Abstract
Purpose
Postherpetic Neuralgia (PHN), recognized as the most common complication of Herpes Zoster, is experiencing an increasing trend in its occurrence. The goal of this study was to identify the independent risk factors for PHN and create a dynamic nomogram using routine clinical characteristics to predict PHN in patients with herpes zoster, for early identification and prevention of PHN.
Patients and Methods
A total of 2420 patients were retrospectively reviewed and divided into training (n=1696) and validation (n=724) cohort using a 7:3 random allocation. Univariable, LASSO and multivariable logistic regression analysis was performed to identified independent risk factors for PHN. A dynamic nomogram was assessed through the area under the receiver operating characteristic curve (AUC), calibration curves and Hosmer-Lemeshow test. The decision curve analysis (DCA) was used to evaluate its clinical validity.
Results
Multivariable logistic regression identified several independent risk factors for PHN, including age, female, diabetes mellitus, malignant tumors, and connective tissue diseases. The area under the curve was 0.698 (95% CI, 0.666–0.730) for training cohort and 0.713 (95% CI, 0.663–0.763) for the validation cohort. Calibration curve revealed a moderate consistency between actual observation and prediction. Decision curve analysis showed a risk threshold of 16% and demonstrated a clinically effective predictive model.
Conclusion
We have developed a user-friendly dynamic nomogram to predict PHN in patients with herpes zoster, which can assist in early identification and prevention of PHN.
Keywords: postherpetic neuralgia, herpes zoster, risk factors, dynamic nomogram
Introduction
Herpes zoster (HZ) is a dermatological infection caused by the varicella-zoster virus, which can affect individuals ranging from children to the elderly.1,2 The incidence rate is at around 4–4.5 cases per 1000 person-years, with a growing prevalence due to the aging global population.3–6 There are studies that have shown the occurrence of HZ is related to age and gender.7,8 Furthermore, a meta-analysis has identified a multitude of chronic underlying diseases as potential risk factors for HZ.9
Postherpetic Neuralgia (PHN), recognized as the most common complication of HZ, is experiencing an increasing trend in its occurrence. According to reports,10 the incidence of PHN following herpes zoster decreases over time, with rates of 38.1% observed one-month post-infection, 27.0% at three months, and 19.0% at six-month. It has also been observed that HZ cases can lead to PHN that usually persists for 4 to 9 weeks, but a portion of PHN patients experience pain for more than a year.11 This pain potentially results in long-term disability. Researchers also found that the associated risk factors for PHN include age, pre-existing comorbidities (including diabetes mellitus and immune-related diseases12), the intensity of pain.13,14 However, there is still a lack of large-scale studies on the risk of developing PHN in patients with underlying chronic comorbidities. Currently, the primary clinical management strategies for PHN are Pharmacotherapy, minimally invasive interventional treatments and psychosocial support.15 Despite the availability of multiple drugs and treatment strategies for PHN, their efficacy can vary widely among individuals. PHN profoundly affects patients’ professional and daily activities, placing a substantial economic burden on both families and society.12,16
Nomogram models offer a quantifiable and visually accessible representation of risk, serving as predictive tools that have gained widespread adoption in various clinical fields in recent years for assessing the risk of diverse diseases.17–19 Specifically, a nomogram for predicting PHN assists clinicians in identifying patients who may require closer monitoring surveillance or more proactive therapeutic strategies.
There is an urgent necessary to continuously update effective predictive tools to alleviate the health burden caused by PHN. This retrospective study indeed aims to identify the independent risk factors for postherpetic neuralgia (PHN) and create a dynamic nomogram using routine clinical characteristics to predict PHN in patients with HZ, for early identification and prevention of PHN.
Materials and Methods
Patient Selection
This study was approved by the Clinical Research Certification Review Committee of the Northern People’s Hospital of Jiangsu Province (NO. 223ky299). As the study design was retrospective, written informed consent from the patients was not required by the review committee. The study conditions were posted on the notice boards in the outpatient and inpatient departments of the hospital to inform patients about the study. This research adhered to the principles of the Declaration of Helsinki and subsequent amendments.
Patients with herpes zoster-related diagnoses in the outpatient Pain Clinic at Northern Jiangsu People’s Hospital from September 2020 to September 2023 were included. The exclusion criteria were as follows: (1) Patients with missing data greater than 10%; (2) Patients not admitted for the first time, with missing data from their initial visit. Finally, we included 2420 PHN patients.
Data Collection
We conducted a single-center retrospective cohort study at Northern Jiangsu People’s Hospital in Jiangsu Province, which is a tertiary academic hospital. In accordance with the definition of PHN, patients who consistent with the diagnostic criteria were included in the PHN group, characterized by the onset of the disease marked by the appearance of a rash or rash area, with a duration of no less than 3 months;4 Those with a shorter duration were placed in the non-PHN group. Data were collected through the electronic medical record system by searching for medical records coded with ICD-10 codes. We conducted a comprehensive review of each record based on clinical relevance and data availability, extracting data, including the following indicators: location and laterality of the pain, gender, age, surgical history, and pre-existing comorbidities such as diabetes mellitus, hypertension, coronary heart disease, pulmonary disease, hepatobiliary disease, urinary system disease, central nervous system disease, gastrointestinal disease, thyroid disease, breast disease, allergy history, malignant tumors, and connective tissue diseases. The clinicians’ accuracy in diagnosing HZ/PHN is high.
Variable Selection
In the study, 2420 PHN patients were randomly divided into a training set with 1696 participants and a validation set with 724 participants, conforming to the theoretical ratio of 7:3. The training cohort was used for the development and establishment of the model. Univariate logistic regression was used to assess the association between clinical characteristics and progression to PHN. Subsequently, factors with statistical significance (P<0.05) were included in the LASSO regression analysis using R software, based on the −2 log-likelihood and binomial type metrics, to further select variables. LASSO regression is a compressed estimation method that aims to reduce the variable set. It constructs a penalty function that can compress the coefficients of variables and reduce some regression coefficients to zero, thereby achieving the purpose of variable selection.20 This process involved a rigorous 10-fold cross-validation to ensure robust results. The variables included in the model were both centered and normalized to optimize performance. Subsequently, we identified the optimal lambda value that yielded the best balance between model complexity and predictive accuracy. The “Lambda_1se” selection resulted in a model that not only performed well but also featured the most streamlined set of independent variables.21 So the LASSO method was used to analyze the data in the training set to select the optimal predictors in the present risk factors including age, gender, PD, DM, hypertension, tumor, CTD. The selected variables were then subjected to multivariate logistic regression analysis to identify the independent risk factors for PHN.
Development and Validation of the Nomogram
Identified independent risk factors were used to construct a nomogram model for predicting PHN and create an online PHN risk calculator, providing a user-friendly tool for risk estimation of PHN. We assessed internal validity of the prediction models using bootstrap validation with 1000 repetitions. The sample size used for the development of the prediction model complied with the standard of 10 events per variable.22 The discriminative ability for predicting PHN in patients with herpes zoster was evaluated by the area under the receiver operating characteristic curve (AUC). Typically, AUC values ranging from 0.6–0.75, 0.75–0.9, and above 0.9 correspond to acceptable, good, and excellent discriminative power, respectively.23 In order to further validate the accuracy of the model, the Hosmer-Lemeshow test was used to assess the goodness of fit of the model, and calibration curves were employed to measure the consistency between predicted and actual probability.24 Decision curve analysis (DCA) was conducted to assess the clinical utility of the nomogram by quantifying the net benefit at different threshold probabilities.25 The nomogram was applied to validation cohort to further assess its stability using AUC, calibration curves and DCA.
Statistical Analysis
Statistical analysis was performed via IBM SPSS Statistics Version 25.0 and R 4.3.2 with the Hmisc, rms, rmda, pROC, regplot, DynNom packages. Continuous variables are presented as mean (standard deviation [SD]) if normally distributed and median (interquartile range [IQR]) if not normally distributed. Categorical variables are presented as number and frequency (%). For categorical variables, the χ2 test or Fisher exact test was used when appropriate. Predictive factors were optimized through univariate logistic regression and LASSO regression analysis, and multivariate logistic regression analysis was further utilized for final variable selection and model establishment. The model’s stability was evaluated using AUC and calibration curves, and its clinical practicability was assessed with DCA. A two-tailed P-value <0.050 was considered statistically significant.
Results
Basic Information of Enrolled Patients
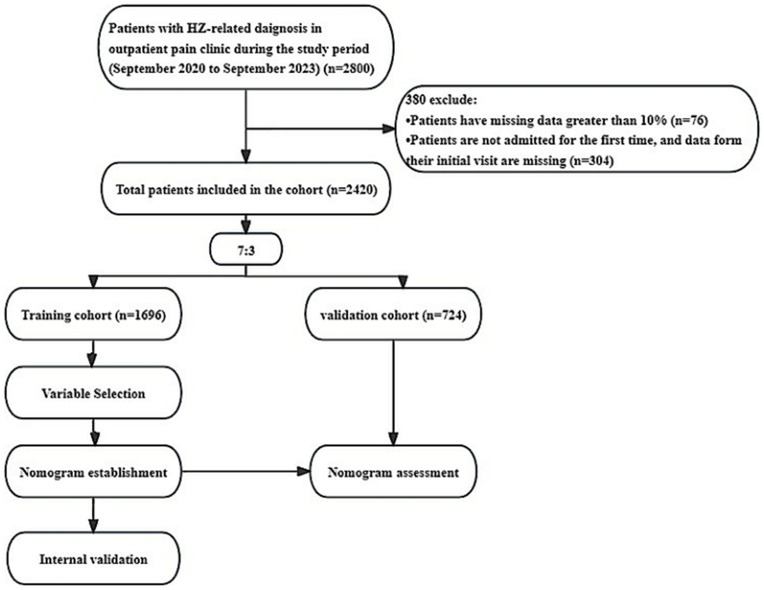
A total of 2420 patients’ data were included in the final analysis after excluding 380 patients. Among the excluded patients, data for 76 patients was missing by more than 10%, and 304 patients were not admitted upon their first visit, and the data from their initial consultation was also missing (Figure 1). In the cohort of 2420 patients, 16.3%, 83%,49.5% and 59.5% were positive patients, negative patients, women, and men, respectively (Table 1). Among the eligible cases, 1696 patients were included in the training cohort. 851 (50.2%) patients were male, and the median age was 62 (IQR, 52–71) years. The validation cohort comprised 724 patients, with a median age of 62 (IQR, 52–71.25) years. Furthermore, we conducted a comparison of baseline data between the training and validation cohorts. Our analysis revealed that the two cohorts exhibited differences in the proportion of urinary system diseases, while no significant differences were observed in other clinical characteristics (Table 2).
Figure 1.
Flowchart and the process of the study. HZ, Herpes Zoster.
Table 1.
General Characteristics of the Patients
| Variables | Overall | Non-PHN Group (n=2027) | PHN Group (n=393) | P |
|---|---|---|---|---|
| Age (yr), median (IQR) | 62.00 (52.00, 71.00) | 60.00 (50.00, 70.00) | 70.00 (60.00, 70.00) | <0.001 |
| Gender, n (%) | <0.001 | |||
| Male | 1223 (50.5) | 1060 (52.3) | 163 (41.5) | |
| Female | 1197 (49.5) | 967 (47.7) | 230 (58.5) | |
| Location, n (%) | 0.895 | |||
| HNF | 307 (12.7) | 256 (12.6) | 51 (13.0) | |
| SNNUL | 390 (16.1) | 330 (16.3) | 60 (15.3) | |
| CNB | 1460 (60.3) | 1217 (60.0) | 243 (61.8) | |
| ANB | 211 (8.7) | 181 (8.9) | 30 (7.6) | |
| BNLL | 52 (2.1) | 43 (2.1) | 9 (2.3) | |
| Laterality, n (%) | 0.309 | |||
| Left | 1187 (49.0) | 985 (48.6) | 202 (51.4) | |
| Right | 1233 (51.0) | 1042 (51.4) | 191 (48.6) | |
| DM, n (%) | <0.001 | |||
| No | 2139 (88.4) | 1815 (89.5) | 324 (82.4) | |
| Yes | 281 (11.6) | 212 (10.5) | 69 (17.6) | |
| Surgical history, n (%) | 0.127 | |||
| No | 1740 (71.9) | 1445 (71.3) | 295 (75.1) | |
| Yes | 680 (28.1) | 582 (28.7) | 98 (24.9) | |
| Hypertension, n (%) | <0.001 | |||
| No | 1746 (72.1) | 1493 (73.7) | 253 (64.4) | |
| Yes | 674 (27.9) | 534 (26.3) | 140 (35.6) | |
| CHD, n (%) | 0.787 | |||
| No | 2225 (91.9) | 1865 (92.0) | 360 (91.6) | |
| Yes | 195 (8.1) | 162 (8.0) | 33 (8.4) | |
| PD, n (%) | 0.002 | |||
| No | 2278 (94.1) | 1921 (94.8) | 357 (90.8) | |
| Yes | 142 (5.9) | 106 (5.2) | 36 (9.2) | |
| HBD, n (%) | 0.17 | |||
| No | 2358 (97.4) | 1979 (97.6) | 379 (96.4) | |
| Yes | 62 (2.6) | 48 (2.4) | 14 (3.6) | |
| USD, n (%) | 0.424 | |||
| No | 2371 (98.0) | 1988 (98.1) | 383 (97.5) | |
| Yes | 49 (2.0) | 39 (1.9) | 10 (2.5) | |
| CNSD, n (%) | 0.268 | |||
| No | 2288 (94.5) | 1921 (94.8) | 367 (93.4) | |
| Yes | 132 (5.5) | 106 (5.2) | 26 (6.6) | |
| GD, n (%) | 0.114 | |||
| No | 2324 (96.0) | 1941 (95.8) | 383 (97.5) | |
| Yes | 96 (4.0) | 86 (4.2) | 10 (2.5) | |
| TD, n (%) | 0.51 | |||
| No | 2369 (97.9) | 1986 (98.0) | 383 (97.5) | |
| Yes | 51 (2.1) | 41 (2.0) | 10 (2.5) | |
| Breast disease, n (%) | 0.402 | |||
| No | 2407 (99.5) | 2015 (99.4) | 392 (99.7) | |
| Yes | 13 (0.5) | 12 (0.6) | 1 (0.3) | |
| Allergy, n (%) | 0.88 | |||
| No | 2355 (97.3) | 1973 (97.3) | 382 (97.2) | |
| Yes | 65 (2.7) | 54 (2.7) | 11 (2.8) | |
| Tumor, n (%) | <0.001 | |||
| No | 2307 (95.3) | 1946 (96.0) | 361 (91.9) | |
| Yes | 113 (4.7) | 81 (4.0) | 32 (8.1) | |
| CTD, n (%) | 0.007 | |||
| No | 2391 (98.8) | 2008 (99.1) | 383 (97.5) | |
| Yes | 29 (1.2) | 19 (0.9) | 10 (2.5) |
Note: The variables are shown as median (interquartile range), or n (%).
Abbreviations: DM, diabetes mellitus; CHD, coronary heart disease; PD, pulmonary disease; HBD, hepatobiliary disease; USD, urinary system disease; CNSD, central nervous system disease; GD, gastrointestinal disease; CTD, connective tissue diseases; HNF, head and face; SNNUL, shoulders, neck, and upper limbs; CNB, chest and back; ANB, abdomen and back; BNLL, buttocks and lower limbs.
Table 2.
Baseline Characteristics of All Patients in the Training and Validation Cohort
| Variables | Overall | Training Cohort (n=1696) | Validation Cohort (n=724) | P |
|---|---|---|---|---|
| Age (yr), median (IQR) | 62.00 (52.00, 71.00) | 62.00 (52.00, 71.00) | 62.00 (52.00, 71.25) | 0.85 |
| Gender, n (%) | 0.587 | |||
| Male | 1223 (50.5) | 851 (50.2) | 372 (51.4) | |
| Female | 1197 (49.5) | 845 (49.8) | 352 (48.6) | |
| Location, n (%) | 0.446 | |||
| HNF | 307 (12.7) | 214 (12.6) | 93 (12.8) | |
| SNNUL | 390 (16.1) | 279 (16.5) | 111 (15.3) | |
| CNB | 1460 (60.3) | 1017 (60.0) | 443 (61.2) | |
| ANB | 211 (8.7) | 144 (8.5) | 67 (9.3) | |
| BNLL | 52 (2.1) | 42 (2.5) | 10 (1.4) | |
| Laterality, n (%) | 0.369 | |||
| Left | 1187 (49.0) | 842 (49.6) | 345 (47.7) | |
| Right | 1233 (51.0) | 854 (50.4) | 379 (52.3) | |
| DM, n (%) | 0.882 | |||
| No | 2139 (88.4) | 1498 (88.3) | 641 (88.5) | |
| Yes | 281 (11.6) | 198 (11.7) | 83 (11.5) | |
| Surgical history, n (%) | 0.887 | |||
| No | 1740 (71.9) | 1218 (71.8) | 522 (72.1) | |
| Yes | 680 (28.1) | 478 (28.2) | 202 (27.9) | |
| Hypertension, n (%) | 0.972 | |||
| No | 1746 (72.1) | 1224 (72.2) | 522 (72.1) | |
| Yes | 674 (27.9) | 472 (27.8) | 202 (27.9) | |
| CHD, n (%) | 0.479 | |||
| No | 2225 (91.9) | 1555 (91.7) | 670 (92.5) | |
| Yes | 195 (8.1) | 141 (8.3) | 54 (7.5) | |
| PD, n (%) | 0.927 | |||
| No | 2278 (94.1) | 1596 (94.1) | 682 (94.2) | |
| Yes | 142 (5.9) | 100 (5.9) | 42 (5.8) | |
| HBD, n (%) | 0.899 | |||
| No | 2358 (97.4) | 1653 (97.5) | 705 (97.4) | |
| Yes | 62 (2.6) | 43 (2.5) | 19 (2.6) | |
| USD, n (%) | 0.016 | |||
| No | 2371 (98.0) | 1654 (97.5) | 717 (99.0) | |
| Yes | 49 (2.0) | 42 (2.5) | 7 (1.0) | |
| CNSD, n (%) | 0.378 | |||
| No | 2288 (94.5) | 1608 (94.8) | 680 (93.9) | |
| Yes | 132 (5.5) | 88 (5.2) | 44 (6.1) | |
| GD, n (%) | 0.536 | |||
| No | 2324 (96.0) | 1626 (95.9) | 698 (96.4) | |
| Yes | 96 (4.0) | 70 (4.1) | 26 (3.6) | |
| TD, n (%) | 0.188 | |||
| No | 2369 (97.9) | 1656 (97.6) | 713 (98.5) | |
| Yes | 51 (2.1) | 40 (2.4) | 11 (1.5) | |
| Breast disease, n (%) | 0.946 | |||
| No | 2407 (99.5) | 1687 (99.5) | 720 (99.4) | |
| Yes | 13 (0.5) | 9 (0.5) | 4 (0.6) | |
| Allergy, n (%) | 0.902 | |||
| No | 2355 (97.3) | 1650 (97.3) | 705 (97.4) | |
| Yes | 65 (2.7) | 46 (2.7) | 19 (2.6) | |
| Tumor, n (%) | 0.802 | |||
| No | 2307 (95.3) | 1618 (95.4) | 689 (95.2) | |
| Yes | 113 (4.7) | 78 (4.6) | 35 (4.8) | |
| CTD, n (%) | 0.494 | |||
| No | 2391 (98.8) | 1674 (98.7) | 717 (99.0) | |
| Yes | 29 (1.2) | 22 (1.3) | 7 (1.0) |
Note: The variables are shown as median (interquartile range), or n (%).
Abbreviations: DM, diabetes mellitus; CHD, coronary heart disease; PD, pulmonary disease; HBD, hepatobiliary disease; USD, urinary system disease; CNSD, central nervous system disease; GD, gastrointestinal disease; CTD, connective tissue diseases; HNF, head and face; SNNUL, shoulders, neck, and upper limbs; CNB, chest and back; ANB, abdomen and back; BNLL, buttocks and lower limbs.
Variable Selection
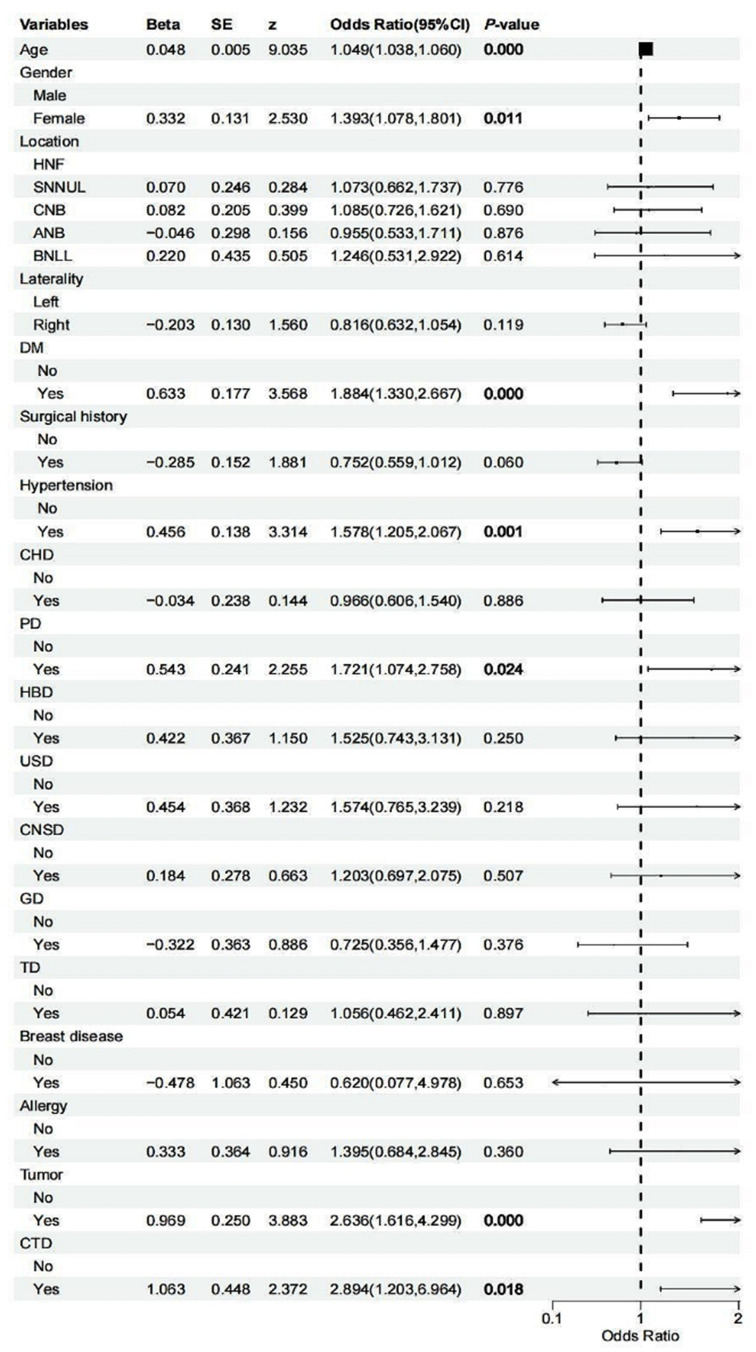
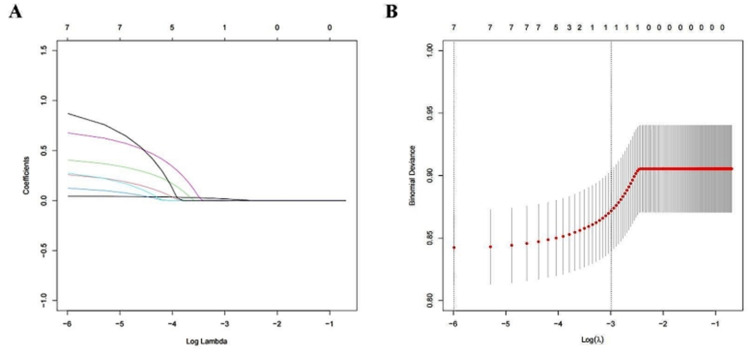
The results of the univariate analysis showed that there were significant differences between the two groups in age (P<0.001), gender (P=0.011), and the coexistence of underlying conditions, including diabetes mellitus (P<0.001), hypertension (P=0.001), pulmonary disease (P=0.024), malignant tumors (P<0.001), and connective tissue diseases (P=0.018). The differences in other indicators were not significant (Figure 2). In the LASSO regression analysis, vertical lines are drawn at the lambda_min (λ=0.003) and the lambda_1se (λ=0.050), and all seven clinical features have non-zero coefficients (Figure 3).
Figure 2.
Univariate logistic regression forest plot.
Abbreviations: DM, diabetes mellitus; CHD, coronary heart disease; PD, pulmonary disease; HBD, hepatobiliary disease; USD, urinary system disease; CNSD, central nervous system disease; GD, gastrointestinal disease; CTD, connective tissue diseases; HNF, head and face; SNNUL, shoulders, neck, and upper limbs; CNB, chest and back; ANB, abdomen and back; BNLL, buttocks and lower limbs.
Figure 3.
LASSO regression analysis for variable selection. (A) LASSO coefficient curve for the seven variables. (B) the process of determining the optimal λ value in the lasso regression analysis through 10-fold cross-validation and the minimum criterion. LASSO, least absolute shrinkage and selection operator.
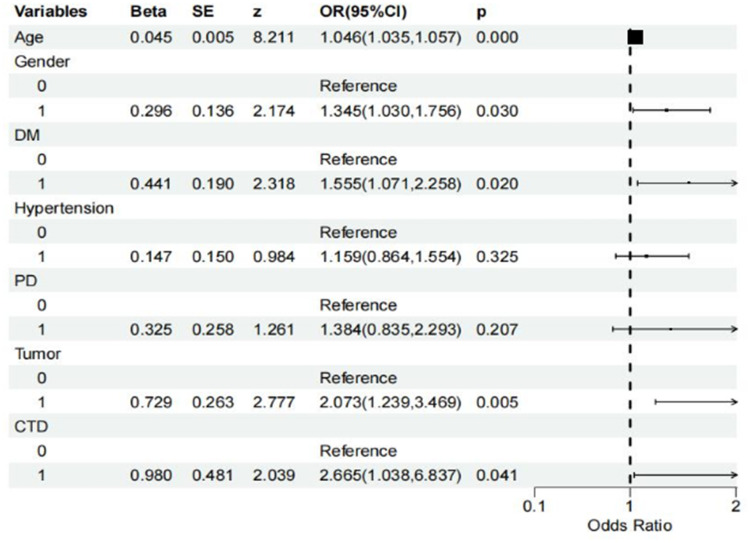
The selected seven predictive variables were used to conduct a multivariate logistic regression analysis. It identified several independent risk factors for PHN, including female, older age, diabetes mellitus, malignant tumors, and connective tissue diseases (P<0.05). Age is a significant factor affecting the risk of PHN. The analysis results show that for each additional year of age, the odds ratio for the occurrence of PHN increases by 4.6% (odds ratio [OR], 1.046; 95% confidence interval [CI], 1.035–1.057, P<0.001). The risk of PHN in females was 34.5% higher compared to males (OR, 1.345; 95% CI, 1.030–1.756, P=0.030). The risk of PHN in diabetic patients increased by 55.5% (OR, 1.555; 95% CI, 1.071–2.258, P=0.020). The risk of PHN in patients with malignant tumors was 2.07 times higher (OR, 2.073; 95% CI, 1.239–3.469, P=0.005). Patients with connective tissue diseases had a 2.67 times higher risk of PHN (OR, 2.665, 95% CI, 1.038–6.837, P=0.041) (Figure 4).
Figure 4.
Multivariable logistic regression forest plot.
Abbreviations: DM, diabetes mellitus; PD, pulmonary disease; CTD, connective tissue diseases.
Constructure Nomogram and Online Calculator
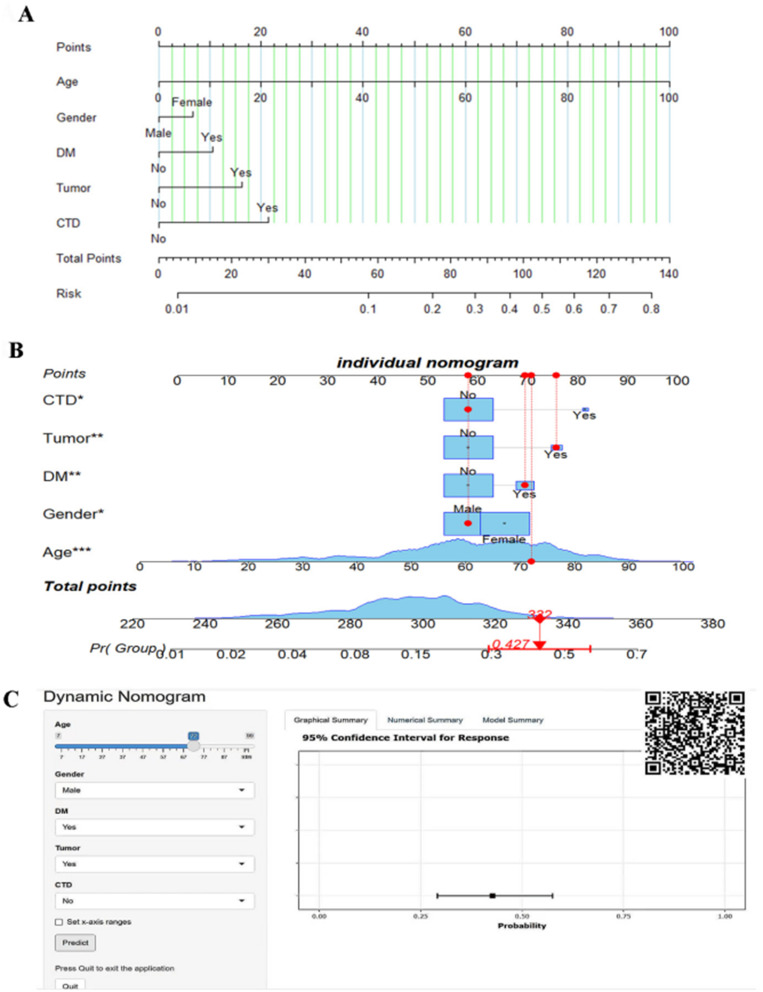
Identified independent risk factors were used to construct a nomogram for predicting the risk of postherpetic neuralgia (PHN) in patients diagnosed with herpes zoster (Figure 5A). For instance, assuming a 72-year-old male with herpes zoster who has comorbid diabetes mellitus and malignant tumor but no history of connective tissue diseases, the dynamic nomogram model estimated a 42.7% risk for developing PHN (Figure 5B). According to model performance, we developed dynamic nomogram model and an online visualization Online calculator for recognizing patients at high risk of PHN (https://phn-predictive-model-dynnom.shinyapps.io/DynNomapp/). It can also be accessed via the QR code in the upper right corner (Figure 5C). Patients were evaluated for the risk of PHN based on independent risk factors for (gender, age, presence of diabetes mellitus, malignant tumors, and connective tissue diseases).
Figure 5.
Nomogram Risk Prediction Model for PHN. *A symbol indicates statistical significance, and the quantity represents the level of significance. (A) A nomogram model for PHN. (B) An individual nomogram. (C) A dynamic nomogram and an online risk calculator for PHN.
Abbreviations: DM, diabetes mellitus; CTD, connective tissue disease.
Nomogram Performance
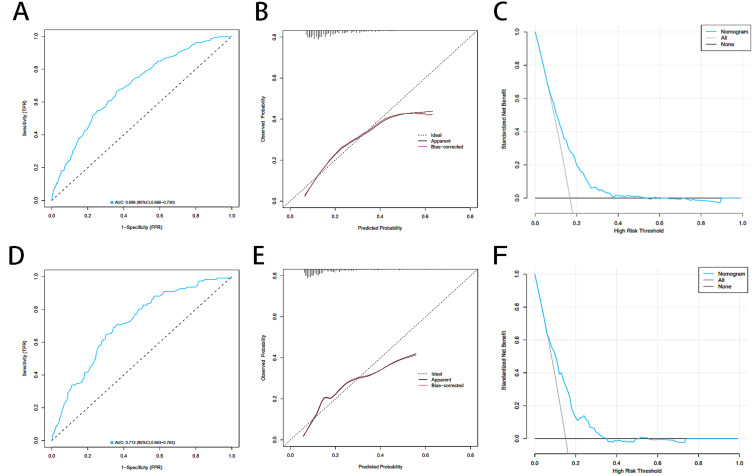
The internal validation of the nomogram model for predicting the risk of PHN in HZ patients was conducted using the Bootstrap, and the C-statistic was 0.692. The predictive performance of the nomogram model was analyzed using the AUC, with the results showing an AUC of 0.698 (95% CI, 0.666–0.730) for the training cohort and an AUC of 0.713 (95% CI, 0.663–0.763) for the validation cohort (Figure 6A and D). This indicates that the model has an acceptable level of discrimination. The Hosmer-Lemeshow test showed a goodness fit (training cohort, P=0.107; validation cohort, P=0.141), indicating that the predicted probabilities of the model are essentially consistent with the actual probabilities, thereby confirming its robust calibration. Additionally, the calibration curves for the training and validation cohorts showed moderate consistency (Figure 6B and E). In summary, the nomogram of the predictive model has acceptable predictive power. The threshold ranges for DCA were derived from the training and validation cohorts based on the sensitivity and specificity of the model. The net benefit is superior to intervening on all patients or not intervening on all patients. Decision curve analysis showed a risk threshold of 16% and demonstrated a clinically effective predictive model (Figure 6C and F).
Figure 6.
Comparison of ROC decision, calibration curves and DCA curves on the training and validation cohorts. The ROC decision on the training (A) and validation cohorts (D). The calibration curves on the training (B) and validation (E) cohorts. The DCA curves on the training (C) and validation (F) cohorts.
Abbreviations: ROC, Receiver operating characteristic curve; DCA, Decision curve analysis.
Discussion
Postherpetic neuralgia (PHN) is the most common complication of HZ. The results of this study show that the incidence of PHN in patients with HZ is 16.3%, which is a significant clinical issue. We analyzed 22 potential variables in 2420 patients with herpes zoster. Five independent risk factors were pinpointed: age, gender, the presence of diabetes mellitus, malignant tumors, and connective tissue disorders. Through LASSO and multivariate analysis, we have developed a dynamically visualized nomogram model and online risk calculator for the risk of PHN in patients with herpes zoster. The diagnostic performance of the nomogram is acceptable (AUC, 0.713), and it shows goodness consistency in the calibration curve. Internal validation of the model using the Bootstrap method (1000 resamples) indicates that the model’s accuracy is acceptable. DCA analysis indicates that the net benefit is superior to intervening on all patients or not intervening on all patients.
In previous reports, age has been the only widely recognized risk factor for PHN, with the incidence of PHN in HZ patients across all age groups ranging from 5–30%.26 Furthermore, the incidence of PHN increases with age in the entire study population,27 which is consistent with our findings. The results of this study indicate that for each additional year of age, the odds ratio for the occurrence of PHN increases by 4.6%. Recent studies have assessed the risk of PHN in 10-year age increments. The findings show that the risk of PHN increases by 1.52 times for every additional decade of age.28 This increase is likely due to the age-related decline in cellular immune function, which may result in sustained high levels of the VZV after reactivation. The ongoing viral activity can lead to continuous nerve damage, potentially causing PHN.29 Therefore, it is particularly important to implement preventive interventions early for the elderly. Additionally, some studies have shown that hypertension is an independent risk factor for the occurrence of PHN,14 but this was not confirmed in our study. T This may be due to age being not only a well-recognized independent risk factor for PHN but also a significant risk factor for hypertension. Therefore, further research is needed to explore the relationship between hypertension and PHN, taking into account age as a potential confounding factor.
When analyzing the risk of PHN based on gender, we observed that women have a higher risk, a result consistent with other published studies.7,30,31 The reason for this may be that women are more likely to report pain and the severity of pain, and they tend to experience longer-lasting pain compared to men. We consider that gender might be a factor influencing the incidence of shingles. Potential mechanisms could involve differences in physiological and immune responses between genders. For instance, women may be more likely to report pain and its severity and may experience more prolonged pain compared to men. Nevertheless, further research is necessary to uncover the underlying causes of this phenomenon.
Our research revealed that patients with HZ who also have diabetes mellitus or connective tissue disorders are at a significantly elevated risk for the development of PHN, with respective risk increases of 55.5% and 166.5% compared to those without these comorbidities. A study reported that diabetes mellitus frequently results in pathological alterations in peripheral blood vessels, which can deprive nerves of essential nutrients and facilitate viral invasion.30 Further evidence supports that underlying diseases such as diabetes mellitus and immune system disorders can diminish a patient’s immune response.32–37 This explains why patients with weakened immune systems or those receiving treatment for autoimmune conditions, including systemic lupus erythematosus, rheumatoid arthritis, and inflammatory bowel disease, are at a higher risk for developing herpes zoster (HZ), experiencing disease recurrence, and encountering complications.38,39
A large-scale retrospective population-based study40 has shown that the presence of various tumor types exerts distinct influences on the risk of developing HZ and PHN. It is noted that patients within the PHN cohort exhibit a more pronounced prevalence of gastrointestinal, respiratory, and hematological cancers. In our study, although we did not specify the types of tumors, we still found that the occurrence of PHN is associated with the presence of tumors, and patients with malignant tumors have a risk of developing PHN that is 2.07 times higher than that of the general population. These results suggest that PHN is related to the development of malignant tumor. However, another study in Germany found no significant association between herpes zoster infection and the development of gastrointestinal cancer.41 Therefore, the relationship between PHN and malignant tumors remains controversial, and future research analyzing the relationship between PHN and cancer risk for specific types of cancer is also clinically significant.
This study has certain limitations. Firstly, the lack of external validation is one of the main limitations of our study. Further research is needed to replicate and externally validate the results of this study. Secondly, our retrospective study may have limitations such as missing values, misclassification, and confounding, which could lead to recall bias. Thirdly, although the accuracy and consistency of the model are acceptable, its AUC did not reach 0.8, which means that the model’s performance has scope for further enhancement. Future work can focus on optimizing the model to augment its precision for practical clinical use. In the future, it is necessary to conduct more multicenter, prospective, large-sample clinical studies, include more patients’ laboratory tests data (such as CRP, immunoglobulin IgG42), treatment methods and psychological mental states,43 compare different algorithmic models, and establish more accurate predictive models.
Conclusion
In the current investigation, we used five routine clinical characteristics to create a dynamic nomogram that predicted PHN. The nomogram, which has an acceptable and moderate discrimination and calibration, could assist clinicians in identifying PHN early and administer timely preventive measures.
Funding Statement
This study was supported by the National Natural Science Foundation of China (82171207), the Natural Science Foundation of Jiangsu Province (BK20231246), the Youth Science and Technology Talents Support Project of Jiangsu Association for Science and Technology (2021-008), and the Youth Excellent Talent Training Project of “333” High-Level Personnel Training Program in Jiangsu Province (2022-3-6-146).
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Dayan RR, Peleg R. Herpes zoster - typical and atypical presentations. Postgrad Med. 2017;129(6):567–571. doi: 10.1080/00325481.2017.1335574 [DOI] [PubMed] [Google Scholar]
- 2.Le P, Rothberg M. Herpes zoster infection. BMJ. 2019;364:k5095. doi: 10.1136/bmj.k5095 [DOI] [PubMed] [Google Scholar]
- 3.Gross GE, Eisert L, Doerr HW, et al. S2k guidelines for the diagnosis and treatment of herpes zoster and postherpetic neuralgia. J Dtsch Dermatol Ges. 2020;18(1):55–78. [DOI] [PubMed] [Google Scholar]
- 4.Rosamilia LL. Herpes zoster presentation, management, and prevention: a modern case-based review. Am J Clin Dermatol. 2020;21(1):97–107. doi: 10.1007/s40257-019-00483-1 [DOI] [PubMed] [Google Scholar]
- 5.Tang J, Tao J, Luo G, Zhu J, Yao M. Analysis of risk factors and construction of a prediction model of motor dysfunction caused by limb herpes zoster. J Pain Res. 2022;15:367–375. doi: 10.2147/JPR.S346564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang Z, Liu X, Suo L, Zhao D, Pan J, Lu L. The incidence of herpes zoster in China: a meta-analysis and evidence quality assessment. Hum Vaccin Immunother. 2023;19(2):2228169. doi: 10.1080/21645515.2023.2228169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morant-Talamante N, Diez-Domingo J, Martínez-úbeda S, Puig-Barberá J, Alemán-Sánchez S, Pérez-Breva L. Herpes zoster surveillance using electronic databases in the Valencian Community (Spain). BMC Infect Dis. 2013;13(1):463. doi: 10.1186/1471-2334-13-463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rząd M, Kanecki K, Lewtak K, Tyszko P, Gorynski P, Nitsch-Osuch A. Hospitalizations of patients with herpes zoster in Poland during 2012-2021: a population-based study. Vaccine. 2024;42(8):1928–1933. doi: 10.1016/j.vaccine.2024.02.022 [DOI] [PubMed] [Google Scholar]
- 9.Steinmann M, Lampe D, Grosser J, et al. Risk factors for herpes zoster infections: a systematic review and meta-analysis unveiling common trends and heterogeneity patterns. Infection. 2024;52(3):1009–1026. doi: 10.1007/s15010-023-02156-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim YN, Kim DW, Kim ED. Efficacy of continuous epidural block in acute herpes zoster: incidence and predictive factors of postherpetic neuralgia, a retrospective single-center study. Medicine. 2016;95(32):e4577. doi: 10.1097/MD.0000000000004577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patil A, Goldust M, Wollina U. Herpes zoster: a review of clinical manifestations and management. Viruses. 2022;14(2):192. doi: 10.3390/v14020192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen L, Li J, Liu H, Yang P, Zuo Y, Ye L. Interventions for zoster-associated pain: a retrospective study based on the clinical database. Front Neurol. 2022;13:1056171. doi: 10.3389/fneur.2022.1056171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li HL, Gong G, Wang JL, Li FB. Analysis of Risk Factors For Postherpetic Neuralgia In Patients With Postmalignancy Herpes Zoster. Pain Physician. 2023;26(4):E397–E403. doi: 10.36076/ppj.2023.26.E397 [DOI] [PubMed] [Google Scholar]
- 14.Hu J, Zhong LZ, Li TT, Jia QY, Li HM. 带状疱疹后神经痛的危险因素研究 [Study of risk factors of postherpetic neuralgia]. Zhonghua Yi Xue Za Zhi. 2022;102(40):3181–3185. Chinese. doi: 10.3760/cma.j.cn112137-20220601-01213 Chinese [DOI] [PubMed] [Google Scholar]
- 15.Finnerup NB, Attal N, Haroutounian S, et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol. 2015;14(2):162–173. doi: 10.1016/S1474-4422(14)70251-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen P, Chen Z, Xiao Y, et al. Characteristics and economic burden of hospitalized patients with herpes zoster in China, before vaccination. Hum Vaccin Immunother. 2023;19(3):2268990. doi: 10.1080/21645515.2023.2268990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu L, Zhang X, Jiang F, et al. Development and validation of nomograms to predict preoperative anxiety and postoperative pain in patients undergoing gynecological surgery: an observational analysis. J Affect Disord. 2023;339:227–236. doi: 10.1016/j.jad.2023.07.058 [DOI] [PubMed] [Google Scholar]
- 18.Chen ZX, Schwartz M, Gu LH, et al. Development and validation of safety and efficacy-associated risk calculator for hepatocellular carcinoma in the elderly after resection (SEARCHER): a multi-institutional observational study. Int J Surg. 2022;106:106842. doi: 10.1016/j.ijsu.2022.106842 [DOI] [PubMed] [Google Scholar]
- 19.Mases A, Beltrán de Heredia S, Gallart L, et al. Prediction of acute myocardial injury in noncardiac surgery in patients at risk for major adverse cardiovascular and cerebrovascular events: a multivariable risk model. Anesth Analg. 2023;137(6):1116–1126. doi: 10.1213/ANE.0000000000006469 [DOI] [PubMed] [Google Scholar]
- 20.Liang W, Liang H, Ou L, et al; China Medical Treatment Expert Group for COVID-19. Development and validation of a clinical risk score to predict the occurrence of critical illness in hospitalized patients with COVID-19. JAMA Intern Med. 2020;180(8):1081–1089. doi: 10.1001/jamainternmed.2020.2033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mo R, Shi R, Hu Y, Hu F. Nomogram-based prediction of the risk of diabetic retinopathy: a retrospective study. J Diabetes Res. 2020;2020:7261047. doi: 10.1155/2020/7261047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Riley RD, Ensor J, Snell KIE, et al. Calculating the sample size required for developing a clinical prediction model. BMJ. 2020;368:m441. doi: 10.1136/bmj.m441 [DOI] [PubMed] [Google Scholar]
- 23.Yao Q, Hu XH, He LL. Evaluation of comprehensive myocardial contractility in children with Kawasaki disease by cardiac magnetic resonance in a large single center. Quant Imaging Med Surg. 2022;12(1):481–492. doi: 10.21037/qims-20-1402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yi M, Meric-Bernstam F, Kuerer HM, et al. Evaluation of a breast cancer nomogram for predicting risk of ipsilateral breast tumor recurrences in patients with ductal carcinoma in situ after local excision. J Clin Oncol. 2012;30(6):600–607. doi: 10.1200/JCO.2011.36.4976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vickers AJ, van Calster B, Steyerberg EW. A simple, step-by-step guide to interpreting decision curve analysis. Diagn Progn Res. 2019;3(1):18. doi: 10.1186/s41512-019-0064-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kawai K, Gebremeskel BG, Acosta CJ. Systematic review of incidence and complications of herpes zoster: towards a global perspective. BMJ Open. 2014;4(6):e004833. doi: 10.1136/bmjopen-2014-004833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Södergren E, Mårdberg K, Nishimwe M, et al. Incidence and burden of herpes zoster in Sweden: a regional population-based register study. Infect Dis Ther. 2024;13(1):121–140. doi: 10.1007/s40121-023-00902-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ding S, Wen S, Kang H, Zhang H, Guo H, Li Y. Association of the incidence of postherpetic neuralgia with early treatment intervention of herpes zoster and patient baseline characteristics: a systematic review and meta-analysis of cohort studies. Int J Infect Dis. 2024;147:107181. doi: 10.1016/j.ijid.2024.107181 [DOI] [PubMed] [Google Scholar]
- 29.Wang XX, Zhang Y, Fan BF. Predicting postherpetic neuralgia in patients with herpes zoster by machine learning: a retrospective study. Pain Ther. 2020;9(2):627–635. doi: 10.1007/s40122-020-00196-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parikh R, Spence O, Giannelos N, Kaan I. Herpes zoster recurrence: a narrative review of the literature. Dermatol Ther. 2024;14(3):569–592. doi: 10.1007/s13555-024-01101-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Du J, Sun G, Ma H, et al. Prevalence and risk factors of anxiety and depression in patients with postherpetic neuralgia: a retrospective study. Dermatology. 2021;237(6):891–895. doi: 10.1159/000512190 [DOI] [PubMed] [Google Scholar]
- 32.Saadatian-Elahi M, Bauduceau B, Del-Signore C, Vanhems P. Diabetes as a risk factor for herpes zoster in adults: a synthetic literature review. Diabet Res Clin Pract. 2020;159:107983. doi: 10.1016/j.diabres.2019.107983 [DOI] [PubMed] [Google Scholar]
- 33.Ning L, Liu R, Li S, et al. Increased risk of herpes zoster infection in patients with inflammatory bowel disease: a meta-analysis of cohort studies. Eur J Clin Microbiol Infect Dis. 2020;39(2):219–227. doi: 10.1007/s10096-019-03706-9 [DOI] [PubMed] [Google Scholar]
- 34.Yasokawa N, Yasuda Y, Chin H, Kurose K, Aoyama Y, Oga T. Generalized herpes zoster and cutaneous metastasis during chemotherapy for non-small cell lung cancer: a case report. Thorac Cancer. 2021;12(1):117–121. doi: 10.1111/1759-7714.13722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang Z, Deng W, Wu Q, Sun L. Tuberculosis, hepatitis B and herpes zoster in tofacitinib-treated patients with rheumatoid arthritis. Immunotherapy. 2019;11(4):321–333. doi: 10.2217/imt-2018-0113 [DOI] [PubMed] [Google Scholar]
- 36.Warner BE, Goins WF, Kramer PR, Kinchington PR. A guide to preclinical models of zoster-associated pain and postherpetic neuralgia. Curr Top Microbiol Immunol. 2023;438:189–221. doi: 10.1007/82_2021_240 [DOI] [PubMed] [Google Scholar]
- 37.Wen SY, Ou-Yang C, Chang C, Chen CC, Chang HY. Impact of type 1 versus type 2 diabetes on developing herpes zoster and post-herpetic neuralgia: a population-based cohort study. Acta Derm Venereol. 2023;103:adv9400. doi: 10.2340/actadv.v103.9400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen SY, Suaya JA, Li Q, et al. Incidence of herpes zoster in patients with altered immune function. Infection. 2014;42(2):325–334. doi: 10.1007/s15010-013-0550-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Muñoz-Quiles C, López-Lacort M, Díez-Domingo J, Orrico-Sánchez A. Herpes zoster risk and burden of disease in immunocompromised populations: a population-based study using health system integrated databases, 2009-2014. BMC Infect Dis. 2020;20(1):905. doi: 10.1186/s12879-020-05648-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sim JH, Cho HS, Kim YD, et al. The association between herpes zoster and increased cancer risk: a nationwide population-based matched control study. Curr Oncol. 2021;28(4):2720–2730. doi: 10.3390/curroncol28040237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leyh C, Roderburg C, Luedde T, Loosen SH, Kostev K. Herpes zoster is not associated with subsequent gastrointestinal cancer: data from over 200,000 outpatients in Germany. J Cancer Res Clin Oncol. 2023;149(19):17115–17121. doi: 10.1007/s00432-023-05432-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Terheyden P, Sunderkötter C, Söhngen FD, et al. Varicella zoster virus-specific hyperimmunoglobulin in the adjuvant treatment of immunocompromised herpes zoster patients: a case series. Dermatol Ther. 2023;13(10):2461–2471. doi: 10.1007/s13555-023-01019-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Forbes HJ, Thomas SL, Smeeth L, et al. A systematic review and meta-analysis of risk factors for postherpetic neuralgia. Pain. 2016;157(1):30–54. doi: 10.1097/j.pain.0000000000000307 [DOI] [PMC free article] [PubMed] [Google Scholar]