etiology of α thalassemia stems from the deficiency in the α-globin gene. The etiology of β-thalassemia primarily stems from point mutations in the β-globin gene [2]. It is by far the most common single gene disease, which can lead to increased infant mortality or birth defects and has attracted wide attention worldly [3,4]. There is a high incidence of thalassemia in Southeast Asia, the Middle East, India and southern China [5].
Quanzhou City, located in the southeastern coastal areas of China, used to be one of the important ports on the ancient "Maritime Silk Road" [6]. Due to its close communication with the Eastern and Western populations in ancient times, it may be one of the reasons for the high carrying rate of thalassemia gene population [5].In 2013, the prevalence of thalassemia in Quanzhou was reported to be 3.06 % [7].In 2019, it was reported that the carrier rate of thalassemia gene in Quanzhou was 12.92 % [8].In recent years, due to the expanding population base and shifting demographics of childbearing age in Quanzhou, there is a likelihood of further changes in the prevalence rate of thalassemia gene carriers. Therefore, it is still very important to investigate the epidemiology of thalassemia in Quanzhou area, monitor the population of childbearing age, and prevent the birth of children with severe thalassemia. This study aimed to analyze the correlation between the genotypes and phenotypes of thalassemia patients in Quanzhou area, so as to provide reference data for genetic counseling and clinical diagnosis in this area.
2. Material and Methods
2.1. Subjects
2997 subjects suspected with thalassemia in Quanzhou First Hospital from January 2019 to June 2022 were selected. The inclusion criteria for the study subjects were as follows: people with small cell hypochromic anemia (Hb, MCV, MCH below normal) indicated by hematological parameters or a family history of thalassemia. A total of 190 males and 2807 females, with a median age of 29 years old (Males:27 years old, Females:30 years old), the maximum recorded age was 93 years old (Males: 93 years old, Females: 90 years old), while the minimum age was 4 days (for both sexes was observed at 4 days).
Blood samples and basic clinical information of the selected subjects were collected. All subjects were registered in Fujian and had no relatives. All of them or their parents had signed the informed consent before the experiment. All experiments were performed in accordance with relevant guidelines and standards.
2.2. Detection of hematological indicators
The blood samples of the subjects are collected and recorded by trained professionals, while an automated information system is to code the samples. The analysis of hematology parameters is conducted in a specialized clinical laboratory with comprehensive and standardized quality control measures implemented throughout the testing process. Additionally, the automatic blood analyzer incorporates self-cleaning mechanisms after each sample collection to prevent cross-contamination. 2 ml of whole venous blood samples (EDTA-k2 anticoagulation) were collected from patients, and blood cells were detected by XS-1000i hematology analyzer (Sysmex, Japan). Another 5 ml of whole venous blood was collected, and serum was collected by centrifugation after natural coagulation. Serum ferritin (SF) concentration was measured by UniCel Dxl 800 automatic immune analyzer (Beckman Coulter, USA) and matching reagents. The biological reference interval of ferritin in the laboratory (23.9–336.2 ng/ml for men and 11.0–306.8 ng/ml for women) was used to diagnose iron deficiency (ID) and iron overload (IO). In men:ID with SF < 23.9 ng/ml and IO with SF > 336.2 ng/ml. In women: ID with SF < 11.0 ng/ml and IO with SF > 306.8 ng/ml.
2.3. Detection of common thalassemia genotype
Referred to industry standards [8], and DNA was extracted by DNA blood extraction kit (Yaneng Biotechnology, Shenzhen, China).Three deletion types of α-thalassemia (--SEA/, -α3.7/, -α4.2/) were analyzed by GAP-PCR (The reaction conditions and primers sequence are shown in Table 1, Table 2). Three non-deletion types of α-thalassemia (αCSα/、αQSα/,αWSα/) and 17 β-thalassemia gene mutations: −28(A > G), −29(A > G), - 30(T > C), −32(C > A), CD14-15(+G), CD17(AAG > TAG), CD26(GAG > AAG), CD27-28(+C), CD31(-C), CD41-42(-TTCT), CD43(GAG > TAG), CD71-72(+A), IVS-Ⅰ- 1(G > T), IVS-Ⅰ-5(G > C), IVS-Ⅱ-654(C > T), 5′UTR Cap+ 40–43(-AAAC), Initiation condon(ATG > AGG), reverse dot hybridization was used for detection (Yaneng Biotechnology, Shenzhen, China).
Table 1.
The condition of Gap PCR.
| Procedure | Temperature (°C) | Time | Cycle number |
|---|---|---|---|
| Unchain/activate | 96 | 1min | 1 |
| Amplification | 96 | 10s | 25 |
| 50 | 10s | ||
| 60 | 2min | ||
| Keep warm | 16 | ∞ | 1 |
Table 2.
The primer of α&β-gene sequencing.
| Gene | Primer | Sequencing | |
|---|---|---|---|
| α-gene | α1 | A1F | CTCCGGCCCAGCACATGAG |
| A1R | AGCTGCAAGGAGGTTCTGACCAT | ||
| α2 | A2F | CTCCGCCGCAGCCAAGTAG | |
| A2R | CAGCTGACGAGAGTGCCTTGGTC | ||
| β-gene | G1 | B1F | CGGCTGTCATCACGAAGACCT |
| B1R | CAGCTCACTCAGTGTGGCTTC | ||
| G2 | B2F | GCTGTTATGTAGAACCCT | |
| B2R | TTGCTATTGCCTCGTCCCAGA | ||
| G3 | B3F | ATGTATCATCGATCTTTGCAC | |
| B3R | GTTTTAAATACGCTGACCTCC | ||
2.4. Detection of rare thalassemia genotype
SALSA multiplex ligation-dependent probe amplification (MLPA) P027.B1 assay (MRC Holland; Amsterdam, The Netherlands) was utilised to detect the deletional fragments of the α-thalassemia genes, and verified by Gap-PCR and breakpoint sequencing. The detection results were processed using the software MRC-Coffalyser version 9.4 (MRC Holland; Amsterdam, The Netherlands).For suspected HKαα alleles were detected by single PCR and nested PCR [9].
2.5. Statistical analysis
Subjects' basic information, thalassemia genotype, hematological parameters and other data were input into Microsoft Excel 2019 (Microsoft; Redmond, WA, USA). The heatmap for each phenotype group was generated using Origin Pro 2024(OriginLab, USA). The data were analyzed using SPSS 26.0 (IBMInc., Chicago, USA). The histogram was created to show the distribution of data, and the normality of continuous variables was tested by Kolmogorov–Smirnov. Normal data were statistically described by mean standard deviation and evaluated by t-test. Non-normal data were described by median (P25,P75). Kruskal-wallis test, Wilcoxon rank sum test or chi-square test were used to compare significance according to data characteristics and test purposes. For chi-square tests with small sample sizes (n < 40), Fisher's exact test is employed. p < 0.05 was statistically significant [10].
3. Results
3.1. Distribution of thalassemia genotypes in Quanzhou area
Among the 2997 subjects suspected with thalassemia, 1076 (35.9 %) subjects were diagnosed as thalassemia carriers or patients. 715 cases were found carrying α-thalassemia mutation (66.45 %, 715/1076) with 668 cases (62.08 %, 668/1076) identified as carriers of the α-thalassemia and 47 cases (4.37 %, 47/1076) diagnosed as α-thalassemia patients. There were 349 cases (32.43 %, 349/1076) with β-thalassemia, among which 345 cases (32.06 %, 345/1076) were β-thalassemia carriers and 4 cases (0.37 %, 4/1076) were β-thalassemia patients.12 cases (1.12 %, 12/1076) carried α-thalassemia mutation compound with β-thalassemia mutation. Among 1076 cases carrying thalassemia mutation, 99.9 % were diagnosed with common thalassemia genotype. Totally, there were 32 common genotypes detected in this study, including--SEA/αα(48.42 %), -α3.7/αα(9.76 %), --SEA/-α3.7(3.53 %), -α4.2/αα(2.14 %), βIVSII−654 (C→T)/βN (13.20 %), βCD41-42 (−TTCT)/βN (10.97 %), βCD17 (A→T)/βN (4.93 %), etc. A case (0.1 %, 1/1076) was found carrying HKαα/--SEA genotype, a rare type of thalassemia (Table 3).
Table 3.
Genotype of thalassemia in Quanzhou area.
| Genotype | Phenotype | Subjects (n) | Frequency (%) | |
|---|---|---|---|---|
| α-thalassemia | ||||
| αQSα/αα | Silent carrier | 9 | 0.84 | |
| αCSα/αα | Silent carrier | 5 | 0.46 | |
| αWSα/αα | Silent carrier | 4 | 0.37 | |
| -α3.7/αα | Silent carrier | 105 | 9.76 | |
| -α4.2/αα | Silent carrier | 23 | 2.14 | |
| --SEA/αα | Minor | 521 | 48.42 | |
| -α3.7/-α3.7 | Minor | 1 | 0.09 | |
| Subtotal | 668 | 62.08 | ||
| --SEA/-α3.7 | Intermedia | 38 | 3.53 | |
| --SEA/-α4.2 | Intermedia | 3 | 0.28 | |
| --SEA/αCSα | Intermedia | 2 | 0.19 | |
| --SEA/αQSα | Intermedia | 2 | 0.19 | |
| --SEA/-α27.6 | Intermedia | 1 | 0.09 | |
| HKαα/-SEA | Intermedia | 1 | 0.09 | |
| Subtotal | 47 | 4.37 | ||
| β-thalassemia | ||||
| βIVS−II−654 (C→T)/βN | Minor | 142 | 13.20 | |
| βCD41-42 (−TTCT)/βN | Minor | 118 | 10.97 | |
| βCD17 (A→T)/βN | Minor | 53 | 4.93 | |
| β−28 (A→G)/βN | Minor | 9 | 0.84 | |
| βCD71-72 (+A)/βN | Minor | 8 | 0.74 | |
| βCD27-28 (+C)/βN | Minor | 5 | 0.46 | |
| βCAP+40-43(−AAAC)/βN | Minor | 1 | 0.09 | |
| βCD43 (G→T)/βN | Minor | 1 | 0.09 | |
| βInt (ATG→AGG)/βN | Minor | 1 | 0.09 | |
| βCD26 (G→A)/βN | Minor | 7 | 0.65 | |
| Subtotal | 345 | 32.06 | ||
| βCD41-42 (−TTCT)/βCD41-42 (−TTCT) | Major | 2 | 0.19 | |
| βIVS−II−654 (C→T)/βCD41-42 (−TTCT) | Major | 1 | 0.09 | |
| βIVS−II−654 (C→T)/βCD26 (G→A) | Major | 1 | 0.09 | |
| Subtotal | 4 | 0.37 | ||
| α with β-thalassemia | ||||
| --SEA/αα/βCD41-42 (−TTCT)/βN | Minor | 3 | 0.28 | |
| --SEA/αα/βIVS−Ⅰ−1 (G→T)/βN | Minor | 1 | 0.09 | |
| -α3.7/αα/βIVS−II−654 (C→T)/βN | Minor | 3 | 0.28 | |
| -α3.7/αα/βCD17 (A→T)/βN | Minor | 1 | 0.09 | |
| -α3.7/αα/βCD41-42 (−TTCT)/βN | Minor | 2 | 0.19 | |
| -α4.2/αα/β−28(A→G) /βN | Minor | 1 | 0.09 | |
| --SEA/-α3.7/βCD41-42 (−TTCT)/βN | Intermedia | 1 | 0.09 | |
| Subtotal | 12 | 1.12 | ||
| Total | 1076 | 100 | ||
Abbreviations: SEA, Southeast Asian deletion; HK, HongKong deletion; CS, Hb Constant Spring; QS, Hb Quong Sze; WS, Hb Westmead; α0 indicates the absence of α-globin peptide chain synthesis. α+ indicates a decrease in α-globin peptide chain synthesis. α means no mutation. β0 indicates the absence of β-globin peptide chain synthesis; β+indicates a decrease in β-globin peptide chain synthesis; N indicates no mutation.
3.2. Correlation analysis of hematologic parameters of thalassemia gene carriers/patients with different phenotypes
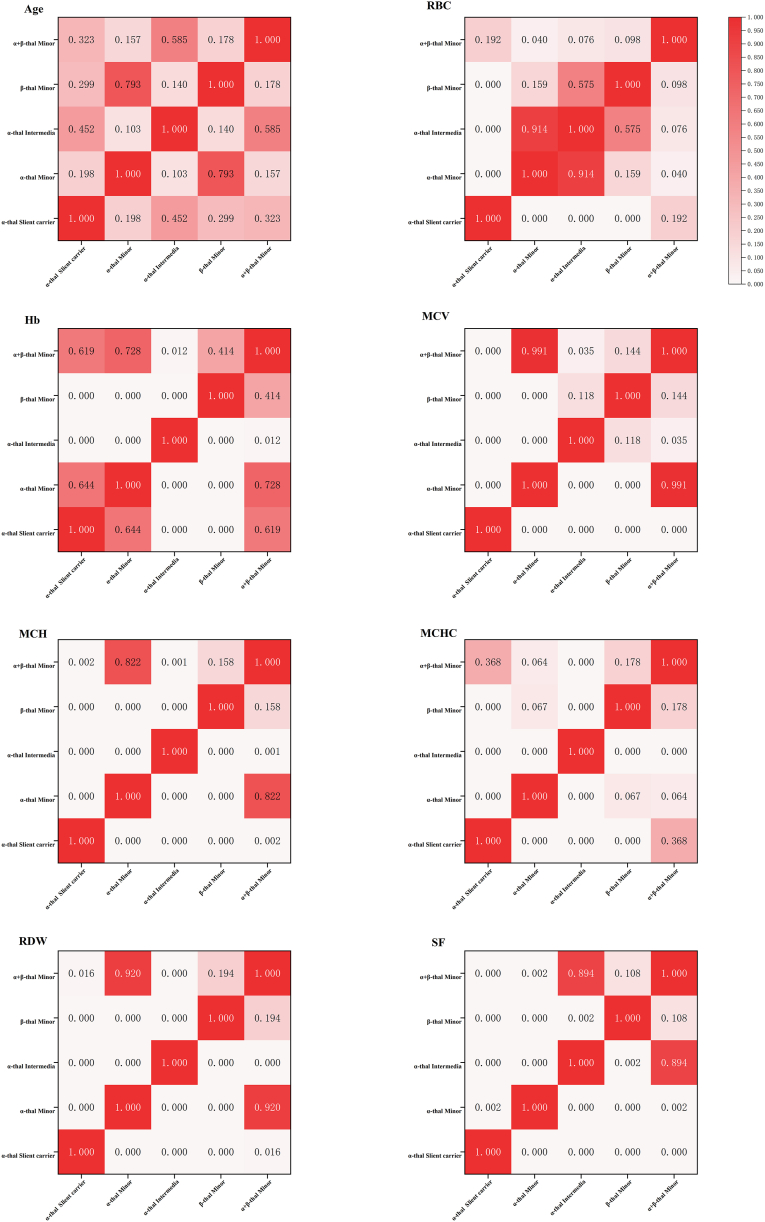
According to the guidelines provided by the Thalassaemia International Federation (TIF) [[11], [12], [13]], we categorized individuals carrying or affected by thalassemia gene mutations into distinct phenotypes(Table 4). The comparative analysis between these groups is presented in Fig. 1 (The sample size of β-thalassemia major were less than 5 cases, the comparison between groups were not included). There was no statistically significant difference in age among the groups (p > 0.05). The red blood cell count (RBC) of individuals with α-thalassemia silent carrier was found to be the lowest, although there was no significant difference compared to those with α&β-thalassemia minor. The α-thalassemia intermedia group exhibited the lowest hemoglobin levels (Hb). In terms of mean corpuscular volume (MCV) and mean corpuscular hemoglobin (MCH), the α-thalassemia silent carrier group demonstrated the highest values. Conversely, the MCHC value of the α-thalassemia intermedia group was found to be the lowest. The RDW values were significantly higher in the groups of α-thalassemia intermedia compared to other groups, whereas the group of α-thalassemia silent carriers exhibited significantly lower RDW values than other groups. Additionally, serum ferritin(SF) levels were significantly lower in both α-thalassemia silent carriers and individuals with α-thalassemia minor compared to other groups.
Table 4.
Phenotypic characteristics of thalassemia in Quanzhou areaa.
| α-thalassemia |
β-thalassemia |
α with β -thalassemiab |
||||
|---|---|---|---|---|---|---|
| Slient carrier (n = 146) | minor (n = 522) | Intermedia (n = 47) | Minor (n = 345) | Major (n = 4) | Minor (n = 11) | |
| Age(year) | 29.00 (25.00,33.00) | 29.00 (14.75,42.00) | 33.50 (5.25,47.75) | 29.00 (11.00,45.00) | 4.00 (1.00,15.25) | 29.00 (27.00,36.00) |
| RBC ( × 1012/L) | 4.28 (3.97,4.60) | 5.22 (4.66,5.61) | 5.30 (4.81,5.78) | 5.17 (4.35,5.82) | 3.12 (2.80,3.98) | 4.63 (4.24,4.94) |
| Hb(g/L) | 105.00 (89.00,116.00) | 107.00 (94.75,115.00) | 90.00 (83.00,96.75) | 102.00 (88.00,110.00) | 84.00 (64.00,103.25) | 108.00 (93.00,116.00) |
| MCV(fL) | 78.00 (67.80,80.70) | 65.80 (61.90,69.00) | 56.60 (54.10,64.28) | 62.40 (59.20,65.30) | 77.20 (71.43,82.98) | 66.80 (62.71,69.40) |
| MCH(pg) | 25.10 (20.40,27.00) | 21.00 (19.50,22.00) | 17.55 (16.00,19.45) | 19.70 (18.80,21.00) | 25.15 (22.80,28.10) | 22.00 (16.34,23.10) |
| MCHC(g/L) | 326.00 (302.00,335.00) | 316.50 (309.00,324.00) | 298.00 (291.25,309.00) | 318.00 (312.00,325.00) | 331.00 (321.00,338.75) | 328.00 (302.14,336.00) |
| RDW-CV (%) | 14.00 (13.30,18.90) | 15.90 (14.80,17.90) | 23.50 (20.90.,24.85) | 17.00 (15.60,18.30) | 19.88 (14.24,24.13) | 15.56 (14.30,20.25) |
| SF(ng/mL) | 15.90 (4.60,40.50) | 47.45 (19.38,125.33) | 181.67 (77.6,42.41) | 78.00 (41.80,257.90) | 793.56 (220.62,1466.63) | 328.73 (43.36,625.49) |
Age and hematological parameters were statistically described by median (P25,P75).
There was only one case of α with β-thalassemia intermedia, which was not described statistically.
Fig. 1.
The heat map of age and phenotype between 5 phenotypes of thalassemia in Quanzhou area. (The sample size of β-thalassemia major were less than 5 cases, the comparison between groups were not included). The abbreviation "thal" was derived from the term "thalassemia". Independent sample Kruskal-Wallis test was used to compare parameters among the groups, the numbers in the box are p values for comparison among the groups, and ∗p < 0.05 has statistical significance.
3.3. Phenotypic analysis of thalassemia with/without iron metabolism abnormality
A total of 1076 patients with thalassemia were divided into thalassemia +ID group, thalassemia alone group and thalassemia + IO group (Table 5). 94 cases were diagnosed with thalassemia +ID,70 cases were diagnosed with thalassemia + IO and 912 cases were diagnosed with thalassemia alone. In both the thalassemia +ID and thalassemia alone groups, the prevalence of α-thalassemia minors was observed to be the highest (53.2 % and 49.6 %, respectively). Conversely, within the thalassemia + IO group, β-thalassemia minor exhibited the largest proportion (48.6 %).
Table 5.
Analysis of iron metabolism in 1076 patients with thalassemia.
| Thalassemia +ID |
Thalassemia Alone |
Thalassemia + IO |
||||
|---|---|---|---|---|---|---|
| Subjects (n) | Frequency | Subjects (n) | Frequency | Subjects (n) | Frequency | |
| α-Thalassemia | ||||||
| Silent carrier | 24 | 25.5 % | 121 | 13.3 % | 1 | 1.4 % |
| minor | 50 | 53.2 % | 452 | 49.6 % | 20 | 28.6 % |
| Intermedia | 0 | 0.0 % | 35 | 3.8 % | 12 | 17.1 % |
| Subtotal | 74 | 78.7 % | 608 | 66.7 % | 33 | 47.1 % |
| β-Thalassemia | ||||||
| minor | 19 | 20.2 % | 292 | 32.0 % | 34 | 48.6 % |
| major | 0 | 0.0 % | 2 | 0.2 % | 2 | 2.9 % |
| Subtotal | 19 | 20.2 % | 294 | 32.2 % | 36 | 51.5 % |
| α+β-thalassemia | ||||||
| minor | 1 | 1.1 % | 9 | 1.0 % | 1 | 1.4 % |
| Intermedia | 0 | 0.0 % | 1 | 0.1 % | 0 | 0.0 % |
| Subtotal | 1 | 1.1 % | 10 | 1.1 % | 1 | 1.4 % |
| Total | 94 | 100 % | 912 | 100 % | 70 | 100.0 % |
We recruited a total of 878 patients with thalassemia minor, including 522 cases of α-thalassemia minor, 345 cases of β-thalassemia minor, and 11 cases of α with β-thalassemia minor. These patients were categorized into three groups: ID group (thalassemia minor + ID), normal group (thalassemia minor alone), and IO group (thalassemia minor + IO). The analysis and comparison of age, sex composition ratio, and hematological parameters among the three groups revealed that the IO group exhibited a significantly higher of age and proportion of male participants compared to the other two groups, while their RBC count was lower than that of the other groups. The Hb levels in the iron metabolism normal group were significantly higher than those in the other two groups, while RDW was significantly lower. Regarding MCV, MCH, and MCHC values, the ID group exhibited significantly lower levels compared to the other two groups (Table 6).
Table 6.
Analysis of age, gender, and hematological parameters analysis of thalassemia minor with/without iron metabolism abnormalitya.
| Thalassemia minor + ID (n = 70) | Thalassemia minor Alone (n = 753) | Thalassemia minor + IO (n = 55) | p-value1 | p-value2 | p-value3 | |
|---|---|---|---|---|---|---|
| Age(year) | 25.00(5.50,33.25) | 27.00.(15.00,33.00) | 54.00(33.0,62.00) | 0.394 | <0.001b | <0.001b |
| Gender (male/female) | 22/48 | 252/501 | 36/19 | 0.729 | <0.001b | <0.001b |
| RBC( × 1012/L) | 5.20(4.58,5.6) | 5.26(4.71,5.72) | 4.35(3.43,5.27) | 0.205 | <0.001b | <0.001b |
| Hb(g/L) | 90.00(83.00,103.00) | 107.00(98.00,116.00) | 89.00(75.00,109.00) | <0.001b | 0.265 | <0.001b |
| MCV(fL) | 59.50(55.30,63.00) | 64.60(61.30,68.00) | 64.90(61.90,71.00) | <0.001b | <0.001b | 0.215 |
| MCH(pg) | 18.10(16.00,19.90) | 20.70(19.30,21.90) | 20.80(19.60,22.10) | <0.001b | <0.001b | 0.522 |
| MCHC(g/L) | 311.00(288.00,318.00) | 318.50(312.00,324.00) | 318.00(309.00,327.00) | <0.001b | <0.001b | 0.408 |
| RDW-CV(%) | 18.60(16.00,22.00) | 15.90(15.00,17.23) | 18.30(15.90,21.20) | <0.001b | 0.656 | <0.001b |
| SF(ng/mL) | 6.00(3.80,9.20) | 59.20(35.88,120.63) | 563.20(486.90,805.56) | <0.001b | <0.001b | <0.001b |
aHematological parameters were statistically described by median (P25, P75), Kruskal-wallis nonparametric test was used for comparison of age and hematological parameters between groups. Bonferroni's method was used for multiple comparisons based on Pearson's chi-square test, the adjusted statistical significance level (α') for the comparison among the three groups was 0.0167.
p-value1:Comparison of hematological parameters between "thalassemia+ID group" and "thalassemia alone group".
p-value2: Comparison of hematological parameters between "thalassemia+ID group" and "thalassemia+IO group".
p-value3: Comparison of hematological parameters between "thalassemia alone group" and "thalassemia+IO group".
b p<0.05 or <0.0167(for Pearson's chi-square test).
3.4. Analysis of age, gender, and hematological parameters in individuals with α-thalassemia minor and β-thalassemia minor exhibiting normal iron metabolism
Among the 753 cases of individuals with α or β-thalassemia minor alone (α-thalassemia minor, β-thalassemia minor, α with β-thalassemia minor), who exhibited normal iron metabolism, there were 452 cases of α-thalassemia minor, 292 cases of β-thalassemia minor, and 9 cases of α with β-thalassemia minor. In order to discern the disparity between α and β thalassemia patients, we meticulously selected individuals with α and β thalassemia minor who exhibited normal iron metabolism (452 cases of α-thalassemia minor, 292 cases of β-thalassemia minor). Subsequently, we conducted a comparative analysis on age, gender, and hematological parameters within these two cohorts.There were no significant difference in diagnosis age/sex composition ratio and RBC between the two groups (p > 0.05). Hb, MCV, MCH levels in α-thalassemia minor alone were higher than those in β-thalassemia minor alone, while RDW and SF levels exhibited an inverse relationshipr(p < 0.05) (Table 7).
Table 7.
Correlation analysis of the relationship between age, gender, and hematological parameters in individuals with α-thalassemia minor or β-thalassemia minor who exhibit normal iron metabolism.
| α-thalassemia minor(n = 452) | β- thalassemia minor(n = 292) | p-valuea | |
|---|---|---|---|
| Age(year) | 29.00(13.00,36.00) | 25.00(8.00,35.00) | 0.350 |
| Gender (male/female) | 151/301 | 100/192 | 0.813 |
| RBC( × 1012/L) | 5.24(4.80,5.67) | 5.31(4.63,5.89) | 0.204 |
| Hb(g/L) | 110.00(101.00,117.00) | 104.00(94.75,114.00) | <0.001b |
| MCV(fL) | 66.50(63.40,69.10) | 61.90(59.18,65.00) | <0.001b |
| MCH(pg) | 21.10(20.10,22.00) | 19.70(18.75,20.90) | <0.001b |
| MCHC(g/L) | 319.00(312.00,325.00) | 318.00(312.75,324.00) | 0.380 |
| RDW-CV(%) | 15.60(14.60,16.70) | 16.45(15.40,17.80) | <0.001b |
| SF(ng/mL) | 55.20(29.48,121.00) | 65.00(46.28,117.20) | 0.028b |
Hematological parameters and Age were statistically described by median (P25,P75), Kruskal-wallis nonparametric test was used to analyze the significance of hematological parameters in each group, with 95 % confidence interval (two-sided). Pearson's chi-square test was used to test the significance of sex composition ratio among all groups.
p < 0.05.
3.5. Correlation analysis of hematological phenotypes with respect to age and gender in thalassemia
Taking -SEA/αα as an example, there were 521 cases with the genotype of -SEA/αα gene. Among these cases, 452 individuals without iron disorder (thalassemia alone). The hematological parameters of these cases were analyzed based on different age and gender. It was found that Hb, MCV, MCH, MCHC and SF levels in cases under 18 years old were lower than those in cases over 18 years old, while the RBC level was higher than that in the latter group(p < 0.05). The levels of Hb, MCV, MCH, and SF in male subjects under 18 years old were found to be significantly lower compared to those above 18 years old (p < 0.05). In the cases of female subjects, certain hematological parameters including MCV, MCH and MCHC were found to be lower in the under 18 years old group compared to the above 18 years old group, however RBC levels exhibited an opposite trend (p < 0.05). For cases under 18 years old, there was no difference in hematological parameters between male and female (p > 0.05).For cases over 18 years old, the RBC, Hb, MCHC and SF levels in male cases were significantly higher than those in female cases (p < 0.05) (Table 8).
Table 8.
Correlation analysis of hematological phenotypes with age and gender in the thalassemia-only group (taking --SEA/αα as an example)a.
| Age groups | Gender | RBC ( × 1012/L) | Hb (g/L) | MCV (fL) | MCH (pg) | MCHC (g/L) | RDW-CV (%) | SF (ng/mL) |
|---|---|---|---|---|---|---|---|---|
| ≤18 years (n = 114) | 5.40 (5.03,5.79) | 106.00 (97.00,112.00) | 62.00 (60.00,64.50) | 19.50 (18.90,20.50) | 317.00 (308.00,321.00) | 15.80 (15.10,17.00) | 40.60 (27.73,59.09) | |
| male (n = 64) | 5.60 (4.99,5.76) | 107.00 (94.50,113.00) | 62.30 (60.35,65.25) | 20.00 (19.05,20.60) | 316.00 (307.50,322.50) | 16.40 (15.05,17.90) | 40.60 (32.80,59.50) | |
| female (n = 50) | 5.35 (5.00,5.80) | 105.50 (97.00,109.50) | 61.75 (59.35,64.33) | 19.30 (18.80,20.13) | 319.00 (307.75,320.25) | 15.50 (15.18,16.53) | 40.55 (24.20,58.90) | |
| ≥19 years (n = 338) | 5.20 (4.68,5.50) | 112.00 (1040.00,119.00) | 67.60 (65.00,70.00) | 21.70 (21.00,22.30) | 320.00 (313.00,326.00) | 15.50 (14.60,16.50) | 66.27 (31.75,128.88) | |
| male (n = 82) | 5.59 (5.08,6.69) | 127.00 (105.50,142.50) | 69.00 (65.50,70.50) | 21.90 (20.80,22.35) | 314.00 (310.00,323.00) | 15.80 (14.50,18.35) | 163.00 (117.44,246.09) | |
| female (n = 256) | 5.10 (4.66,5.40) | 110.00 (104.00,116.00) | 67.30 (65.00,69.50) | 21.55 (20.98,22.33) | 321.00 (313.00,326.00) | 15.45 (14.60,16.33) | 49.05 (25.65,89.81) | |
| Comparison of Hematological parameters between different age groupsa | ||||||||
| p1 | 0.041b | 0.005b | 0.000b | 0.000b | 0.036b | 0.107 | 0.016b | |
| Comparison of hematological parameters in males berween different age groupsa | ||||||||
| P2 | 0.238 | 0.004b | 0.000b | 0.000b | 0.869 | 0.538 | 0.000b | |
| Comparison of hematological parameters in females berween different age groupsa | ||||||||
| P3 | 0.015b | 0.066 | 0.000b | 0.000b | 0.042b | 0.621 | 0.460 | |
| Comparison of hematological parameters between male and female in each age groupa | ||||||||
| P4 (≦18 years of age group: Male VS Female) | 0.951 | 0.851 | 0.362 | 0.251 | 0.902 | 0.107 | 0.481 | |
| P5 (≥19 years of age group: Male VS Femal) | 0.001b | 0.001b | 0.171 | 0.833 | 0.026b | 0.350 | 0.000b | |
Age and hematological parameters were statistically described by median (P25,P75). hematological parameters were compared by Wilcoxon rank sum test, with 95 confidence intervals (two-sided).
p < 0.05.
4. Discussion
Thalassemia is an autosomal recessive disorder, characterized by defects in the globin gene that result in reduced or absent synthesis of globin peptide chains, leading to microcytic hypochromic anemia [3,5]. It is crucial to differentiate thalassemia from iron deficiency anemia (IDA) and other disorders related to iron metabolism. The recent studies on thalassemia have demonstrated the significant scientific value of the genotype-phenotype correlation in genetic counseling, diagnosis, and treathalassemiaent of this disorder [[14], [15], [16], [17]].However, the phenotypic manifestations of thalassemia vary across different regions due to the specific distribution of genotypes [5].
In this study, the genotypes and phenotypes of 1076 thalassemia carriers or patients in Quanzhou City were analyzed to complement the research in this area. Among the 2997 subjects suspected with thalassemia in Quanzhou area, a total of 1076 cases were diagnosed as thalassemia carriers or patients, resulting in a detection rate of 35.9 % (1076/2997), which was higher than the detection rate reported in 2013 [7] and 2019 [8], which might be attributed to variations in the included populations. Notably, --SEA/αα emerged as the most prevalent α-thalassemia genotype, while βIVSII−654(C→T)/βN was identified as the most common β-thalassemia genotype. These findings aligned with those reports conducted in 2013 [7] and 2019 [8]. When categorizing thalassemia based on TIF recommendations, the α-thalassemia silent carrier exhibited the mildest anemia symptoms, as evidenced by significantly higher MCV, MCH, and MCHC values compared to other groups, along with a significantly lower RDW. The relatively mild anemia symptoms of the α-thalassemia silent carrier can be attributed to the absence or dysfunction of only one out of its four alpha-globin genes, which determines the clinical condition of α-thalassemia [13].
Currently, there was no available literature investigating of thalassemia combined with iron metabolism disorder or exploring the correlation between the hematological parameters and age, gender for subjects with thalassemia in this region. In our study, 8.74 %(94/1076) of cases were diagnosed with thalassemia + ID, 6.50 %(70/1076) of cases were diagnosed with thalassemia + IO and 84.76 %(912/1076) of cases were diagnosed with thalassemia alone. We observed that in the individuals of thalassemia minor, the age at diagnosis for thalassemia + IO was significantly higher compared to the other two groups(thalassemia +ID and thalassemia alone group)(54 vs 25 and 27 years old), which might be attributed to the formation mechanism of iron overload formation [18] and the changes of ferritin reference interval with age [19,20]. According to the "Guideline for the laboratory diagnosis of iron deficiency in adults (excluding pregnancy) and children(2022). " [21], we evaluated the degree of anemia in patients. Subjects with thalassemia alone exhibited milder of anemia, characterized by higher RBC and Hb levels; but, lower RDW values than those in subject with thalassemia combined with ID or IO(RDW is an indicator reflecting the heterogeneity of red blood cell size, which is inversely proportional to the degree of anemia [22]).
Interestingly, in the cohort of thalassemia minor alone cases(452 cases of α-thalassemia minor, 292 cases of β-thalassemia minor), whose iron metabolism were normal, individuals carrying the α-thalassemia gene exhibited milder anemia symptoms compared to those carrying the β-thalassemia gene. α-thalassemia minor showed higher levels of Hb, MCV, and MCH than their β-thalassemia minor counterparts, while RDW and SF levels were lower in the former group. This might be attributed to the different locations and degrees of globin gene defects (α-thalassemia gene mutations are mainly deletion type, while β-thalassemia gene mutations are mainly point mutations [5,23,24]), resulting in distinct clinical manifestations such as anemia, hemolysis, and compensatory erythrocyte increase. The comparison of hematologic parameters between α and β patients yielded contrasting results with carriers: the levels of MCV, MCH, and MCHC were found to be significantly lower in α-thalassemia patients compared to β-thalassemia patients, while RDW exhibited higher values. Consequently, the MCV, MCH, and MCHC levels were found to be higher in β-thalassemia patients compared to β-thalassemia gene carriers, whereas RBC, RDW and SF values were lower in the former group. It was worth noting that these observations might also be attributed to the limited sample size of β-thalassemia patients, leading to a significant sampling error. For cases with α-thalassemia, we observed that carriers exhibited higher levels of Hb, MCV, MCH, and MCHC while the RDW and SF levels were lower compared to patients. These findings suggested that α-thalassemia carriers experienced milder anemia symptoms than α-thalassemia patients. The aforementioned observation was in line with the established knowledge that carriers of the thalassemia gene exhibit a lesser degree of anemia compared to patients [25].
In the analysis of hematological phenotype selected 452 cases with --SEA/αα genotype and without any evidence of iron metabolism disorder to ensure the exclusion of its potential influence on phenotype. The correlation analysis of age, gender and hematological parameters revealed that: Hb, MCV, MCH, MCHC and SF levels were higher in subjects over 18 years old compared to those under 18 years old. The observed difference in SF between the two groups aligns with the currently accepted theory that suggested a gradual worsening of ineffective hematopoiesis and hemolysis caused by thalassemia with the increasing age [25]. The observed differences in Hb, MCV, MCH, and MCHC between the two groups are consistent with recent findings in the field. Stevens Gretchen et al., 2013 reported that the reference interval for hemoglobin in children is globally lower than that in adults [26], and Strand Martin Frank et al., 2022 reported an age-related increasing trend in red blood cell parameters [27]. Currently, there was a lack of reference intervals for hematological parameters among individuals under 18 years old residing in Quanzhou area. In order to enhance the comprehensiveness of this study, future research will focus on collecting hematological data from residents of different ages in Quanzhou, and establishing age-specific reference intervals for hematological parameters. The levels of RBC, Hb, MCHC and SF of males over 18 years old were significantly higher than those in females within the same group (p < 0.05). According to the regulation of "Reference intervals for blood cell analysis" WS/T 405–2012 of China, the reference intervals for RBC, Hb and other parameters in adult males exhibited higher values compared to observed in females [28]. Due to the secretion of more male hormones, the erythrocyte production is promoted, and the erythrocyte parameters are higher compared to females [29,30]. Given the absence of a reference interval for SF in Quanzhou region, we adopted the laboratory biological reference interval for SF (male: 23.9–336.2 ng/ml; Female: 11.0–306.8 ng/ml). Current literature consistently demonstrated higher SF levels in adult males compared to females, attributed to factors such as menstrual blood loss and dietary habits [31].”
In our analysis of hematological parameters, we did not consider the impact of pathology, and physiological factors such as pregnancy and infection on the reference intervals. We used laboratory reference intervals (varying by sex) to diagnose iron deficiency. However, studies have shown significant variations in serum ferritin (SF) levels among healthy individuals of different ages, genders, menopausal status, and obesity [[30], [31], [32]]. Elevated levels of ferritin have been observed during infection [29]. Some scholars have suggested using C-reactive protein (CRP) as an indicator to exclude infection when certifying the biologic reference interval of SF [33]. In future studies, we will thoroughly consider the influencing factors of pregnancy, and infection on SF levels. We will also utilize the ROC curve to identify the optimal cut-off value for diagnosing iron deficiency or iron overload in patients with thalassemia, providing a robust diagnostic foundation for the management of iron metabolism in these patients.
5. Conclusion
In summary, we analyzed data from 1076 thalassemia carriers and patients in the Quanzhou area and discovered that anemia in individuals with thalassemia + ID were observed to be more severe than those with thalassemia alone or thalassemia + IO. Moreover, the anemia severity of carriers with α-thalassemia alone was found to be less pronounced compared with carriers with β-thalassemia alone and the hematologic phenotype of carriers with the same thalassemia genotype was found to be correlated with age and gender.
CRediT authorship contribution statement
Nan Huang: Writing – original draft, Data curation. Yufang Wang: Funding acquisition. Hailong Huang: Writing – review & editing. Zixuan Chen: Validation, Investigation. Zhishan Zhang: Project administration.
Ethics approval and consent to participate
This study was reviewed and approved by the Ethical Committee of Quanzhou First Hospital Affiliated to Fujian Medical University([2018]NO.223), December 27, 2018. All of them or their parents had signed the informed consent before the experiment voluntarily.
Declaration of data authenticity
All data of the tables submitted have been created by the authors who confirm that the data are original with no duplication and have not been previously published in whole or in part.
Data availability statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Funding information
This study was funded by the grants from the Medical and Health Field Guiding Science and Technology Program of Quanzhou in 2021(grant no. 2021N123S).
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:Yufang Wang reports financial support was provided by Quanzhou science and Technology Bureau.
Acknowledgements
The authors thank the patients and volunteers for their support of this study and the Medical and health field Guiding science and technology Program of Quanzhou in 2021 (grant no. 2021N123S) funding for this study.
Contributor Information
Hailong Huang, Email: huanghailong@fjmu.edu.cn.
Zhishan Zhang, Email: 554882707@qq.com.
References
- 1.Huang Hailong, Chen Meihuan, Chen Lingji, et al. Prenatal diagnosis of thalassemia in 695 pedigrees from southeastern China: a 10-year follow-up study. J. Clin. Lab. Anal. 2021;35 doi: 10.1002/jcla.23982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rachmilewitz E.A., Giardina P.J. How I treat thalassemia. Blood. 2011;118(13):3479–3488. doi: 10.1182/blood-2010-08-300335. [DOI] [PubMed] [Google Scholar]
- 3.Weatherall D.J. Thalassemia as a global health problem: recent progress toward its control in the developing countries. Ann. N. Y. Acad. Sci. 2010;1202:17–23. doi: 10.1111/j.1749-6632.2010.05546.x. [DOI] [PubMed] [Google Scholar]
- 4.Torti L. Life beyond alpha-thalassaemia: we are moving forward. Br. J. Haematol. 2022;199(1):11–13. doi: 10.1111/bjh.18362. [DOI] [PubMed] [Google Scholar]
- 5.Zhuang J., Jiang Y., Wang Y., et al. Molecular analysis of α-thalassemia and β-thalassemia in Quanzhou region southeast China. J. Clin. Pathol. 2020;73(5):278–282. doi: 10.1136/jclinpath-2019-206179. [DOI] [PubMed] [Google Scholar]
- 6.Lin H.Q., Wu J.Y., Chen M.L., et al. Prevalence of dyslipidemia and prediction of 10-year CVD risk among older adults living in southeast coastal regions in China: a cross-sectional study. Clin. Interv. Aging. 2019;14:1119–1129. doi: 10.2147/CIA.S207665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu L.P., Huang H.L., Wang Y., et al. Zhonghua yi xue yi chuan xue. Za Zhi. 2013;30(4):403–406. doi: 10.3760/cma.j.issn.1003-9406.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 8.Jiang Y.C., Chen Z.X., Chen Y.B., Zhang Z.S. Zhongguo shi yan xue ye xue. Za Zhi. 2019;27(6):1943–1948. doi: 10.19746/j.cnki.issn.1009-2137.2019.06.038. [DOI] [PubMed] [Google Scholar]
- 9.Zhang M., Huang H., Chen M., et al. Frequencies and hematological manifestations of the HKαα allele in southern Chinese population. Int. J. Clin. Exp. Pathol. 2019;12(8):3058–3062. PMID: 31934145; PMCID: PMC6949723. [PMC free article] [PubMed] [Google Scholar]
- 10.Pereira-Maxwell F. Taylor and Francis; 2017-09-15. Medical Statistics[M] [Google Scholar]
- 11.Cappellini M.D., Farmakis D., Porter J., Taher A., editors. 2021 Guidelines: for the Management of Transfusion Dependent Thalassaemia (TDT) fourth ed. Thalassaemia International Federation; Nicosia (Cyprus): 2023. [PubMed] [Google Scholar]
- 12.Taher A.T., Musallam K.M., Cappellini M.D. third ed. Thalassaemia International Federation; 2023. Guidelines for the Management of Non-transfusion-dependent β-Thalassaemia. Nicosia (Cyprus) [PubMed] [Google Scholar]
- 13.Amid A., Lal A., Coates T.D., Fucharoen S., editors. Guidelines for the Management of α-Thalassaemia. Thalassaemia International Federation; Nicosia (Cyprus): 2023. [PubMed] [Google Scholar]
- 14.Cao A., Galanello R., Rosatelli M.C. Genotype-phenotype correlations in beta-thalassemias. Blood Rev. 1994;8(1):1–12. doi: 10.1016/0268-960x(94)90002-7. [DOI] [PubMed] [Google Scholar]
- 15.George E., Ann T.J. Genotype-phenotype diversity of beta-thalassemia in Malaysia: treathalassemiaent options and emerging therapies. Med. J. Malaysia. 2010;65(4):256–260. PMID: 21901940. [PubMed] [Google Scholar]
- 16.Shoujaa A., Moasses F., Mukhalalaty Y., Murad H., Al-Quobaili F. Genotype/phenotype correlation of β-thalassemia in Syrian patients: a cross-sectional study. Hemoglobin. 2020;44(1):42–46. doi: 10.1080/03630269.2019.1709207. Epub 2020 Jan 6. PMID: 31903828. [DOI] [PubMed] [Google Scholar]
- 17.Pan Yali, Chen Meihuan, Zhang YanHong, et al. Analysis of genotype-phenotype correlation in patients with α-thalassemia from Fujian province, Southeastern China. J. Clin. Lab. Anal. 2022;36 doi: 10.1002/jcla.24696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hsu Christine C., Senussi Nizar H., Fertrin Kleber Y., et al. Iron overload disorders. Hepatol Commun. 2022;6:1842–1854. doi: 10.1002/hep4.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Singh S., Geddam J.J.B., Reddy G.B., et al. Folate, vitamin B12, ferritin and haemoglobin levels among women of childbearing age from a rural district in South India. BMC Nutr. 2017;3:50. doi: 10.1186/s40795-017-0173-z. Published 2017 Jun 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen N., Li Z., Huang Y., et al. Iron parameters in pregnant women with beta-thalassaemia minor combined with iron deficiency anaemia compared to pregnant women with iron deficiency anaemia alone demonstrate the safety of iron supplementation in beta-thalassaemia minor during pregnancy. Br. J. Haematol. 2022;196(2):390–396. doi: 10.1111/bjh.17827. [DOI] [PubMed] [Google Scholar]
- 21.Fletcher A., Forbes A., Svenson N., Wayne Thomas D., A British Society for Haematology Good Practice Paper Guideline for the laboratory diagnosis of iron deficiency in adults (excluding pregnancy) and children. Br. J. Haematol. 2022;196(3):523–529. doi: 10.1111/bjh.17900. [DOI] [PubMed] [Google Scholar]
- 22.Hoffmann JJ, Nabbe KC, van den Broek NM. Effect of age and gender on reference intervals of red blood cell distribution width (RDW) and mean red cell volume (MCV). Clin Chem Lab Med2015;53(12):2015-2019 doi:10.1515/cclm-2015-0155. [DOI] [PubMed]
- 23.Origa raffaella, β-thalassemia. Genet. Med. 2017;19:609–619. doi: 10.1038/gim.2016.173. [DOI] [PubMed] [Google Scholar]
- 24.Shafique F., Ali S., Almansouri T., et al. Thalassemia, a human blood disorder. Braz. J. Biol. 2021;83 doi: 10.1590/1519-6984.246062. [DOI] [PubMed] [Google Scholar]
- 25.Ruan D.D., Gan Y.M., Lu T., et al. Genetic diagnosis history and osteoarticular phenotype of a non-transfusion secondary hemochromatosis. World J Clin Cases. 2020;8(23):5962–5975. doi: 10.12998/wjcc.v8.i23.5962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stevens G.A., Finucane M.M., De-Regil L.M., et al. Global, regional, and national trends in haemoglobin concentration and prevalence of total and severe anaemia in children and pregnant and non-pregnant women for 1995-2011: a systematic analysis of population-representative data. Lancet Glob Health. 2013;1(1):e16–e25. doi: 10.1016/S2214-109X(13)70001-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Strand M.F., Fredriksen P.M., Lindberg M. Hematology reference intervals in 6-12-year-old children: the health-oriented pedagogical project (HOPP) Scand. J. Clin. Lab. Invest. 2022;82(5):404–409. doi: 10.1080/00365513.2022.2100820. [DOI] [PubMed] [Google Scholar]
- 28.Affiliated Hospital of China Medical University . National Health Commission of the People’s Republic of China; Beijing: 2012. Clinical Laboratory Center of National Health Commission, Zhongshan Hospital Affiliated to Fudan University, et al(China).Reference intervals for blood cell analysis. [Google Scholar]
- 29.Tzounakas V.L., Anastasiadi A.T., Drossos P.V., et al. Sex-related aspects of the red blood cell storage lesion. Blood Transfus. 2021;19(3):224–236. doi: 10.2450/2020.0141-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen S., Liu Y., Cai L., et al. Erythropoiesis changes with increasing age in the elderly Chinese. Int J Lab Hematol. 2021;43(5):1168–1173. doi: 10.1111/ijlh.13615. [DOI] [PubMed] [Google Scholar]
- 31.Rushton D.H., Barth J.H. What is the evidence for gender differences in ferritin and haemoglobin? Crit. Rev. Oncol. Hematol. 2010;73(1):1–9. doi: 10.1016/j.critrevonc.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 32.Mahroum N., Alghory A., Kiyak Z., et al. Ferritin - from iron, through inflammation and autoimmunity, to COVID-19. J. Autoimmun. 2022;126 doi: 10.1016/j.jaut.2021.102778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eie A.M.Ø., Strand M.F., Fredriksen P.M., Lindberg M. Population-based reference intervals for ferritin, iron, transferrin and transferrin saturation and prevalence of iron deficiency in 6-12-year-old children: the Health Oriented Pedagogical Project (HOPP) Scand. J. Clin. Lab. Invest. 2021;81(3):208–212. doi: 10.1080/00365513.2021.1884893. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.