Abstract
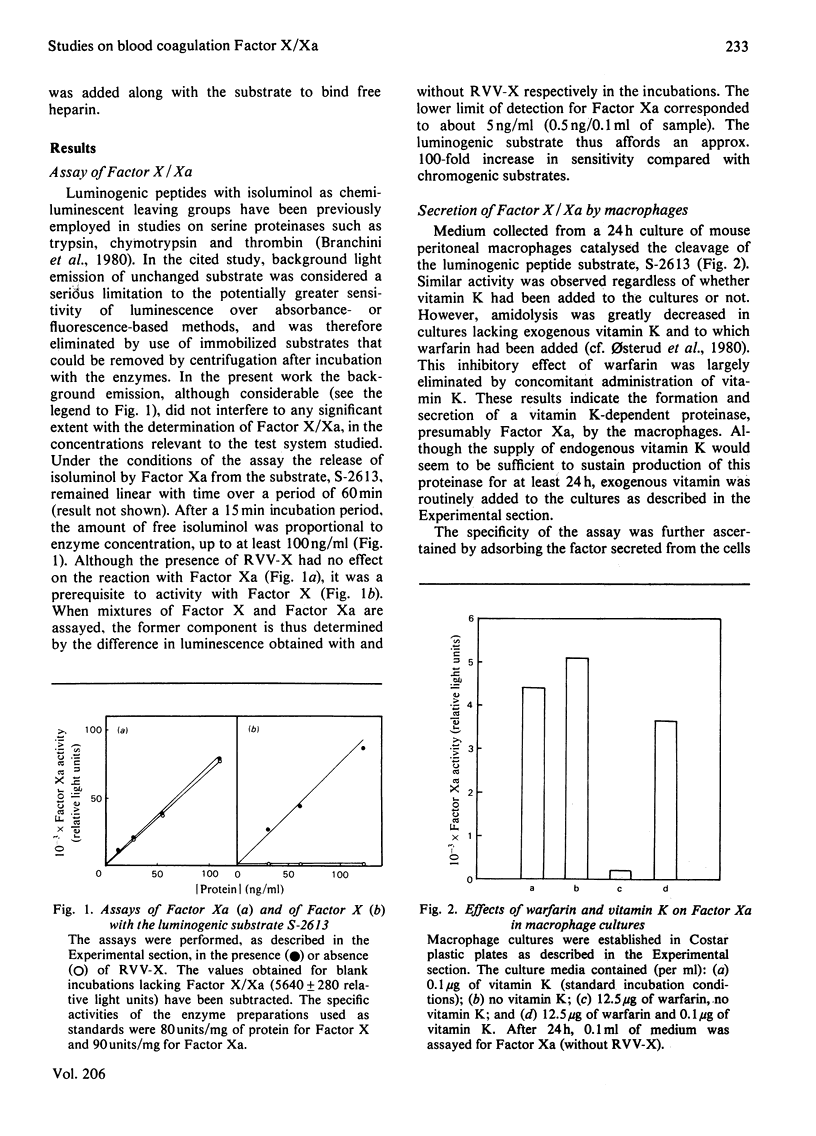
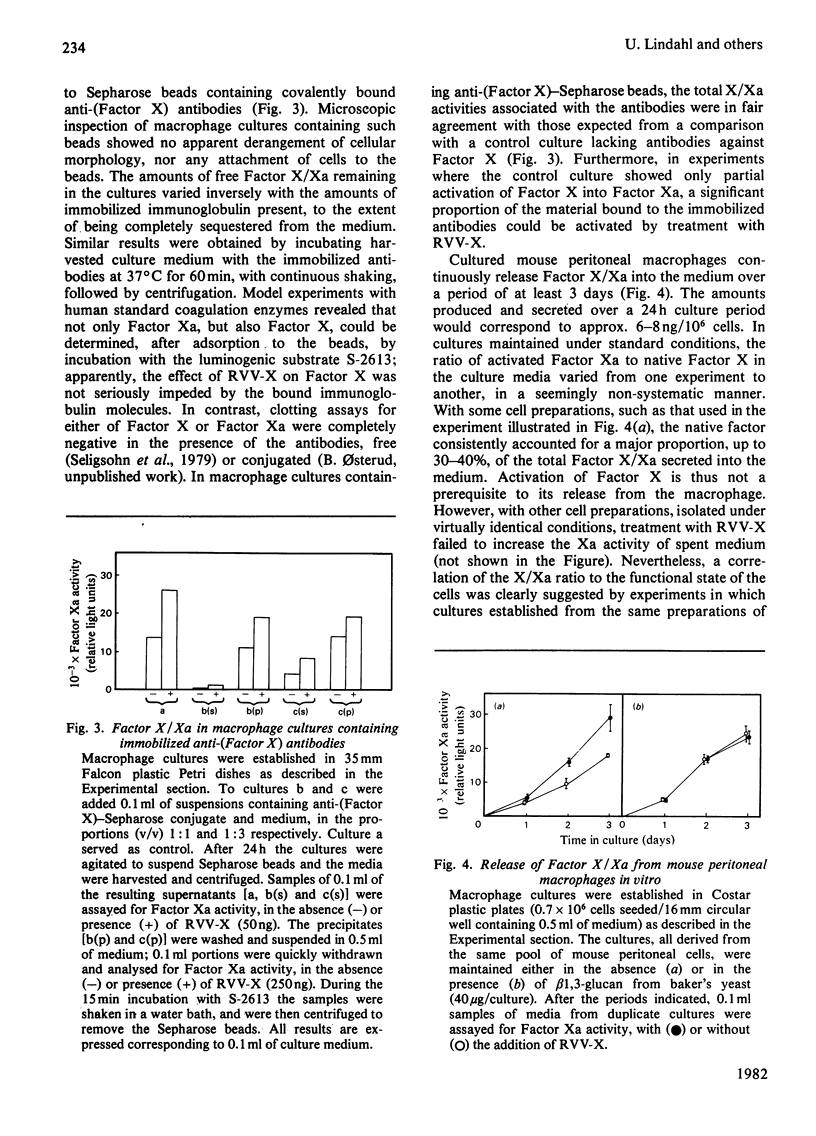
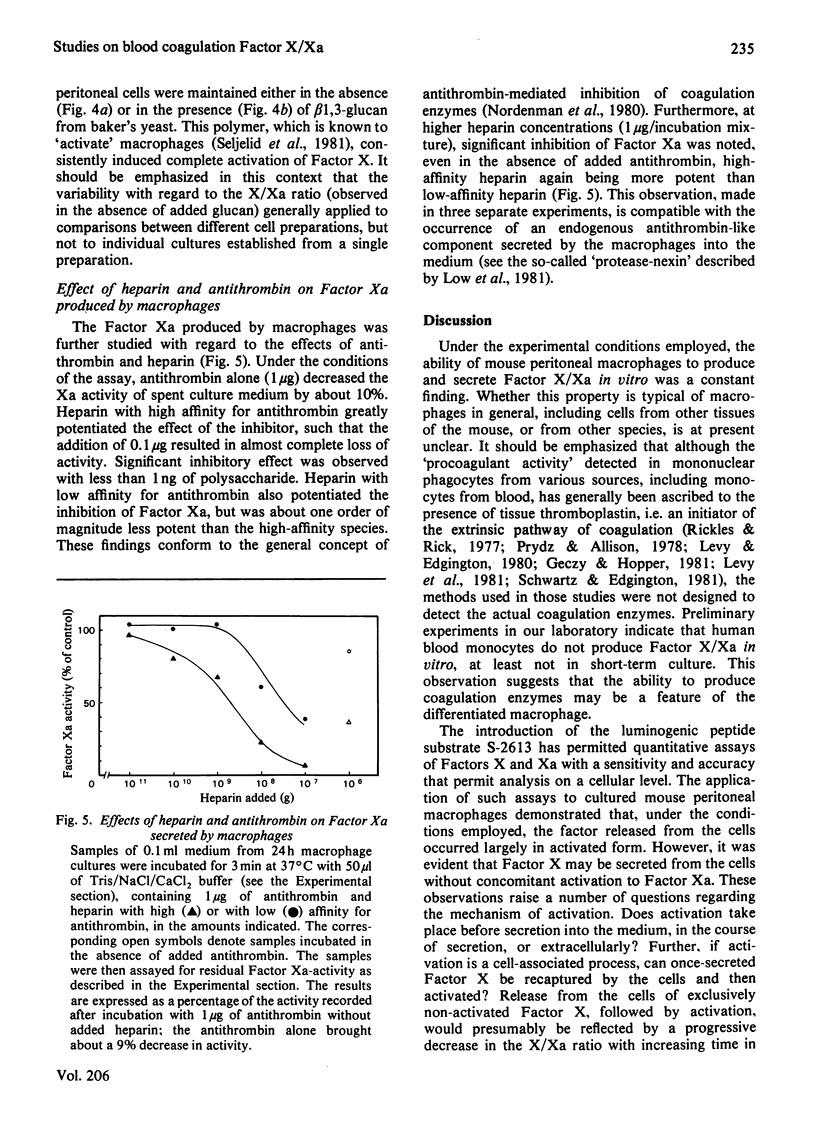
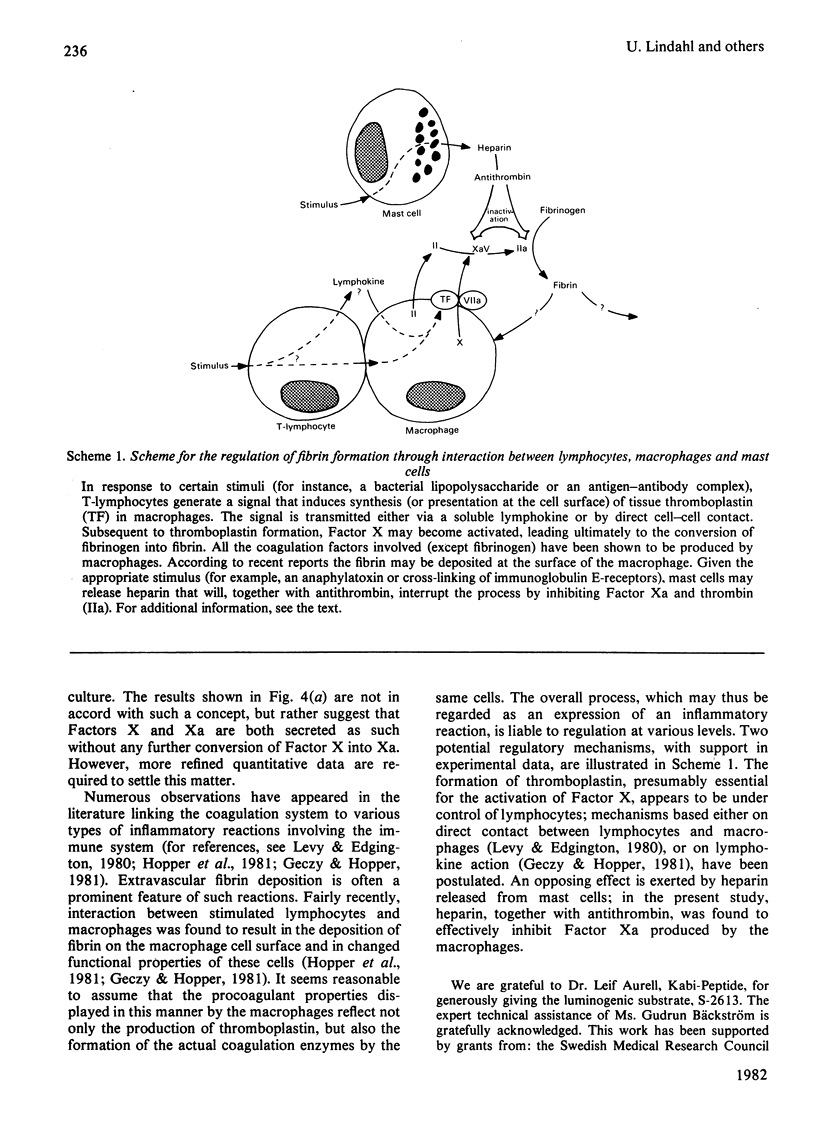
The formation and secretion of coagulation Factor X/Xa by mouse peritoneal macrophages was studied with a luminogenic peptide substrate (S-2613; t-butyloxycarbonylisoleucylglutamyl-γ-piperidylglycylarginylisoluminol). Amidolysis was quantified by measuring the light emitted during oxidation of isoluminol, released by Factor Xa. A lower detection limit of about 0.5ng of Factor Xa was established; the assay was linear with enzyme concentration up to at least 100ng/ml. Factor X was determined after treatment with the Factor X-activating component of Russell's-viper (Vipera russelli) venom. Macrophages, cultured in the absence of serum, released Factor X/Xa into the culture medium. The concentration of coagulation enzyme in the medium increased in an essentially linear fashion over a period of at least 3 days, at a rate corresponding to 6–8ng produced/24h per 106 cells. The ratio of Factor Xa/X+Xa varied from about 60 to 100%, showing that activation of Factor X to Xa is not prerequisite to release of the enzyme from the cells. Factor Xa activity was suppressed in the presence of warfarin [3-(α-acetonylbenzyl)-4-hydroxycoumarin; 12.5μg/ml of medium], but could be restored by adding vitamin K (0.1μg/ml) along with the warfarin. Cultures to which Sepharose beads containing covalently bound anti-(Factor X) antibodies had been added showed decreased amounts of free Factor X/Xa in the culture medium. The missing activity could be demonstrated by incubating the recovered conjugate with the substrate peptide S-2613. Factor Xa produced by the macrophages was efficiently inactivated by heparin in the presence of antithrombin, heparin with high affinity for antithrombin being more effective than the corresponding low-affinity species.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bengtsson G., Olivecrona T., Hök M., Riesenfeld J., Lindahl U. Interaction of lipoprotein lipase with native and modified heparin-like polysaccharides. Biochem J. 1980 Sep 1;189(3):625–633. doi: 10.1042/bj1890625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branchini B. R., Salituro F. G., Hermes J. D., Post N. J. Highly sensitive assays for proteinases using immobilized luminogenic substrates. Biochem Biophys Res Commun. 1980 Nov 17;97(1):334–339. doi: 10.1016/s0006-291x(80)80172-0. [DOI] [PubMed] [Google Scholar]
- COHN Z. A., BENSON B. THE DIFFERENTIATION OF MONONUCLEAR PHAGOCYTES. MORPHOLOGY, CYTOCHEMISTRY, AND BIOCHEMISTRY. J Exp Med. 1965 Jan 1;121:153–170. doi: 10.1084/jem.121.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geczy C. L., Hopper K. E. A mechanism of migration inhibition in delayed-type hypersensitivity reactions. II. Lymphokines promote procoagulant activity of macrophages in vitro. J Immunol. 1981 Mar;126(3):1059–1065. [PubMed] [Google Scholar]
- Hopper K. E., Geczy C. L., Davies W. A. A mechanism of migration inhibition in delayed-type hypersensitivity reactions. I. Fibrin deposition on the surface of elicited peritoneal macrophages on vivo. J Immunol. 1981 Mar;126(3):1052–1058. [PubMed] [Google Scholar]
- Iscove N. N., Melchers F. Complete replacement of serum by albumin, transferrin, and soybean lipid in cultures of lipopolysaccharide-reactive B lymphocytes. J Exp Med. 1978 Mar 1;147(3):923–933. doi: 10.1084/jem.147.3.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson C. M., Nemerson Y. Blood coagulation. Annu Rev Biochem. 1980;49:765–811. doi: 10.1146/annurev.bi.49.070180.004001. [DOI] [PubMed] [Google Scholar]
- Kisiel W., Hermodson M. A., Davie E. W. Factor X activating enzyme from Russell's viper venom: isolation and characterization. Biochemistry. 1976 Nov 2;15(22):4901–4906. doi: 10.1021/bi00667a023. [DOI] [PubMed] [Google Scholar]
- Levy G. A., Edgington T. S. Lymphocyte cooperation is required for amplification of macrophage procoagulant activity. J Exp Med. 1980 May 1;151(5):1232–1244. doi: 10.1084/jem.151.5.1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist P. A., Fujikawa K., Davie E. W. Activation of bovine factor IX (Christmas factor) by factor XIa (activated plasma thromboplastin antecedent) and a protease from Russell's viper venom. J Biol Chem. 1978 Mar 25;253(6):1902–1909. [PubMed] [Google Scholar]
- Low D. A., Baker J. B., Koonce W. C., Cunningham D. D. Released protease-nexin regulates cellular binding, internalization, and degradation of serine proteases. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2340–2344. doi: 10.1073/pnas.78.4.2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manners D. J., Masson A. J., Patterson J. C. The structure of a beta-(1 leads to 3)-D-glucan from yeast cell walls. Biochem J. 1973 Sep;135(1):19–30. doi: 10.1042/bj1350019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- March S. C., Parikh I., Cuatrecasas P. A simplified method for cyanogen bromide activation of agarose for affinity chromatography. Anal Biochem. 1974 Jul;60(1):149–152. doi: 10.1016/0003-2697(74)90139-0. [DOI] [PubMed] [Google Scholar]
- Nordenman B., Nordling K., Björk I. A differential effect of low-affinity heparin on the inhibition of thrombin and factor Xa by antithrombin. Thromb Res. 1980 Feb 1;17(3-4):595–600. doi: 10.1016/0049-3848(80)90100-0. [DOI] [PubMed] [Google Scholar]
- Osterud B., Bögwald J., Lindahl U., Seljelid R. Production of blood coagulation factor V and tissue thromboplastin by macrophages in vitro. FEBS Lett. 1981 May 5;127(1):154–160. doi: 10.1016/0014-5793(81)80363-8. [DOI] [PubMed] [Google Scholar]
- Osterud B., Lindahl U., Seljelid R. Macrophages produce blood coagulation factors. FEBS Lett. 1980 Oct 20;120(1):41–43. doi: 10.1016/0014-5793(80)81041-6. [DOI] [PubMed] [Google Scholar]
- Osterud B., Rapaport S. I. Activation of factor IX by the reaction product of tissue factor and factor VII: additional pathway for initiating blood coagulation. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5260–5264. doi: 10.1073/pnas.74.12.5260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prydz H., Allison A. C. Tissue thromboplastin activity of isolated human monocytes. Thromb Haemost. 1978 Jun 30;39(3):582–591. [PubMed] [Google Scholar]
- Rickles F. R., Rick P. D. Structural features of Salmonella typhimurium lipopolysaccharide required for activation of tissue factor in human mononuclear cells. J Clin Invest. 1977 Jun;59(6):1188–1195. doi: 10.1172/JCI108743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz B. S., Edgington T. S. Lymphocyte collaboration is required for induction of murine monocyte procoagulant activity by immune complexes. J Immunol. 1981 Aug;127(2):438–443. [PubMed] [Google Scholar]
- Schwartz B. S., Levy G. A., Curtiss L. K., Fair D. S., Edgington T. S. Plasma lipoprotein induction and suppression of the generation of cellular procoagulant activity in vitro: two procoagulant activities are produced by peripheral blood mononuclear cells. J Clin Invest. 1981 Jun;67(6):1650–1658. doi: 10.1172/JCI110201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seijelid R., Bögwald J., Lundwall A. Glycan stimulation of macrophages in vitro. Exp Cell Res. 1981 Jan;131(1):121–129. doi: 10.1016/0014-4827(81)90413-4. [DOI] [PubMed] [Google Scholar]
- Seligsohn U., Osterud B., Brown S. F., Griffin J. H., Rapaport S. I. Activation of human factor VII in plasma and in purified systems: roles of activated factor IX, kallikrein, and activated factor XII. J Clin Invest. 1979 Oct;64(4):1056–1065. doi: 10.1172/JCI109543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seljelid R., Bäckström G., Lindahl U. Proteinase activity in macrophage cultures. Effects of heparin and antithrombin. Exp Cell Res. 1980 Oct;129(2):478–481. doi: 10.1016/0014-4827(80)90519-4. [DOI] [PubMed] [Google Scholar]


