Figure 8.
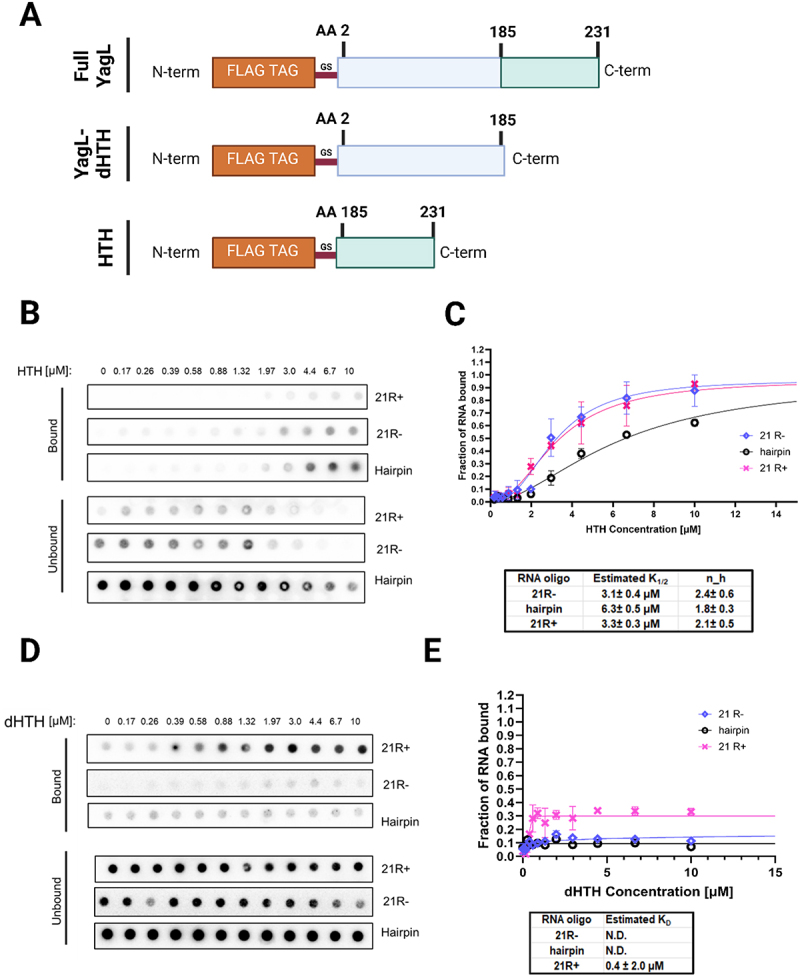
The predicted HTH domain of YagL is responsible for its RNA-binding activity. (A) schematic visualization of the two YagL protein truncations constructed to determine their contribution to RNA-binding. YagL-dHTH includes the N-terminus domain of the protein minus a predicted HTH domain located at the C-terminus of the protein. HTH consists of the last 46 amino acids of YagL. Nucleotide sequences of these protein truncations are included in supplementary table S4. (B&D) filter binding assays were performed to evaluate binding of the HTH and dHTH protein truncations to two single-stranded 21mer RNA sequences, ‘21 R+’ and ‘21 R-’, and a small, structured RNA, ‘hairpin’. (C&E) the bound and unbound fractions were quantified to generate binding curves for the HTH and the dHTH truncated protein respectively. For the HTH truncation, sigmoidal curves were obtained, suggesting cooperative binding of ~2 functional units (hill coefficients: 2.4 ± 0.6 (21 R-), 1.8 ± 0.3(hairpin), and 2.1 ± 0.5 (21 R+). Image created with Biorender.com.
