Abstract
Objective
Orbital apex syndrome (OAS) is a rare condition with multiple cranial nerve involvement caused by varied etiologies. It is not only a threat to the patient’s vision but also life-threatening due to the intracranial spread of infection, if not diagnosed early and treated accurately. To study the outcome of endoscopic sinus surgery (ESS) for OAS secondary to sinusitis concerning resolution of ptosis, improvement of ophthalmoplegia, visual prognosis, intracranial spread of infection, and mortality.
Methods
A retrospective review of patients with OAS secondary to sinusitis who underwent ESS from 2011 to 2021 was tabulated and analyzed.
Results
Twenty-seven patients (mean age: 55.11+/-16 years; male 62%) were included in this study. At presentation, blurring of vision (81%), headache (66%), diplopia (63%) ptosis (63%) were the most common symptoms, and ophthalmoplegia (100%) was the most common sign. Five patients had no perception of light and the rest had various degrees of vision impairment. The most common etiopathology of sinusitis was fungal sinusitis (12 mucormycosis and four aspergillus). The final visual prognosis at three months follow-up post-ESS showed vision stabilization (no improvement or worsening) in 13 (48%) patients, improvement in seven (26%) patients, and vision deterioration in two (7%) patients. There was a significant improvement in ptosis (70%) and ophthalmoplegia (85%). There was no intracranial spread of infection or recurrence with a mortality rate of 3.7% (one patient).
Conclusion
ESS coupled with appropriate antimicrobials effectively treats OAS secondary to sinusitis with decreased morbidity and mortality.
Keywords: Orbital apex syndrome, sinusitis, complication, endoscopic sinus surgery, mucormycosis, ocular vision, blepharoptosis, ophthalmoplegia
Introduction
The pyramid-shaped human orbital apex (OA) has a complex anatomy and is closely related to the superior orbital fissure (SOF), the optic canal (OC), and the cavernous sinus (CS) (1). The orbital apex syndrome (OAS) also called Jacod syndrome is characterized by the involvement of cranial nerves (CN) in the SOF and OC, namely the oculomotor nerve (III CN), the trochlear nerve (IV CN), the abducens nerve (VI CN), the ophthalmic division of the trigeminal nerve (V CN), and the optic nerve (II CN), respectively. A varied variety of clinical conditions, neoplastic, inflammatory, infectious, iatrogenic, or traumatic can cause this rare syndrome (2). OAS is not only a threat to the patient’s vision but also life-threatening if it spreads through the ophthalmic vessels and bony fissures into intracranial structures (3).
OAS secondary to sinusitis is mostly due to infections and accounts for only 15% of the heterogeneous etiologies (4). Fungal and bacterial infections from the adjacent paranasal sinuses, namely the ethmoid and sphenoid sinuses, spread to the OA. This can be treated by endoscopic sinus surgery (ESS) and culture-specific antimicrobials if diagnosed early, and hence morbidity and mortality due to this syndrome can be reduced. Radiological imaging, computerized tomography (CT), and magnetic resonance imaging (MRI) help locate the lesion, some etiologies, and clinical features for differential diagnosis (2).
The literature available on OAS secondary to sinusitis is limited only to case reports in our country. Our study is one of the first retrospective decadal reviews done with the primary objective of studying the outcome of ESS for OAS secondary to sinusitis due to infection and to study etiopathology and varied clinical presentations.
Methods
We reviewed the medical records at Ramaiah Medical College Hospital, a tertiary care center, kept between January 1st, 2011, and December 31st, 2021, and identified the patients who have undergone ESS for OAS secondary to sinusitis due to infection and included those that satisfy the criteria (Table 1).
Table 1. Inclusion and exclusion criteria for patient enrollment in the study.
|
Inclusion criteria • Aged 18 years or more. • Diagnosed case of orbital apex syndrome secondary to sinusitis due to infection. • Radiological imaging either computerized tomography or magnetic resonance imaging. • Undergone endoscopic sinus surgery. • Three-month follow-up period post-endoscopic sinus surgery. |
|
Exclusion criteria • Orbital apex syndrome secondary to other etiology. |
A tabulation of the patient’s demographics (gender and age), comorbidities, clinical manifestations (rhino orbital symptoms and signs), the eye involved, radiological imaging findings (CT/MRI), diagnostic nasal endoscopic findings, microbiology and histopathology findings, initial and final vision at three-month follow-up post-ESS (Snellen’s visual acuity chart), etiology, intraoperative findings, prognosis at three-month follow-up post-ESS in terms of resolution of ptosis, improvement of ophthalmoplegia, vision improvement, the intracranial spread of infection, and mortality.
OAS secondary to sinusitis was diagnosed based on clinical manifestations, radiological imaging, microbiology, and histopathology findings. Treatment included medical and surgical measures. Medical treatment was initiated with empirical intravenous (IV) antibiotics on admission and changed later according to the culture sensitivity and histopathology results. Saline irrigation, topical and systemic decongestants, and anti-inflammatory agents were started. The diabetic patients underwent initial evaluation by an endocrinologist and the glycemic control during the hospital stay was achieved by a combination of short and long-acting insulin. The patients were followed up daily by the endocrinologist with blood sugar readings.
Surgical intervention was done within 24 hours of the diagnosis and tailored based on radiological imaging and intra-operative findings. All patients underwent ESS with or without orbital decompression, optic nerve decompression, endoscopic medial maxillectomy, and subtotal maxillectomy. ESS began with a repeat diagnostic nasal endoscopy followed by uncinectomy, middle meatal antrostomy, anterior and posterior ethmoidectomy, and sphenoidotomy. The lamina papyracea, the skull base, and the opticocarotid recess were identified. Medial orbitotomy and decompression were done using a blunt elevator to dissect the lamina papyracea in the anteroposterior direction to the medial OA and the periorbita was incised using a sickle knife. Selected patients underwent optic nerve decompression by removing the bone around the OC. Endoscopic medial maxillectomy involved the removal of the uncinate process, the bulla, the inferior and middle turbinate, and the medial wall of the maxilla to clear the disease from the pterygopalatine fossa and pterygoid plates. Patients with palatal involvement underwent subtotal maxillectomy in which the floor of the maxillary sinus (hard palate) was resected in addition to one other wall. The disease was cleared from the SOF. Discharge was sent for culture sensitivity, crusts for potassium hydroxide mount, tissue for histopathology, and necrotic tissue was debrided prudently. Patients diagnosed with mucormycosis underwent repeated debridement as needed.
Treatment outcome was evaluated based on the resolution of ptosis, improvement in ophthalmoplegia, visual prognosis-stabilization of vision (no improvement or worsening), vision improvement, vision deterioration, the intracranial spread of infection, and mortality at three months follow-up post-ESS.
Statistical Analysis
Our study was approved by the Ramaiah Medical College Institutional Ethics Committee (no: MSRMC/EC/AP-04/06-2023, date: 23.06.2023). The study data was analyzed using IBM SPSS Statistics for Windows v29.0.2.0 (IBM Corp: Armonk, NY). Fisher’s exact test was used to analyze categorical variables presented as numbers and percentages. Student’s t-test was used to analyze continuous variables presented as mean and standard deviation (SD). Descriptive statistics of ptosis, ophthalmoplegia, visual prognosis, intracranial spread of infection, and mortality were analyzed and summarized in terms of percentage. McNemars test was used to compare the resolution of ptosis, improvement in ophthalmoplegia, and visual prognosis pre- and post-intervention, and a p-value of <0.05 was considered statistically significant.
Results
A total of 27 patients were included in our study and their mean age was 55 (SD: +/-16) years. Most patients were male (62%), and 22 (82%) had diabetes mellitus. Among the 22 diabetic patients, four were diagnosed at admission, 13 were on oral hypoglycemics and seven were treated with insulin for an average duration of six years. Five had chronic kidney disease secondary to diabetic nephropathy. Among these five patients, four were in medical management and one was undergoing hemodialysis. At presentation three patients had a history of coronavirus disease-2019 (COVID-19) (11%) and two (7%) were COVID-positive.
All patients had unilateral eye involvement and presented with a variety of rhino-orbital symptoms. The most common symptoms included blurring of vision (81%), headache (66%), diplopia (63%), ptosis (63%), nasal obstruction (48%), and facial pain (48%) (Table 2).
Table 2. Clinical features in patients at presentation.
|
Presenting symptoms |
Number of patients |
Percent (%) |
|
Blurring of vision |
22 |
81 |
|
Headache |
18 |
66.0 |
|
Ptosis |
17 |
63.0 |
|
Diplopia |
17 |
63.0 |
|
Nasal obstruction |
13 |
48.0 |
|
Facial pain |
13 |
48.0 |
|
Periorbital edema |
10 |
37.0 |
|
Facial puffiness |
6 |
22.0 |
|
Nasal discharge |
6 |
22.0 |
|
Eye pain |
6 |
22.0 |
|
Loss of vision |
5 |
18.5 |
|
Congestion of eye/chemosis |
3 |
11.0 |
|
Watering of eye |
1 |
3.0 |
|
Proptosis |
1 |
3.0 |
On physical examination, there were various degrees of ophthalmoplegia (limitation of extraocular movements) in all patients. Five patients presented with no perception of light and the vision evaluated using a Snellen visual acuity chart ranged from 20/200 to 20/40 (Table 3). Sixty-three percent of patients had a positive relative afferent pupillary defect.
Table 3. Patient vision (Snellen visual acuity chart) at presentation.
|
Vision (feet) |
Number of patients |
Percentage (%) |
|
No perception of light |
5 |
18.5 |
|
Perception of light |
5 |
18.5 |
|
20/200 |
3 |
11.1 |
|
20/70 |
4 |
14.8 |
|
20/50 |
3 |
11.1 |
|
20/40 |
7 |
25.9 |
All patients underwent a thorough ear, nose, and throat examination with a diagnostic nasal endoscopy on presentation. Ten patients demonstrated necrotic tissue, blackish crusts, and discoloration of the nasal mucosa. Radiological imaging was done in all patients, 19 patients with CT only, three patients with MRI only, and five patients with both CT and MRI. Ipsilateral sphenoid and ethmoidal involvement were seen in 24 patients, two patients had infiltrative lesions, and 13 patients had bony erosion with calcifications on imaging (Figure 1).
Figure 1.
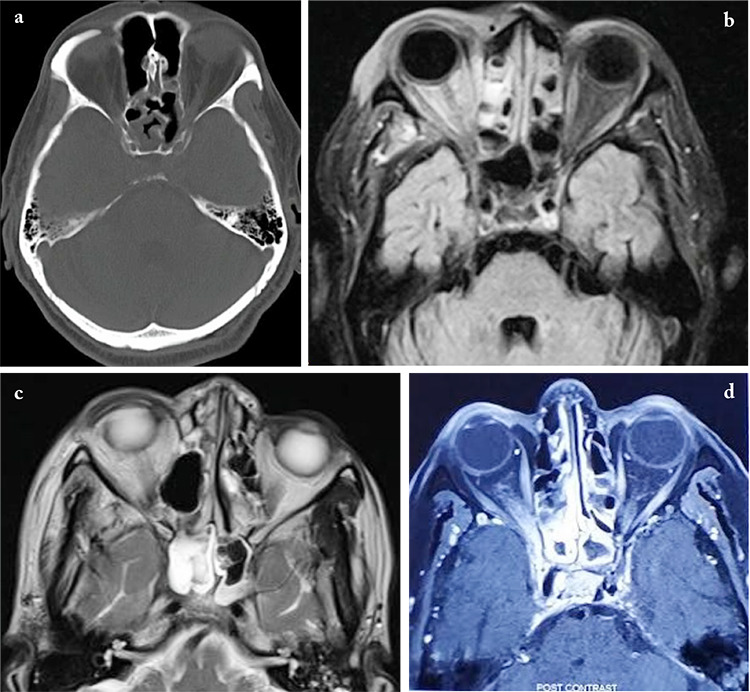
Radiological imaging (CT/MRI) presentations of orbital apex syndrome secondary to sinusitis (a-d)
a) Axial non-contrast CT scan of orbit showing right sphenoid sinus mucosal thickening with involvement of right orbital apex. b) Axial T2 Flair of MRI orbit showing high signal changes in bilateral ethmoid air cells with involvement of right orbital apex. c) Axial T2-weighted MRI scan of orbit showing right eye proptosis with circumferential mucosal thickening of right sphenoid sinus and extension to the right orbital apex. d) Axial T1-weighted post-contrast scan of MRI orbit showing enhancement of right orbital apex, optic nerve, posterior ethmoidal, and sphenoid sinus
CT: Computerized tomography, MRI: Magnetic resonance imaging
All patients underwent ESS with or without modifications with tissue biopsy for definitive diagnosis (Table 4). Twenty patients had disease extending medial to the medial wall of the orbit and underwent orbital decompression. One patient with OC erosion underwent optic nerve decompression. Endoscopic medial maxillectomy helped clear the disease in three patients. One patient with palatal involvement underwent subtotal maxillectomy. The duration from symptom onset to surgery ranged between 10 to 42 days.
Table 4. Type of surgery.
|
Surgery |
Number of patients |
Percentage (%) |
|
Endoscopic sinus surgery |
27 |
100.0 |
|
Orbital decompression |
20 |
74.0 |
|
Optic nerve decompression |
1 |
3.7 |
|
Medial maxillectomy |
3 |
11.1 |
|
Subtotal maxillectomy |
1 |
3.7 |
Histopathology and microbiological results showed fungal sinusitis to be the leading cause in 16 patients with aseptate fungal elements–mucormycosis in 12 and septate fungal elements–aspergillus in four patients. The remaining 11 patients had bacterial sinusitis with Staphylococcus aureus as the most common isolate (10 patients) and only one patient had Staphylococcus epidermidis. Antimicrobial therapy was revised based on the etiology to culture-sensitive IV antibiotics or antifungal agents as per the isolate (IV amphotericin B, voriconazole, itraconazole). Intranasal amphotericin wash was done, and a check endoscopy was performed every third day for two weeks followed by weekly for four weeks for crust removal and planning debridement in patients diagnosed with fungal sinusitis. At the time of discharge, these patients were on prophylactic antimicrobials and hypoglycemic medication to maintain strict glycemic control. They were also on regular follow-up with the endocrinologist with blood sugar readings.
All patients at their follow-up visit after three months post-ESS were evaluated and among the 17 patients who presented with ptosis, 12 (70%) exhibited complete recovery which was statistically significant (p<0.001). All 27 patients included in our study had various degrees of ophthalmoplegia at presentation and 23 (85%) showed improvement post-ESS which was statistically significant (p<0.001).
The final visual acuity (VA) at three-month follow-ups post-ESS showed vision stabilization (no improvement or worsening) in 13 patients (48%), vision improved in seven patients (26%), and vision deteriorated in two patients (7%). Statistical analysis showed no significant difference between initial and post-intervention VA (p=0.05), however, there was no intracranial spread of infection or recurrence in any of the patients. Though not statistically significant, patients with bacterial sinusitis had better improvement in VA when compared to patients with fungal etiology. Worsening of vision post-surgical intervention was seen in two patients who had not undergone orbital decompression. The patient who underwent optic nerve decompression had no perception of light at presentation and had no improvement in vision postoperatively. However, there was no further spread of infection intracranially or recurrence during the follow-up period in this patient. The patients who underwent endoscopic medial maxillectomy and subtotal maxillectomy had a diagnosis of mucormycosis and did not have any intracranial spread of infection or mortality. The patient who underwent sub-total maxillectomy received an obturator three months after surgery. One patient who was diagnosed with mucormycosis and was receiving medical treatment post-ESS and debridement expired on postoperative day four due to an acute cerebrovascular accident thus resulting in a mortality rate of 3.7% in our study (Table 5).
Table 5. Patient vision (Snellen visual acuity chart) on follow-up of 3 months.
|
Initial vision (feet) |
Number of patients |
Improvement |
Final vision (feet) |
|
No perception of light |
5 |
0 |
|
|
Perception of light |
5 |
1 |
20/40 |
|
20/200 |
3 |
1 |
20/30 |
|
20/70 |
4 |
2 |
20/40 |
|
20/30 | |||
|
20/50 |
3 |
1 |
20/20 |
|
20/40 |
7 |
2 |
20/20 |
|
20/20 |
Discussion
Our study analyzed the presenting rhino-orbital symptoms and outcome in terms of prognosis in ptosis, ophthalmoplegia, vision, intracranial spread of infection, and mortality due to OAS secondary to sinusitis by ESS that could aid in early clinical suspicion, diagnosis and decrease the morbidity and mortality due to this rare syndrome. The literature available on this condition is limited and restricted to case studies due to different etiologies causing OAS (5, 6, 7). This, we believe, is the first case series of our population.
The most common presenting complaints of OAS are blurring of vision and ophthalmoplegia despite the varied etiologies (1). The most common presenting complaints in our study were blurring of vision (81%), headache (66%), diplopia (63%), ptosis (63%), nasal obstruction (48%), and facial pain (48%). The most common clinical signs were ophthalmoplegia and positive relative afferent pupillary defect. The signs and symptoms indicate the involvement of neural structures which are closely related to this area and consistent with other studies (8, 9, 10).
Even though neuro-ophthalmic symptoms develop at an earlier stage, there is a delay in definitive diagnosis of OAS secondary to sinusitis (11, 12). The duration from symptom onset to surgery ranged from 10 to 42 days as most patients ignored the initial sinonasal symptoms. Our study highlights the other accompanying sinonasal symptoms that can aid in the early diagnosis of sinusitis as etiology, such as headache, nasal obstruction, facial pain, and nasal discharge.
Aryasit et al. (12) in their retrospective study on OAS concluded by stating, “Imaging should be performed on all patients to locate the site of lesion and determine the etiology. Imaging is also a tool for surgical planning”. All patients in our study had radiological imaging, which aided the accurate diagnosis–the first step towards treatment. The infection spreads from the adjacent paranasal sinuses to the OA. The majority of our patients had ipsilateral involvement of the sphenoid and ethmoidal sinuses with OA involvement on imaging. Bilateral involvement of the OA is possible, although all our patients had unilateral involvement (13).
All our patients underwent ESS with no complications. We were able to reach the narrow OA crevice with the help of endoscopes and achieve disease clearance, acquire tissue for histopathology, and identification of microorganisms which aided in implementing emergent definitive treatment. It also helped in the debridement of the necrotic tissue from the surrounding area, and decompression of the orbit and/or optic nerve which prevented the ascent of infection to the CS (intracranially) and decreased the morbidity and mortality due to this condition. Gu et al. (14) in their report on six cases of OAS caused by sinus disease state that “Endoscopic sinus surgery is a safe and effective method for OAS caused by sinus disease, which is the primary therapy for the disease”.
Infectious etiology accounts for only 15% of the cases of OAS and is most commonly due to sinusitis (4). Fungal infection is most commonly due to aspergillus and mucor (15). The most common bacterial isolates belong to staphylococcal species, Streptococcus pneumoniae, and Gram-negative bacilli like Pseudomonas aeruginosa, Klebsiella, and proteus species (16). Diabetics are susceptible to these infections. Most cases in our study were due to fungal sinusitis, mucormycosis (12/16 patients), and four patients due to aspergillus species (4/16 patients). Invasive fungal sinusitis commonly seen in immunocompromised patients often presents as OAS with the presence of necrotic tissue in the OA, which was also a finding of our study (4, 15). The most common bacterial isolate in our study was Staphylococcus aureus (10/11 patients) and one patient with Staphylococcus epidermidis, a commensal that is recognized as a pathogen in immunocompromised patients (17). Patients with rhino-orbital mucormycosis need extensive surgical and medical treatment to maximize outcomes (18). All our patients underwent ESS with repeated debridement if necessary and received specific antimicrobials. IV liposomal amphotericin B was initiated for mucormycosis (5–10 mg/day to a final daily dose of 0.5 to 0.7 mg/kg depending on the cardio-renal status, maximum cumulative dose of 2.5 g) followed by posaconazole 300 mg once daily for 4–6 weeks. Voriconazole and itraconazole for aspergillus species 200 mg twice daily for two months and culture-sensitive antibiotics for bacterial sinusitis. Corticosteroids are commonly used in OAS secondary to inflammatory or traumatic etiology or when the definitive etiology is not known. Our patients did not receive systemic corticosteroids.
Lee et al. (4) in their retrospective study on 20 diagnosed cases of OAS have documented that despite aggressive multidisciplinary management, improvement in VA is poor after treatment but there is a relationship between the cause of the disease and visual prognosis. Treatment directed towards underlying etiologies experienced improved VA. Our study showed comparable results concerning VA. We documented a stabilization of vision and prevention of further deterioration of vision in most patients when the treatment was directed at the etiology. Clearance of disease from the narrow crevices by extended surgery helped prevent the spread intracranially but showed no significant improvement in pre- and post-intervention VA.
Yamanoi et al. (19), in their report on three cases of intracranial aspergillosis with OAS, documented an improvement in ophthalmoplegia in two of the cases. In our study, all patients presented with some degree of ophthalmoplegia which is the most common sign of OAS, and 23 patients (85%) had a recovery when the treatment was aimed at the etiology (12). Ptosis improved in 12 (70%) out of the 17 patients who presented with the condition.
All the patients in our study population followed the institutional postoperative anticoagulation protocol. Excluding the two COVID patients, all patients did not receive any anticoagulants given early postoperative mobilization. The COVID patients received antiplatelets i.e., Aspirin 75 mg once daily from 24 hours postoperatively for three weeks and low molecular weight heparin once daily as 1 mg/kg during hospital stay. However, we documented one death on postoperative day four in a COVID positive Mucormycosis patient due to an acute cerebrovascular accident which was attributed to the prothrombotic state created by COVID-19 (20). We did not document any intracranial spread of infection in any of the patients, which is a known complication of OAS, possibly due to the accurate diagnosis and treatment tailored to the etiology. ESS helped in the clearance of the disease, thereby reducing the morbidity and mortality in our study.
The patient data was collected over a decade, but still, a limited number of patients were studied due to the rarity of OAS secondary to sinusitis. We suggest large multicentric studies on OAS secondary to sinusitis due to infection.
Conclusion
Prompt diagnosis and intervention by ESS with appropriate culture-sensitive antimicrobials help reverse some of the CN damage, prevent further deterioration of vision, and primarily prevent the extension of infection intracranially in OAS secondary to sinusitis. There is a decrease in morbidity and mortality when treatment is directed at the etiology of OAS.
Main Points
• Data emerging from orbital apex syndrome (OAS) secondary to sinusitis due to infectious etiology is largely restricted to isolated case reports and this is the first case series in our population.
• Associated symptoms such as headache, nasal obstruction, facial pain, and nasal discharge are often overlooked as suggestive of OAS secondary to infection-causing sinusitis. However, these can aid in early diagnosis.
• After surgical intervention, an improvement in ptosis and ophthalmoplegia can be seen in most patients.
• Early endoscopic sinus surgery with modifications helps in the clearance of the disease from the narrow crevices and prevents further spread of infection intracranially thereby reducing the morbidity and mortality of this condition.
Acknowledgments
The authors wish to acknowledge the faculty from the Department of Ophthalmology and Critical Care for their support in the management of these patients.
Footnotes
Ethics Committee Approval: Our study was approved by the Ramaiah Medical College Institutional Ethics Committee (no: MSRMC/EC/AP-04/06-2023, date: 23.06.2023).
Informed Consent: Retrospective study.
Authorship Contributions
Surgical and Medical Practices: C.C., S.B., H.N.R., S.B.P., S.P.D.R., T.U., S.K.R., Concept: C.C., S.B., H.N.R., S.P.D.R., S.K.R., Design: C.C., S.B., S.B.P., S.K.R., Data Collection and/or Processing: C.C., S.B., H.N.R., S.P.D.R., T.U., Analysis and/or Interpretation: C.C., S.B., S.B.P., S.K.R., Literature Search: C.C., S.B., H.N.R., S.P.D.R., T.U., Writing: C.C., S.B., S.B.P., T.U.
Conflict of Interest: The authors have no conflicts of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Badakere A, Patil-Chhablani P. Orbital apex syndrome: a review. Eye Brain. 2019;11:63–72. doi: 10.2147/EB.S180190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yeh S, Foroozan R. Orbital apex syndrome. Curr Opin Ophthalmol. 2004;15(6):490–8. doi: 10.1097/01.icu.0000144387.12739.9c. [DOI] [PubMed] [Google Scholar]
- 3.Xiong M, Moy WL. Orbital apex syndrome resulting from mixed bacterial sphenoid sinusitis. Eur J Case Rep Intern Med. 2018;5(7):000905. doi: 10.12890/2018_000905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee PH, Shao SC, Lee WA. Orbital apex syndrome: a case series in a tertiary medical center in Southern Taiwan. Front Med. 2022;9:845411. doi: 10.3389/fmed.2022.845411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Besada E, Hunter M, Bittner B. An uncommon presentation of orbital apex syndrome. Optometry. 2007;78(7):339–43. doi: 10.1016/j.optm.2007.04.086. [DOI] [PubMed] [Google Scholar]
- 6.Zafar MA, Waheed SS, Enam SA. Orbital aspergillus infection mimicking a tumour: a case report. Cases J. 2009;2:7860. doi: 10.4076/1757-1626-2-7860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chaudhary A, Ramchand T, Frohman LP, Liu JK, Eloy JA. Miller Fisher variant of Guillain-Barré syndrome masquerading as acute sphenoid sinusitis with orbital apex syndrome. Laryngoscope. 2012;122(5):970–2. doi: 10.1002/lary.23248. [DOI] [PubMed] [Google Scholar]
- 8.Thurtell MJ, Chiu ALS, Goold LA, Akdal G, Crompton JL, Ahmed R. Neuro-ophthalmology of invasive fungal sinusitis: 14 consecutive patients and a review of the literature. Clin Exp Ophthalmol. 2013;41:567–76. doi: 10.1111/ceo.12055. [DOI] [PubMed] [Google Scholar]
- 9.Espinoza GM. Orbital inflammatory pseudotumors: etiology, differential diagnosis, and management. Curr Rheumatol Rep. 2010;12:443–7. doi: 10.1007/s11926-010-0128-8. [DOI] [PubMed] [Google Scholar]
- 10.Li Y, Lip G, Chong V, Yuan J, Ding Z. Idiopathic orbital inflammation syndrome with retro-orbital involvement: a retrospective study of eight patients. PLoS One. 2013;8(2):e57126. doi: 10.1371/journal.pone.0057126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cho SW, Lee WW, Ma DJ, Kim JH, Han DH, Kim HJ. Orbital apex lesions: a diagnostic and therapeutic challenge. J Neurol Surg B Skull Base. 2018;79(04):386–93. doi: 10.1055/s-0037-1612616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aryasit O, Preechawai P, Aui-Aree N. Clinical presentation, aetiology and prognosis of orbital apex syndrome. Orbit. 2013;32(2):91–4. doi: 10.3109/01676830.2013.764439. [DOI] [PubMed] [Google Scholar]
- 13.Kim DH, Jeong JU, Kim S, Kim ST, Han GC. Bilateral orbital apex syndrome related to sphenoid fungal sinusitis. Ear Nose Throat J. 2023;102(12):NP618–20. doi: 10.1177/01455613211024768. [DOI] [PubMed] [Google Scholar]
- 14.Gu Q, Li J, Fan J, He G. [Report of 6 orbital apex syndrome caused by sinus diseases.] Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2013;27:67–9. [PubMed] [Google Scholar]
- 15.Jiang N, Zhao G, Yang S, Lin J, Hu L, Che C. A retrospective analysis of eleven cases of invasive rhino-orbito-cerebral mucormycosis presented with orbital apex syndrome initially. BMC. Ophthalmol . 2016;16:10. doi: 10.1186/s12886-016-0189-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kusunoki T, Kase K, Ikeda K. A case of orbital apex syndrome due to Pseudomonas aeruginosa infection. Clin Pract. 2011;1(4):e127. doi: 10.4081/cp.2011.e127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blum RA, Rodvold KA. Recognition and importance of Staphylococcus epidermidis infections. Clin Pharm . 1987;6(6):464–75. [PubMed] [Google Scholar]
- 18.Anders UM, Taylor EJ, Martel JR, Martel JB. Acute orbital apex syndrome and rhino-orbito-cerebral mucormycosis. Int Med Case Rep J. 2015;8:93–6. doi: 10.2147/IMCRJ.S83036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamanoi T, Shibano K, Soeda T, Hoshi A, Matsuura Y, Sugiura Y. Intracranial invasive aspergillosis originating in the sphenoid sinus: a successful treatment with high-dose itraconazole in three cases. Tohoku J Exp Med. 2004;203(2):133–9. doi: 10.1620/tjem.203.133. [DOI] [PubMed] [Google Scholar]
- 20.Kulkarni R, Pujari SS, Gupta D, Ojha P, Dhamne M, Bolegave V. Cerebrovascular involvement in mucormycosis in COVID-19 pandemic. J Stroke Cerebrovasc Dis. 2022;31(2):106231. doi: 10.1016/j.jstrokecerebrovasdis.2021.106231. [DOI] [PMC free article] [PubMed] [Google Scholar]


