FIGURE 2.
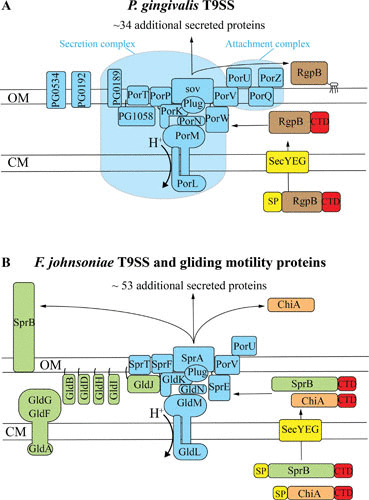
T9SS and gliding motility proteins. Proteins in blue are associated with the T9SS, and proteins in green are motility proteins that are not directly associated with the T9SS. Orthologous T9SS proteins between panels A and B are shown in the same relative positions, color, and shapes. F. johnsoniae GldK, GldL, GldM, GldN, SprA, SprE, and SprT correspond to P. gingivalis PorK, PorL, PorM, PorN, Sov, PorW, and PorT, respectively. Black lines are lipid tails on lipoproteins. Proteins secreted by the T9SS have predicted N-terminal signal peptides (yellow) that target them to the Sec system for export across the cytoplasmic membrane (CM) and C-terminal domains (red) that target them to the T9SS for secretion across the outer membrane (OM). Proteins are not drawn to scale, and stoichiometry of components is not illustrated. (A) P. gingivalis T9SS proteins. Where protein names were not available, locus tags (from P. gingivalis strain W83) were used. The gingipain protease RgpB is shown covalently attached to the outer membrane acidic lipopolysaccharide (A-LPS). Secretion complex and attachment complex are indicated by the large and small blue barrels, respectively. (B) F. johnsoniae T9SS and gliding motility proteins. SprB is a motility adhesin that is propelled by some of the other proteins shown. SprF is required for secretion of SprB but not for secretion of other proteins. SprF and nine other F. johnsoniae proteins are related to P. gingivalis PorP. F. johnsoniae PorV is required for secretion of ChiA and many other proteins, but not for secretion of SprB.
