Abstract
Background:
Hypotension following induction of general anesthesia (GA) is commonly observed. Ultrasound (US) measurement of collapsibility index (CI) of the inferior vena cava (IVC) for predicting postinduction hypotension has been studied. As there is limited data available comparing the diagnostic accuracy of subclavian vein (SCV) versus IVC-CI, we performed this observational study.
Methods:
A total of 132 adult patients scheduled for elective surgery under GA were enrolled. US measurements of three readings of maximum and minimum diameters of SCV and IVC were recorded during both quiet and deep breathing, and the mean of three values was calculated. CI was derived using the formula: (dmax – dmin) × 100/dmax. Subsequently, GA was administered using standard technique, irrespective of the findings of SCV and IVC measurements. The administered drugs and dosage were recorded. Hemodynamic parameters were collected at baseline and then at every minute for the first 20 min. The primary objective was to compare the diagnostic accuracies of SCV-CI and IVC-CI for prediction of postinduction hypotension during quiet breathing. The secondary objectives were to compare the diagnostic accuracies during deep breathing and find the correlation between IVC-CI and SC-CI during quiet and deep breathing, incidence of hypotension, and time required to acquire US images.
Results:
Fifty-seven patients developed postinduction hypotension. During quiet breathing, SCV-CI ≥10% had a sensitivity of 68% and specificity of 56% (area under curve [AUC] [95% confidence interval {CI}] of 0.659 [0.56–0.75]; P = 0.002), while IVC-CI ≥34% had a sensitivity of 70% and specificity of 59% (AUC [95% CI] of 0.672 [0.58–0.76]; P = 0.001) for prediction of postinduction hypotension. During deep breathing, both SCV-CI and IVC-CI had moderate accuracy (P = 0.001 for both). Pearson’s correlation showed a significant positive correlation between SCV-CI and IVC-CI with a correlation coefficient (r) of 0.313 during quiet breathing and 0.379 during deep breathing (P < 0.001). The time required for acquiring US images was significantly less for SCV compared to IVC during both quiet and deep breathing (P < 0.001 for both).
Conclusion:
Both SCV-CI and IVC-CI were found to have good and comparable diagnostic accuracy for the prediction of postinduction hypotension. We also found a significant positive correlation between SCV-CI and IVC-CI. In comparison to IVC, US scanning of SCV took lesser time to acquire the images.
Keywords: Collapsibility index, inferior vena cava, postinduction hypotension, subclavian vein, ultrasound
Introduction
Hypotension following the induction of general anesthesia (GA) is commonly observed with a reported incidence as high as 50%.[1,2] The anesthetic drugs used for the induction of GA lead to cardiovascular depression and peripheral vasodilatation resulting in hemodynamic instability and decreased organ perfusion.[2,3] Patients with underlying cardiovascular disease and hypovolemia are at a higher risk of having an exaggerated hypotensive response, resulting in increased perioperative morbidity and mortality.[4,5,6,7]
Various objective indices such as perfusion index and its derived parameters have been used to predict postinduction hypotension, but none is found to have a good predictive value.[8] Ultrasound (US)-guided measurements of inferior vena cava (IVC) diameter and its respiratory variability known as collapsibility index (CI) have been found to have a good sensitivity and specificity in predicting postinduction hypotension.[9,10,11] However, in patients with abdominal distension, obesity, and pregnancy, it becomes difficult to scan IVC using US. To overcome this problem, measuring the CI of subclavian vein (SCV) during deep breathing has been reported by a few studies to have good reliability.[12,13]
However, there is limited data available comparing the diagnostic accuracy of US-guided SCV-CI versus IVC-CI in predicting postinduction hypotension.[13] Hence, in this observational study, we compared the CI of these two major veins during both quiet and deep breathing. Our hypothesis was that there would be no difference in the diagnostic accuracies of SCV-CI and IVC-CI for the prediction of postinduction hypotension during both quiet and deep breathing. The primary objective of our study was to compare the diagnostic accuracies of SCV-CI and IVC-CI for prediction of postinduction hypotension during quiet breathing. The secondary objectives were to compare the diagnostic accuracies during deep breathing and find the correlation between IVC-CI and SC-CI during quiet and deep breathing, incidence of hypotension following the induction of GA, and the total time required to acquire US images and for measurement of the venous diameters.
Methodology
Ethical approval for this study (AIIMS/IEC/2020/3159) was provided by the university’s Institutional Review Board, AIIMS Jodhpur, India (Chairperson Prof. Pravin Sharma) on September 23, 2020, and written informed consent was obtained from all subjects participating in the trial. The trial was registered before patient enrollment at clinicaltrials.gov (CTRI/2020/12/029511, principal investigator: Sadik Mohammed, date of registration: December 2, 2020). Our study adheres to the principles of declaration of Helsinki, and the manuscript follows the applicable STrengthening the Reporting of OBservational studies in Epidemiology guidelines. This study was conducted between December 2020 and May 2022.
Patients of either gender, aged more than 18 years, and scheduled to undergo elective surgery under GA were enrolled. Patients with autonomic nervous system disorders, patients with implanted pacemaker/cardioverter, noncooperative patients, and pregnant patients were excluded. During the preoperative visit, patients’ demographics and baseline vital parameters were recorded. Detailed history was taken, and general physical and systemic examination was performed. Routine laboratory investigations were carried out as per the institutional protocol.
US scanning technique
On the day of surgery, US-guided measurements of SCV and IVC parameters were recorded with the patients lying supine and spontaneously breathing. All the measurements were performed using US machine (Venue Go; GE Healthcare, Chicago, IL, USA) with a high-frequency (6–13 MHz) linear transducer set to vascular mode for SCV and a phased array transducer (2–5 MHz) set to abdominal for IVC scanning.
For SCV scanning, the transducer was placed just below the right clavicle in a perpendicular orientation and the vessels (the axillary vein [AV] and artery in short axis), pleura, and rib were identified. The probe was then rotated clockwise while keeping the vessels in the center of the screen till the long-axis view of AV was obtained. Pulse wave Doppler was used to differentiate the vein from the artery. After obtaining the long-axis view, the probe was moved until the vein was no longer visible medially, as the acoustic shadow of the clavicle would obscure the view below it (hammer sign) [Figure 1a]. For IVC scanning, the transducer was placed in the subxiphoid area with the pointer oriented cephalad. The probe was moved laterally to the right and cranially till a two-dimensional long-axis image of IVC, as it enters the right atrium, was obtained [Figure 1b]. Pulse wave Doppler was used to differentiate IVC from the aorta.
Figure 1.
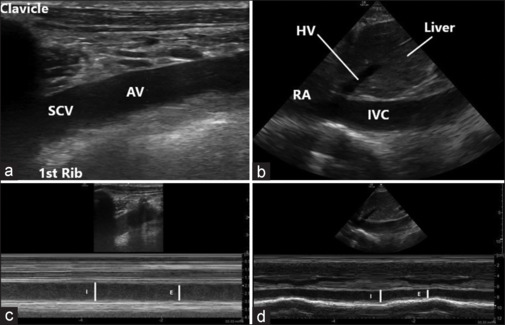
Ultrasound images: (a) 2D image of the subclavian vein as a continuation of axillary vein at the outer border of the first rib; (b) 2D image of the inferior vena cava as it enters the right atrium; (c) M-mode image with the cursor placed over the subclavian vein just proximal to the outer border of the first rib; (d) M-mode image with the cursor placed over the inferior vena cava just distal to the entry of the hepatic vein. 2D=two-dimensional, AV=axillary vein, E=expiration, HV=hepatic vein, I=inspiration, IVC=inferior vena cava, RA=right atrium, SCV=subclavian vein
Variations in venous diameters were assessed during inspiratory and expiratory phases of the respiratory cycle using M-mode, with the cursor placed medial to the lower border of the first rib for SCV [Figure 1c] and 1 cm proximal to the opening of hepatic vein for IVC [Figure 1d]. The measurements were taken during both quiet and deep breathing. Maximum and minimum diameters over a single respiratory cycle were measured using built-in software.
All the US scans were performed by two investigators (SM and SC) having more than 3 years of experience in using point-of-care US, and were not involved in further management of the patients and in data collection. All the scans were reviewed by another experienced investigator (GB). To ensure consistent measurements, we performed three scans of both SCV and IVC in all our patients and the mean of three values was recorded. If there was a difference of more than 0.2 cm in diameter between any two of the measurements, that patient’s data was excluded from the study. CI was calculated using the mean of three values and was expressed as a percentage using the formula: CI = (dmax – dmin) × 100/dmax. Time to acquire US images was defined as the time between probe placement at the desired site and the acquisition of still M-mode image on US machine.
Administration of GA
Demographic data including age, sex, height, weight, and body mass index (BMI) were recorded. Subsequently, standard monitoring was attached and GA was administered. Induction was performed using standard technique in all the patients, irrespective of the finding of SCV and IVC measurements. For the induction of GA, we administered inj. midazolam, inj. fentanyl, and inj. propofol to all the patients and their dosages were recorded. The airway management was facilitated using a nondepolarizing neuromuscular blocking drug (inj. atracurium). After abolition of the verbal response, airway was secured using either a supraglottic airway device or an endotracheal tube. Heart rate and blood pressure (BP) readings were collected just before induction (baseline) and then at every minute after induction for the first 20 min. Technique used for the measurement of BP (noninvasive or invasive) was also noted. No patient received any intravenous (IV) fluid before the induction of GA.
Episode of hypotension was defined as a mean arterial pressure (MAP) of lower than 60 mmHg and/or more than 30% decrease in MAP value from the baseline. Severe hypotension was defined as MAP less than 55 mmHg and/or >40% fall in MAP value from the baseline, and prolonged hypotension was defined as hypotension lasting for ≥2 min. These were treated using either fluid boluses or incremental doses of vasopressors.
Statistical analysis
Sample size was calculated based on the sensitivity and specificity of IVC-CI for predicting postinduction hypotension. Zhang and Critchley[9] reported a sensitivity of 78.6% and a specificity of 91.7% for IVC-CI at an optimum cutoff value of 43%. To detect a difference of 10% in the sensitivity of IVC-CI and SCV-CI, at 5% significance and 80% power, the required sample size was a maximum of 178 subjects and a minimum of 78 subjects (maximum and minimum probability of disagreement between sensitivity was 0.17 and 0.10, respectively).[14] The mean of these two values was 128, and we recruited 132 patients in our study.
Data collected during the study was compiled using Microsoft Excel spreadsheets. The normality of data was tested with Kolmogorov–Smirnov one-sample test. Data were presented as mean ± standard deviation (SD) for continuous variables and as absolute numbers or percentages for categorical variables. The development of clinically significant hypotension after induction was analyzed with respect to patient characteristics, hemodynamics, and SCV and IVC measurements using Student’s t-test or χ2 test as appropriate. The receiver operating characteristic curve for the episode of hypotension as well as for severe postinduction hypotension and CI of SCV and IVC were constructed and compared. Pearson correlation coefficient (r) was used to test the relationship between SCV and IVC measurements during both quiet and deep breathing.
Results
A total of 164 patients were screened, of which 132 patients’ data was analyzed after applying the inclusion and exclusion criteria [Figure 2]. We had to exclude 13 patients from our study as we were unable to visualize IVC clearly, but we were able to visualize SCV in all these patients. The demographic characteristics (age, weight, height, BMI, gender), American Society of Anesthesiologists physical status, fasting duration, and baseline hemodynamic parameters were comparable between the two groups (with and without postinduction hypotension) (P > 0.05) [Table 1].
Figure 2.
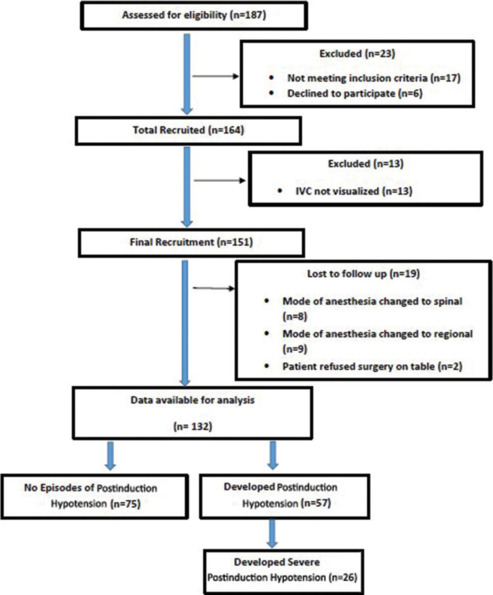
Flow of patients during the study period
Table 1.
Patient demographic characteristics, ASA physical status, fasting duration, and baseline vital parameters between the two groups
| Parameters | Total (n=132) | Hypotension |
Mean difference (95% CI) | P | |
|---|---|---|---|---|---|
| Yes (n=57) | No (n=75) | ||||
| Age (in years) | 39.54±16.7 | 40.65±18.1 | 38.69±15.7 | 1.96 (−7.78 to 3.88) | 0.50 |
| Weight (in kg) | 61.23±11.4 | 59.64±12.4 | 62.44±10.4 | -2.81 (-1.2 to 6.8) | 0.17 |
| Height (in cm) | 167.27±9.3 | 166.77±9.6 | 167.65±9.1 | -0.88 (-2.3 to 4.1) | 0.59 |
| BMI (kg/m2) | 21.93±4.2 | 21.28±3.8 | 22.41±4.5 | -1.13 (-0.34 to 2.6) | 0.13 |
| Gender (M/F)a | 86/46 | 36/21 | 50/25 | - | 0.67 |
| ASA physical status (I/II/III)a | 83/46/03 | 37/18/02 | 46/28/01 | - | 0.33 |
| Fasting duration (in hours) | 8.74±1.4 | 9.00±1.5 | 8.55±1.3 | 0.45 (-0.94 to 0.03) | 0.06 |
| HR (per min) | 82.92±12.2 | 83.93±12.1 | 82.15±12.3 | 1.78 (−6.02 to 2.46) | 0.40 |
| SBP (mmHg) | 125.0±12.6 | 125.47±12.6 | 124.75±12.8 | 0.73 (−5.13 to 3.7) | 0.74 |
| DBP (mmHg) | 77.83±11.2 | 78.49±14.3 | 77.32±8.2 | 1.17 (-0.68 to 6.21) | 0.11 |
| MAP (mmHg) | 93.56±9.88 | 94.3±11.5.4 | 93.0±8.5 | 1.3 (−4.76 to 2.12) | 0.45 |
aData is expressed as mean±SD or numbers. ASA=American Society of Anesthesiologists, BMI=Body mass index, CI=Confidence interval, DBP=Diastolic blood pressure, HR=Heart rate, MAP=Mean arterial pressure, SBP=Systolic blood pressure, SD=Standard deviation
The lowest MAP recorded after induction of GA was used to calculate the percentage decrease in MAP from baseline for each patient. Of the recruited 132 patients, 57 (43.2%) patients developed episodes of postinduction hypotension, of which 26 patients developed severe hypotension. None of the patients had prolonged hypotension. BP was measured using noninvasive technique in 110 patients, and the remaining 22 patients had invasive arterial catheter placed. The type of anesthetic drug and dosage used had no significant effect on the incidence or severity of postinduction hypotension. Similarly, we found no difference between the groups with respect to the type of the airway device used, number of attempts and time required to secure the airway, and the amount of fluid administered intraoperatively.
Various SCV and IVC parameters were measured with US and their comparison between the two groups are shown in Table 2. Both SCV-CI (P = 0.001) and IVC-CI (P = 0.001) were found to have good predictive ability in detecting the likelihood of postinduction hypotension during both quiet and deep breathing. During quiet breathing, SCV-CI ≥10% had a sensitivity of 68% and specificity of 56% (AUC [95% confidence interval {CI}] of 0.659 [0.56–0.75]; P = 0.002), while IVC-CI ≥34% had a sensitivity of 70% and specificity of 59% (AUC [95% CI] of 0.672 [0.58–0.76]; P = 0.001) for prediction of postinduction hypotension [Figure 3a]. Similarly, during deep breathing, SCV-CI ≥27% had a sensitivity of 71% and specificity of 51% (AUC [95% CI] of 0.662 [0.57–0.76]; P = 0.002), while IVC-CI ≥50% had a sensitivity of 70% and specificity of 56% (AUC [95% CI] of 0.679 [0.59–0.77]; P < 0.001) for prediction of postinduction hypotension [Figure 3b]. For prediction of severe postinduction hypotension during quiet breathing, the optimum cutoff value, sensitivity, and specificity for SCV-CI were ≥11%, 73%, and 56%, respectively, while for IVC-CI, they were ≥34%, 76%, and 67%, respectively [Figure 3c]. Similarly, during deep breathing, the values for SCV-CI were ≥33%, 73%, and 53%, respectively, while for IVC-CI, they were ≥55%, 73%, and 60%, respectively [Figure 3d].
Table 2.
Comparison of ultrasound measured SCV and IVC parameters between the two groups
| Parameters | Total (n=132) | Hypotension |
Mean difference (95% CI) | P | |
|---|---|---|---|---|---|
| Yes (n=57) | No (n=75) | ||||
| SCV quiet breathing | |||||
| Max diameter (cm) | 0.97±0.20 | 0.98±0.22 | 0.96±0.18 | 0.02 (-0.09-0.05) | 0.53 |
| Min diameter (cm) | 0.84±0.22 | 0.82±0.25 | 0.86±0.19 | -0.04 (-0.04-0.11) | 0.30 |
| Collapsibility index (%) | 14.36±10.64 | 17.88±12.79 | 11.69±7.73 | 6.19 (−9.74-2.64) | 0.001 |
| SCV deep breathing | |||||
| Max diameter (cm) | 0.98±0.20 | 0.99±0.22 | 0.97±0.18 | 0.02 (-0.25-0.19) | 0.43 |
| Min diameter (cm) | 0.61±0.27 | 0.56±0.29 | 0.65±0.25 | -0.09 (0.00-0.18) | 0.05 |
| Collapsibility index (%) | 39.51±21.02 | 46.33±21.91 | 34.32±18.86 | 12.01 (-19.04-4.97) | 0.001 |
| IVC quiet breathing | |||||
| Max diameter (cm) | 1.88±0.4 | 1.76±0.3 | 1.97±0.4 | -0.21 (0.08-0.33) | 0.001 |
| Min diameter (cm) | 1.31±0.4 | 1.13±0.3 | 1.46±0.4 | -0.33 (0.19-0.46) | 0.001 |
| Collapsibility index (%) | 30.63±14.6 | 35.32±13.9 | 27.06±14.3 | 8.26 (-13.16--3.35) | 0.001 |
| IVC deep breathing | |||||
| Max diameter (cm) | 1.91±0.38 | 1.79±0.35 | 1.99±0.38 | -0.19 (0.07 to 0.32) | 0.003 |
| Min diameter (cm) | 0.91±0.45 | 0.77±0.48 | 1.02±0.39 | -0.25 (0.10 to 0.40) | 0.001 |
| Collapsibility index (%) | 54.20±18.06 | 60.19±18.03 | 49.64±16.82 | 10.55 (-16.58-−4.52) | 0.001 |
Data is expressed as mean±SD. Values in bold denote significant P. CI=Confidence interval, IVC=Inferior vena cava, SCV=Subclavian vein, SD=Standard deviation
Figure 3.
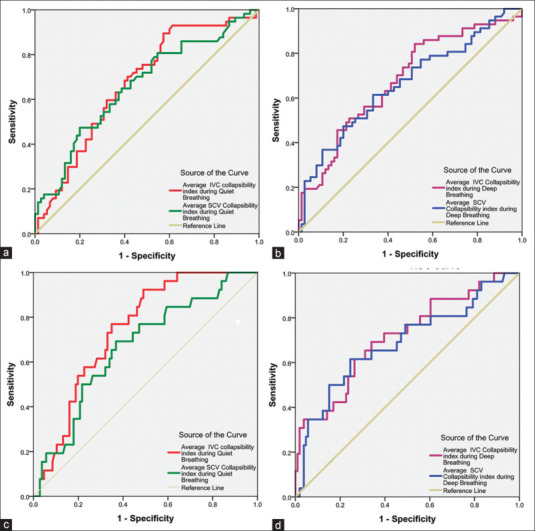
ROC curves showing the ability of subclavian vein and inferior vena cava collapsibility index to predict (a) postinduction hypotension during quiet breathing; (b) postinduction hypotension during deep breathing; (c) severe postinduction hypotension during quiet breathing; (d) severe postinduction hypotension during deep breathing. ROC = receiver operating characteristic
Pearson’s correlation showed significant positive correlation between SCV-CI and IVC-CI with a correlation coefficient (r) of 0.313 during quiet breathing and 0.379 during deep breathing (P < 0.001) [Figure 4a and b].
Figure 4.
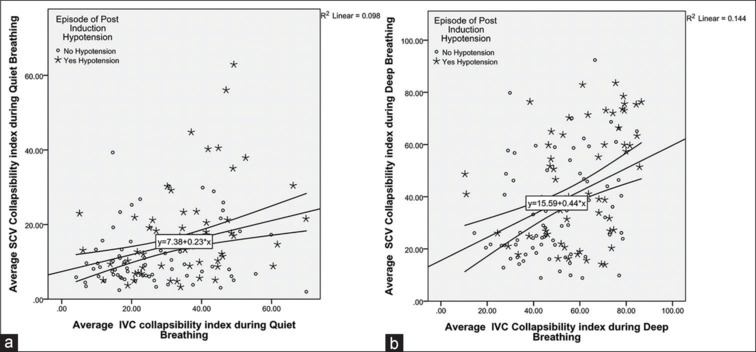
Scattered plots (Pearson’s correlation) showing average collapsibility index of the subclavian vein versus inferior vena cava (a) during quiet breathing and (b) during deep breathing
Time required to acquire the US images and to measure the venous diameters was significantly lesser for SCV compared to IVC during both quiet and deep breathing. During quiet breathing, we required 2.37 ± 0.71 (mean ± SD) min for SCV compared to 3.2 ± 0.9 min for IVC, with a mean difference (95% CI) of 0.82 (0.66–0.98) min (P < 0.001). Similarly, during deep breathing, we needed 2.14 ± 0.63 min for SCV compared to 2.83 ± 0.94 min for IVC, with a mean difference (95% CI) of 0.7 (0.55–0.84) min (P < 0.001).
Discussion
In the present observational study, we found that the minimum diameters of both SCV and IVC significantly decreased during deep breathing, resulting in increase in CI. This showed a good diagnostic accuracy for prediction of postinduction hypotension. In comparison to IVC, it was easy to visualize SCV in all our patients. Moreover, obtaining the US images and measurement of the diameters of SCV required significantly lesser time.
Hypotension is commonly observed following the induction of GA and can have serious consequences. In routine clinical practice, hypotension is treated using fluids and vasopressors.[15,16] In an ideal scenario, we should be able to predict the occurrence of hypotension even before it occurs, so that appropriate measures such as choosing an appropriate induction agent, administering a lower dosage, and preloading with fluids can be undertaken.[17,18]
The incidence of postinduction hypotension reported in the literature ranges from 15% to 60%.[1,2] This wide variation is observed because the definition of postinduction hypotension is not standardized. Moreover, the incidence and severity of hypotension is influenced by many factors such as age, ethnicity, preoperative fasting status, comorbidities, type and dose of anesthetic agent used, etc.[17] Incidence of hypotension observed in our study is comparable to the findings of other studies.[9,13] Zhang and Critchley[9] found an incidence of 46.7% in their patients following the induction of GA using propofol. Similarly, Au et al.[11] also observed an incidence of 55% following the administration of propofol, but hypotension was defined in their study as systolic BP <90 mmHg. In contrast, Choi et al.[12] observed an incidence of only 24.7% following the induction of GA. This difference in incidence could be attributed to the dosage of medications administered in their study, which was much lesser compared to our study.
We observed significant decrease in the minimum diameters of both the larger veins of the body, leading to higher CI during deep breathing. Our findings are in accordance with the results of other studies.[16] Similar to our study, Rose et al.[13] evaluated the CI of SCV and IVC for predicting postinduction hypotension. The authors found a cutoff value of 36% for SCV-CI with a sensitivity of 90% and specificity of 87% and a cutoff value of 37% for IVC-CI with a sensitivity of 94% and specificity of 84% during deep breathing. Zhang and Critchley[9] observed an AUC of 0.89 (0.62–0.95) for IVC-CI (P < 0.0001) and found an optimal cutoff value of 43% with a sensitivity of 93.3% (68.1%–99.8%) and specificity of 81.5% (76.2%–99.9%) in patients without any preexisting cardiovascular disease. Similarly, Szabó et al.[10] evaluated the role of IVC parameters during quiet breathing and found an IVC-CI of >50% with greater specificity (90.0% [95% CI: 78.2%–96.7%]) and low sensitivity (45.5% [95% CI: 28.1%–63.7%]). In another study by Choi et al.,[12] a higher CI for SCV was observed during both quiet (P = 0.009) and deep inspiration (P = 0.002). However, after adjusting for the confounding variables, the authors found SCV-CI to be a significant predictor of decrease in MAP only during deep breathing (P < 0.001) and not during quiet breathing (P = 0.127).
We found limited number of studies looked at the time required to obtain US images. Rose et al.[13] required lesser time to acquire and measure SCV data (mean time of 40.37 sec) compared to IVC data (48.44 sec, P < 0.0001). Similarly, Kent et al.[18] required lesser time to acquire and analyze the diameters of SCV (70 sec) in comparison to IVC (99 sec, P < 0.02). In contrast to the findings of these two studies, we required a little longer time to obtain US images and measure the venous diameters. This difference could be attributed to the number of scans obtained, as in our study, we performed three scans for each vein in all our patients, whereas a single scan was performed in the above two studies.
With respect to the anesthetic drugs and dosage administered, our results are comparable to the findings of the other studies.[11,13] However, Choi et al.[12] required much lower dosage of medications compared to our study, and in one study, etomidate was used for the induction of GA.[9]
Previous studies have reported inability to visualize IVC in approximately 15%–20% of the patients (due to abdominal pathologies, obesity, and pregnancy), which is in accordance with our study findings.[9,15,16] However, we were able to scan SCV in all our patients. This might have been possible because SCV drains into the superior vena cava just behind the sternoclavicular joint, and hence is less susceptible to get influenced by external compression. Similar to our findings, Choi et al.[12] were also able to adequately visualize SCV in 77 out of the 79 patients enrolled in their study. This high feasibility can be regarded as a strength of SCV-CI measurement, which implies its potential for wider application. Measuring the CI of either of these veins can be employed in routine clinical practice to know which patients are likely to become hypotensive, particularly those who are over the age of 50 years and with underlying cardiovascular disease, so that appropriate preventive and corrective measures can be undertaken.
Our study has a few notable limitations. Firstly, as this is an observational trial, bias to trial design cannot be completely excluded. Secondly, we did not include patients undergoing emergency surgeries and those having an underlying significant cardiovascular disease. Therefore, our findings need to be validated at the extremes of CI. However, as we took the mean of three scans and excluded those patients’ data with a difference of more than 0.2 cm in diameter between any two of the measurements, our results are reliable. Thirdly, as we did not collect the data regarding the type of surgery the patients were scheduled for, our study results cannot be applied across all surgical specialties. But as we used propofol in our study, which is the most commonly used IV induction agent in majority of the patients worldwide, our results can be generalized to a larger population.
In summary, for predicting postinduction hypotension, SCV-CI and IVC-CI had comparable and moderately good diagnostic accuracies during both quiet and deep breathing. We found a significant positive correlation between SCV-CI and IVC-CI. In comparison to IVC, US scanning of SCV was easier and required significantly lesser time for obtaining US images for the measurement of its diameters.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Smischney NJ, Beach ML, Loftus RW, Dodds TM, Koff MD. Ketamine/propofol admixture (ketofol) is associated with improved haemodynamics as an induction agent: A randomised, controlled trial. J Trauma Acute Care Surg. 2012;73:94–101. doi: 10.1097/TA.0b013e318250cdb8. [DOI] [PubMed] [Google Scholar]
- 2.Wesselink EM, Kappen TH, Torn HM, Slooter AJC, van Klei WA. Intraoperative hypotension and the risk of postoperative adverse outcomes: A systematic review. Br J Anaesth. 2018;121:706–21. doi: 10.1016/j.bja.2018.04.036. [DOI] [PubMed] [Google Scholar]
- 3.Latson TW, Ashmore TH, Reinhart DJ, Klein KW, Giesecke AH. Autonomic reflex dysfunction in patients presenting for elective surgery is associated with hypotension after anaesthesia induction. Anesthesiology. 1994;80:326–37. doi: 10.1097/00000542-199402000-00013. [DOI] [PubMed] [Google Scholar]
- 4.Jain U, Laflamme CJA, Aggarwal A, Ramsay JG, Comunale ME, Ghoshal S, et al. Electrocardiographic and hemodynamic changes and their association with myocardial infarction during coronary artery bypass surgery. Anesthesiology. 1997;86:576–91. doi: 10.1097/00000542-199703000-00009. [DOI] [PubMed] [Google Scholar]
- 5.Reich DL, Bodian CA, Krol M, Kuroda M, Osinski T, Thys DM. Intraoperative hemodynamic predictors of mortality, stroke and myocardial infarction following coronary artery bypass surgery. Anesth Analg. 1999;89:814–22. doi: 10.1097/00000539-199910000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Bijker JB, van Klei WA, Vergouwe Y, Eleveld DJ, van Wolfswinkel L, Moons KG, et al. Intraoperative hypotension and 1-year mortality after noncardiac surgery. Anesthesiology. 2009;111:1217–26. doi: 10.1097/ALN.0b013e3181c14930. [DOI] [PubMed] [Google Scholar]
- 7.Walsh M, Devereaux PJ, Garg AX, Kurz A, Turan A, Rodseth RN, et al. Relationship between intraoperative mean arterial pressure and clinical outcomes after noncardiac surgery: Toward an empirical definition of hypotension. Anesthesiology. 2013;119:507–15. doi: 10.1097/ALN.0b013e3182a10e26. [DOI] [PubMed] [Google Scholar]
- 8.Cheung CC, Campbell AMN, Frost S, Gilbert K, Michota F, Seal D, et al. Predictors of intraoperative hypotension and bradycardia. Am J Med. 2015;128:532–8. doi: 10.1016/j.amjmed.2014.11.030. [DOI] [PubMed] [Google Scholar]
- 9.Zhang J, Critchley AHL. Inferior vena cava ultrasonography before general anaesthesia can predict hypotension after induction. Anesthesiology. 2016;124:580–9. doi: 10.1097/ALN.0000000000001002. [DOI] [PubMed] [Google Scholar]
- 10.Szabó M, Bozó A, Darvas K, Horváth A, Iványi DZ. Role of inferior vena cava collapsibility index in the prediction of hypotension associated with general anaesthesia: An observational study. BMC Anesthesiol. 2019;19:139. doi: 10.1186/s12871-019-0809-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Au AK, Steinberg D, Thom C, Shirazi M, Papanagnou D, Ku SB, et al. Ultrasound measurement of inferior vena cava collapse predicts propofol induced hypotension. Am J Emerg Med. 2016;34:1125–8. doi: 10.1016/j.ajem.2016.03.058. [DOI] [PubMed] [Google Scholar]
- 12.Choi MH, Chae JS, Lee HJ, Woo JH. Pre-anaesthesia ultrasonography of the subclavian/infraclavicular axillary vein for predicting hypotension after inducing general anaesthesia: A prospective observational study. Eur J Anaesthesiol. 2020;37:474–81. doi: 10.1097/EJA.0000000000001192. [DOI] [PubMed] [Google Scholar]
- 13.Rose N, Chandra M, Nishanth CC, Srinivasan R. Preoperative ultrasonographic evaluation of subclavian vein and inferior vena cava for predicting hypotension associated with induction of general anaesthesia. Anesth Essays Res. 2022;16:54–9. doi: 10.4103/aer.aer_9_22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Connor RJ. Sample size for testing differences in proportions for the paired-sample design. Biometrics. 1987;43:207–11. [PubMed] [Google Scholar]
- 15.Mohammed S, Syal R, Bhatia P, Chhabra S, Chouhan RS, Kamal M. Prediction of post-induction hypotension in young adults using ultrasound-derived inferior vena cava parameters: An observational study. Indian J Anaesth. 2021;65:731–7. doi: 10.4103/ija.IJA_1514_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaptein EM, Cantillep A, Kaptein JS, Oo Z, Thu MB, Thwe PP, et al. Comparison of respiratory variations of subclavian vein and inferior vena cava in hospitalised patients with kidney disease. Int J Nephrol Renovasc Dis. 2020;13:329–39. doi: 10.2147/IJNRD.S280458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ceruti S, Minotti B, De Vivo S, De Christophoris P, Anselmi L, Saporito A. PROtocolized care to reduce HYpotension after spinal anaesthesia (ProCRHYSA randomised trial): Study protocol for a randomised controlled trial. Contemp Clin Trials Commun. 2016;4:39–45. doi: 10.1016/j.conctc.2016.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kent A, Bahner DP, Boulger CT, Eiferman DS, Adkins EJ, Evans DC, et al. Sonographic evaluation of intravascular volume status in the surgical intensive care unit: A prospective comparison of subclavian vein and inferior vena cava collapsibility index. J Surg Res. 2013;184:561–6. doi: 10.1016/j.jss.2013.05.040. [DOI] [PubMed] [Google Scholar]


