Table 6.
SARs Associated with Compound 3a
| position/scaffold | SAR |
|---|---|
|
| |
|
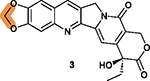
Methylene group is essential for inhibiting DDX5, down-regulating survivin expression and dispensing of topoisomerase 1 inhibition.78 |
|
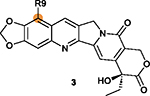
R9 position on ring I is tolerant of modifications with the substituted benzene (R-Ph) ring decreasing cytotoxicity (e.g., 59 and 60).164,168 |
|
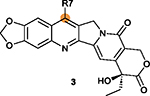
R7 on ring II is tolerant of modifications with
different rings; Five-membered heterocyclic rings are generally better than a six-member pyridine or benzene ring.82 |
|
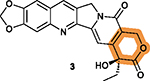
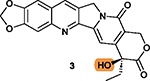
The lactone ring V may be unstable under acidic or basic conditions that may undermine 3’s activity and stability.170 |
|
The free hydroxy group on ring V is critical for
cytotoxicity;167 Protection by hydrophilic groups via ester bond such as that in 61 and compounds 62–65 reduces cytotoxicity.167,169 |
The highlighted elements are the points of discussion.
